Abstract
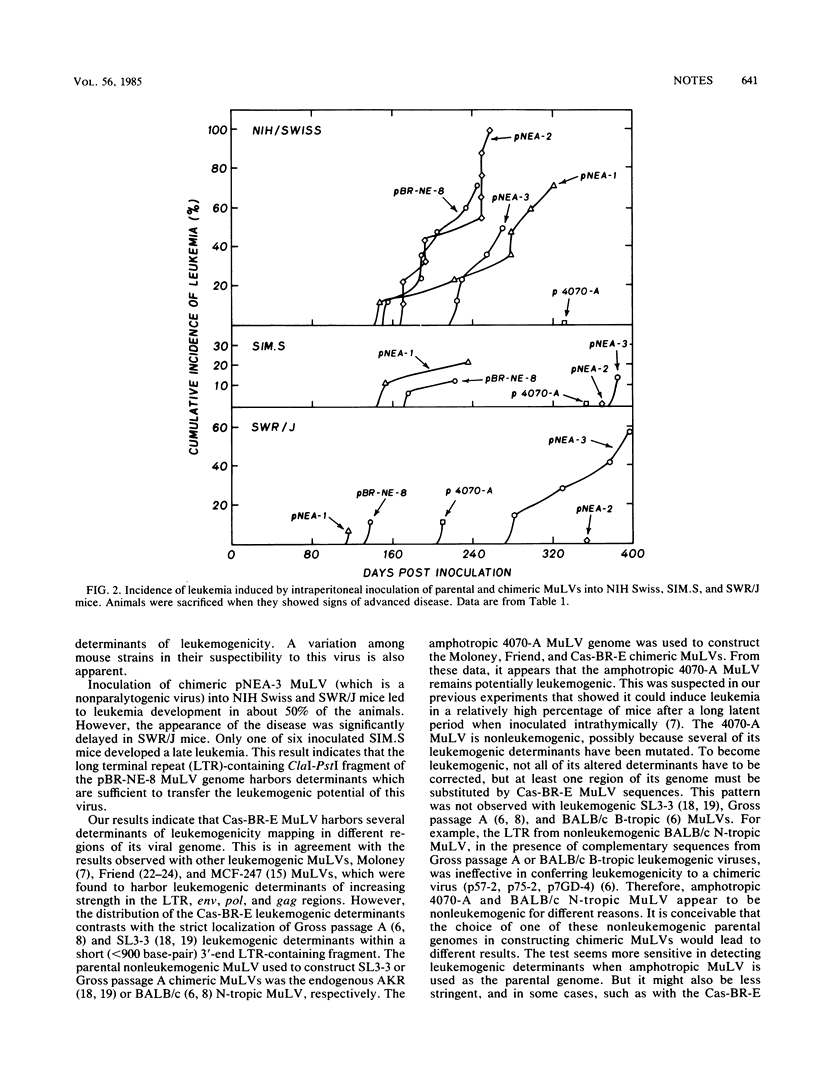
The infectious virus derived from the molecularly cloned genome of the neurotropic ecotropic murine Cas-BR-E retrovirus was previously shown to have retained the ability to induce hind-limb paralysis and leukemia when inoculated into susceptible mice (P. Jolicoeur, N. Nicolaiew, L. DesGroseillers, and E. Rassart, J. Virol. 45:1159-1163, 1983). To map the viral sequences encoding the leukemogenic determinant(s) of this virus, we used chimeric viral genomes constructed in vitro between cloned viral DNAs from the leukemogenic Cas-BR-E murine leukemia virus (MuLV) and from the related nonleukemogenic amphotropic 4070-A MuLV. Infectious chimeric MuLVs, recovered from NIH 3T3 cells microinjected with these DNAs, were inoculated into newborn NIH Swiss, SIM.S, and SWR/J mice to test their leukemogenic potential. We found that each chimeric MuLV, harboring either the long terminal repeat, the gag-pol, or the pol-env region of the Cas-BR-E MuLV genome, was leukemogenic, indicating that this virus harbors several determinants of leukemogenicity mapping in different regions of its genome. This result suggests that the amphotropic 4070-A MuLV has multiple regions along its genome which prevent the expression of its leukemogenic phenotype, and it also shows that substitution of only one of these regions for Cas-BR-E MuLV sequences is sufficient to make it leukemogenic.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. L., Scott J. L., Pal B. K., Estes J. D., Gardner M. B. Immunopathology of natural and experimental lymphomas induced by wild mouse leukemia virus. Am J Pathol. 1981 Sep;104(3):272–282. [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Villemur R., Jolicoeur P. The high leukemogenic potential of Gross passage A murine leukemia virus maps in the region of the genome corresponding to the long terminal repeat and to the 3' end of env. J Virol. 1983 Jul;47(1):24–32. doi: 10.1128/jvi.47.1.24-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson T. N., Langdon W. Y., Hoffman P. M., Hartley J. W., Morse H. C., 3rd Histologic and cell surface antigen studies of hematopoietic tumors induced by Cas-Br-M murine leukemia virus. J Natl Cancer Inst. 1984 Feb;72(2):447–454. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Davidson W. F., Ruscetti S. K., Chused T. M., Morse H. C., 3rd Wild mouse ecotropic murine leukemia virus infection of inbred mice: dual-tropic virus expression precedes the onset of paralysis and lymphoma. J Virol. 1981 Aug;39(2):597–602. doi: 10.1128/jvi.39.2.597-602.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Shimizu C. S., Rasheed S., Pal B. K., Gardner M. B. Characterization of genome structure of amphotropic and ecotropic wild mouse retroviruses. J Virol. 1982 Feb;41(2):605–614. doi: 10.1128/jvi.41.2.605-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Lenz J., Haseltine W. A. Localization of the leukemogenic determinants of SL3-3, an ecotropic, XC-positive murine leukemia virus of AKR mouse origin. J Virol. 1983 Aug;47(2):317–328. doi: 10.1128/jvi.47.2.317-328.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Oliff A., Linemeyer D., Ruscetti S., Lowe R., Lowy D. R., Scolnick E. Subgenomic fragment of molecular cloned Friend murine leukemia virus DNA contains the gene(s) responsible for Friend murine leukemia virus-induced disease. J Virol. 1980 Sep;35(3):924–936. doi: 10.1128/jvi.35.3.924-936.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., McKinney M. D., Agranovsky O. Contribution of the gag and pol sequences to the leukemogenicity of Friend murine leukemia virus. J Virol. 1985 Jun;54(3):864–868. doi: 10.1128/jvi.54.3.864-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S. A 2.4-kilobase-pair fragment of the Friend murine leukemia virus genome contains the sequences responsible for friend murine leukemia virus-induced erythroleukemia. J Virol. 1983 Jun;46(3):718–725. doi: 10.1128/jvi.46.3.718-725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Signorelli K., Collins L. The envelope gene and long terminal repeat sequences contribute to the pathogenic phenotype of helper-independent Friend viruses. J Virol. 1984 Sep;51(3):788–794. doi: 10.1128/jvi.51.3.788-794.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


