Abstract
Myeloid dendritic cells (MDC) play an important role in antigen-specific immunity and tolerance. In transplantation setting donor-derived MDC are a promising tool to realize donor-specific tolerance. Current protocols enable generation of tolerogenic donor MDC from human monocytes during 1-week cultures. However, for clinical application in transplantation medicine, a rapidly available source of tolerogenic MDC is desired. In this study we investigated whether primary human blood MDC could be transformed into tolerogenic MDC using dexamethasone (dex) and lipopolysaccharide (LPS). Human blood MDC were cultured with dex and subsequently matured with LPS in the presence or absence of dex. Activation of MDC with LPS after pretreatment with dex did not prevent maturation into immunostimulatory MDC. In contrast, simultaneous treatment with dex and LPS yielded tolerogenic MDC, that had a reduced expression of CD86 and CD83, that poorly stimulated allogeneic T-cell proliferation and production of T helper 1 (Th1) cytokines, and primed production of the immunoregulatory cytokine interleukin-10 (IL-10) in T cells. In vitro, however, these tolerogenic MDC did not induce permanent donor-specific hyporesponsiveness in T cells. Importantly, tolerogenic MDC obtained by LPS stimulation in the presence of dex did not convert into immunostimulatory MDC after subsequent activation with different maturation stimuli. In conclusion, these findings demonstrate that combined treatment with dex and LPS transforms primary human blood MDC into tolerogenic MDC that are impaired to stimulate Th1 cytokines, but strongly prime the production of the immunoregulatory cytokine IL-10 in T cells, and are resistant to maturation stimuli. This strategy enables rapid generation of tolerogenic donor-derived MDC for immunotherapy in clinical transplantation.
Keywords: corticosteroid, dendritic cells, tolerance, transplantation
Introduction
Induction of immunological tolerance, specific for transplant- or self-antigens while maintaining reactivity to other antigens, is an important goal in transplantation medicine and auto-immunity.
Myeloid dendritic cells (MDC) are the most potent antigen-presenting cells (APC) and have the unique ability to initiate primary T-cell responses.1 However, whereas mature MDC have a potent capacity to stimulate T cells, immature MDC poorly stimulate effector T cells and this is associated with the induction tolerance.1
This unique property of immature MDC has been utilized in experimental animal transplantation models in which transfer of immature donor-derived MDC prolonged allograft survival.2,3 However, in order to use immature MDC as immunotherapy in humans it is pivotal that these MDC are refractory to maturation stimuli, because humans are constantly exposed to inflammatory stimuli from pathogens and other environmental danger signals that can trigger MDC maturation, leading to T-cell activation instead of T-cell tolerance.4
Glucocorticoids are widely used as potent immunosuppressive and anti-inflammatory drugs to prevent allograft rejection and to treat autoimmune and allergic diseases.5,6 Glucocorticoids affect growth, differentiation and function of many cell types, such as T cells, macrophages, monocytes and MDC.7,8 Several studies have investigated the feasibility of using glucocorticoids for developing tolerogenic MDC. It has been demonstrated that glucocorticoids are able to induce tolerogenic MDC when present during differentiation of human monocytes into MDC. The resulting monocyte-derived MDC (Mo-MDC) remained immature upon exposure to maturation signals, as demonstrated by a low expression of the costimulatory molecules and a reduced T-cell stimulatory capacity.8,9 Importantly, these MDC were also able to induce hyporesponsiveness in alloreactive memory T cells by lack of costimulation and active suppression by B7-H1 and interleukin-10 (IL-10).10
In the transplantation setting, a major obstacle for induction of tolerance is the high frequency of pre-existing alloreactive memory T cells that recognize donor human leucocyte antigen (HLA) via the direct pathway, in other words presented on donor cells.11,12 To induce hyporesponsiveness in these T cells, immunotherapy with donor-derived tolerogenic Mo-MDC is an interesting option. However, in clinical transplantation practice, immunotherapy with glucocorticoid-induced tolerogenic Mo-MDC will be limited to living donations, as culturing these MDC from monocytes will minimally take 6 days. Because most acute rejections occur within days, immunotherapy must be started directly after transplantation. A faster way of obtaining tolerogenic MDC could be the use of immature donor blood MDC that do not require 6 day long differentiation. However, to our knowledge, no studies have been carried out on the effects of glucocorticoids on primary human blood MDC.
Here we studied whether pretreatment of freshly isolated primary immature human blood MDC with the synthetic glucocorticoid dexamethasone (dex), or combined treatment with dex and the Toll-like receptor-4 (TLR4) agonist lipopolysaccharide (LPS), leads to the induction of stable tolerogenic MDC. Immunophenotype, cytokine production and T-cell stimulatory capacity of dex-treated MDC were assessed. In addition, it was determined whether dex-treated human blood MDC were able to induce hyporesponsiveness in allogeneic T cells. To investigate whether glucocorticoid treatment had a sustained effect, dex-treated blood MDC were restimulated with different maturation stimuli.
Materials and methods
Antibodies
The following monoclonal antibodies (mAb) were used: immunoglobulin G1 (IgG1)-fluoroscein isothiocyanate (FITC), IgG1-phycoerythrin (PE), IgG1-APC, IgG1-PerCP-cy5.5, IgG2a-PerCP, CD4-PerCP, CD19-FITC, CD20-APC, CD40-APC, CD14-PE, HLA-DR-PerCP, and CD86-APC from Becton and Dickinson (Heidelberg, Germany); CD45-FITC, CD3-PE, CD56-APC, CD80-FITC from Beckman Coulter Immunotech (Marseille, France); CD8-APC from Dako (Glostrup, Denmark); CD83-FITC from Caltag Laboratories (Burlingame, CA); anti-BDCA-1 PE, CD19 and CD14 microbeads, anti-PE microbeads from Miltenyi Biotec (Bergisch Gradbach, Germany).
Isolation of human blood MDC and T cells
By means of Ficoll Isopaque gradient separation (Amersham Biosciences, Roosendaal, The Netherlands), peripheral blood mononuclear cells (PBMC) were isolated from buffycoats obtained from healthy blood donors (Sanquin Blood Bank, The Netherlands). The study was approved by the Dutch blood transfusion organization Sanquin, and all donors gave written imformed consent to use their buffy coat for research according to the ethical rules of blood donation in the Dutch blood donation law. MDC were isolated by positive selection of BDCA-1+ cells after B-cell depletion using magnetic microbeads, as previously described.13 Purity of the isolated MDC was 96 ± 4% (BDCA-1+ CD20− cells determined by flow cytometry) and viability was 96 ± 2% determined by Trypan blue exclusion.
A batch of T cells was purified from buffy coat PBMC by incubation with CD14-PE, anti-BDCA1-PE and CD19 microbeads, and subsequently with anti-PE microbeads for 15 min at 4°. T cells were enriched by negative selection over a Large Separation column using a MidiMACS separation device (Miltenyi Biotec) and contained 87% CD3+ T-cells and 11% CD56+ cells.
Generation of control-, predex- and dex MDC
Isolated MDC (300 000 MDC/well in 200 μl) were cultured with or without 1 μm dexamethasone (dex) (Pharmacy, ErasmusMC, Rotterdam) for 18 hr in RPMI+ with 10% fetal calf serum (FCS; Hyclone, Logan, UT), penicillin (100 U/ml), and streptomycin (100 ug/ml; Gibco BRL Life Technologies, Breda, The Netherlands) supplemented with 500 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Leucomax; Novartis Pharma, Arnhem, The Netherlands). Thereafter MDC were extensively washed and matured with 100 pg/ml LPS (Sigma, Zwijndrecht, The Netherlands) for 24 hr. At this point MDC that had been cultured with dex were divided into two conditions; part of the MDC were matured in absence of dex (predex MDC) and part of the MDC were matured in presence of 1 μm dex (dex MDC). MDC cultured and matured in absence of dex are referred to as control MDC. After maturation MDC were immunophenotyped, and their cytokine production and allogeneic T-cell stimulatory capacity were assessed. Recovery and viability were determined by trypan blue exclusion.
Second stimulation of control and dex MDC
Control and dex MDC (300 000 MDC/well in 200 μl) were given a second maturation stimulus consisting of either 100 pg/ml LPS, 50 pg/ml tumour necrosis factor-α (TNF-α) and 50 pg/ml IL-1β (both from Strathmann Biotech, Hannover, Germany) or CD40L-transfected J558 plasmacytoma cells14 (5000 J558 cells) for 24 hr. After 24 hr of culture with the second maturation stimulus MDC were harvested and their immunophenotype, cytokine production and T-cell stimulatory capacity were assessed.
Immunophenotyping of MDC
The following antibody combinations were used to determine maturation of MDC: anti-BDCA1-PE in combination with CD80-FITC, anti-HLA-DR-PerCP, CD86-APC, CD83-FITC, CD40-APC. Non-viable MDC were excluded from analysis using 7-AAD (Becton and Dickinson). Appropriate isotype-matched control antibodies were used. Optimal dilutions of all antibodies were established in preliminary experiments. The data were analysed on a FACScalibur using CellQuest pro software (Becton Dickinson, CA).
Allogeneic T-cell stimulatory capacity of control, predex and dex MDC
Graded numbers of MDC (40, 20,10 and 5 × 103 MDC/200 μl) were incubated with or without dex to generate control, predex and dex MDC. After 18 hr MDC were washed twice, after which 1·5 × 105 purified allogeneic T cells were added to the MDC. Alternatively, to exclude an effect of MDC death on T-cell stimulation, control, predex and dex MDC were recounted after they had been generated, and graded numbers (10, 5 and 2·5 × 103 MDC/well) were cultured with 1·5 × 105 allogeneic T cells. In addition, restimulated control and dex MDC (20 and 10 × 103 MDC/well) were cultured with 1·5 × 105 allogeneic T cells. After 5 days T-cell proliferation was assessed by measuring the incorporation of [3H]-thymidine (Radiochemical Centre, Amersham, Little Chalfont, UK); 0·5 μCi was added per well and cultures were harvested 18 hr later. T cells stimulated by phytohaemagglutinin (5 μg/ml, Murex, Paris, France) served as a positive control. In addition, culture supernatants were collected at different time points of the MDC T-cell cultures for analysis of cytokine production.
Generation of mature Mo-MDC
Monocytes were isolated from buffy coat PBMC by positive selection using CD14 microbeads and a Large Separation column (both from Miltenyi Biotec) according to the manufacturer's protocol. The purity of monocytes determined by flowcytometry with CD14-PE was 99 ± 0·2%. Monocytes were cultured at a concentration of 1·5 × 106 cells per 2 ml in RPMI+ supplemented with FCS, 50 U/ml GM-CSF and 125 U/ml IL-4 and cultured for 5 days at 37°, 5% CO2. After 2 days medium was refreshed and after 5 days 100 ng/ml LPS was added together with fresh GM-CSF and IL-4, and cells were cultured for an additional 24 hr. At day 6 the cells were harvested, quantified and maturation of Mo-MDC was assessed by flow cytometry with anti-BDCA-1 PE, HLA-DR-PerCP, CD40-APC, CD80-FITC and CD86-APC mAb together with appropriate isotype controls. Mo-MDC were then frozen to be used as APC in restimulation experiments.
Restimulation of MDC-stimulated T cells
Control and dex MDC (2 × 103 MDC) were cultured with 1·5 × 105 purified allogeneic T cells for 7 days. On day 7 the culture medium was refreshed in order to rest the T cells. On day 10 T cells were harvested and quantified per condition. Mature Mo-MDC, generated from the same donor as the MDC used in the primary stimulation, were thawed and used to restimulate the T cells. T-cells (7·5 × 104) were co-cultured with 7·5 × 103 mature Mo-MDC for 4 days, after which [3H]-thymidine incorporation was determined. Supernatants from day 4 of the restimulation cultures were harvested and frozen to determine cytokine production.
Determination of cytokine production
Levels of IL-12, IL-10, IL-6 and TNF-α in the supernatants of control, predex and dex MDC (300 000 MDC/200 μl), and of restimulated control or dex MDC were determined by standard enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience, San Diego, CA). IL-2, IL-4, IL-10 and interferon-γ (IFN-γ) production in co-cultures of MDC with allogeneic T cells were determined by the cytometric bead array technology using human cytokine flex sets according to the manufacturer's instructions (Becton Dickinson). The data were analysed on a FACSarray using CBA analysis software (Becton Dickinson).
Statistical analysis
Differences between groups of unpaired samples were statistically analysed using the Mann–Whitney test. The Wilcoxon Signed Ranks test was used to test whether differences between groups of paired samples. Analyses were performed using spss version 11.0 software. A P-value < 0·05 was considered to be statistically significant. All data are presented as means ± standard error of the mean (SEM).
Results
Dex partially blocks maturation of human blood MDC
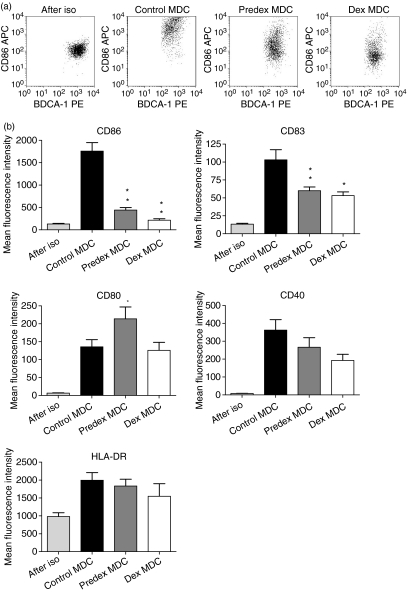
To study whether pretreatment of freshly isolated human blood MDC with dex prevented maturation, MDC were cultured overnight in the presence of dex. After 18 hr the MDC were washed and stimulated with LPS for another 24 hr. Non-treated MDC (control MDC) matured upon stimulation with LPS as demonstrated by an increased expression of CD86, CD80, CD83, CD40 and HLA-DR (Fig. 1a and b). Pretreatment of MDC with dex (predex MDC) partially prevented maturation upon LPS activation, as shown by a lower expression of expression of CD86 and CD83. HLA-DR and CD40 upregulation were not suppressed and CD80 expression was increased compared to control MDC (Fig. 1b).
Figure 1.
Effect of dex treatment on immunophenotype of human blood MDC. Control and predex MDC were generated by incubating isolated human blood MDC in the presence or absence of 1 μm dex for 18 hr and subsequently maturing them with 100 pg/ml LPS. Before addition of LPS the MDC were extensively washed to remove residual dex. Dex MDC were generated in the continuous presence 1 μm dex during both preincuation and LPS stimulation. (a) Representative dotplots of CD86 expression on freshly isolated (after iso), control, predex and dex MDC. (b) The immunophenotypic characteristics of freshly isolated (after iso), control, predex and dex MDC as analysed by flow cytometry. Depicted is the mean fluorescence intensity of the various markers. Data represent the means with SEM of 10 separate experiments. *P < 0·04, **P < 0·02 for the comparison of predex or dex MDC versus control MDC.
However, when MDC were treated with dex before and during LPS activation (dex MDC) CD80 expression was not enhanced and upregulation of CD86 and CD83 were also partially inhibited (Fig. 1a and b). Similar data for these markers were obtained when analysing the percentages of positive MDC (data not shown). The cytokine profiles of the control, predex and dex MDC were similar; during LPS stimulation they produced almost no detectable IL-10, IL-12 or IL-6 (data not shown) and their TNF-α production was not significantly different from each other (978 ± 102 pg/ml; 823 ± 79 pg/ml; 657 ± 156 pg/ml respectively).
Presence of dex during MDC activation primes IL-10 production and reduces Th1 cytokine production in T cells
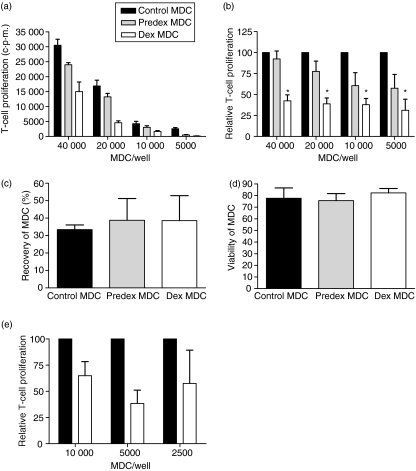
Since both predex and dex MDC had a lowered expression of co-stimulatory molecules needed for T cell activation, their allogeneic T-cell stimulatory capacity was assessed. Pretreatment with dex did not suppress the capacity of LPS-stimulated MDC to induce allogeneic T-cell proliferation. Presence of dex during MDC activation was required for suppression of their T-cell stimulatory capacity (Fig. 2a and b).
Figure 2.
Continuous presence of dex during MDC maturation results in an impaired T-cell stimulatory capacity of MDC. Control, predex and dex MDC were generated by incubating isolated human blood MDC in the presence or absence of 1 μm dex for 18 hr and subsequently maturing them with 100 pg/ml LPS in the presence or absence of 1 μm dex for 24 hr. Before addition of allogeneic T cells, MDC were extensively washed to remove dex from the cultures. Allogeneic T-cell proliferation was assessed after 5 days of incubation with graded number of MDC by [3H]-thymidine incorporation. (a) A representative experiment showing T-cell proliferation induced by control, predex and dex MDC from the same donor. (b) Relative T-cell activation by predex and dex MDC compared to control MDC in eight experiments. To exclude variations in absolute proliferation between experiments, [3H]-thymidine incorporation in T cells stimulated with control MDC was normalized to 100% for each MDC number, thereby allowing easy pairwise comparison. Proliferation of T cells stimulated by predex and dex MDC was calculated by the following formula: Counts of T cells stimulated with predex or dex MDC divided by counts of T cells stimulated by control MDC times 100%. Data represent mean with SEM of eight separate experiments. *P < 0·01 for comparison of dex MDC versus control MDC. (c) Percentage recovery of control, predex, and dex MDC from wells after LPS activation. (d) Viability of MDC, determined by trypan blue exclusion, after LPS activation. (e) Relative T-cell activation by recounted dex MDC compared to recounted control MDC. MDC were recounted after LPS stimulation and graded numbers of MDC were used to stimulate allogeneic T cells. Data represent mean with SEM of five separate experiments.
To exclude that the observed reduced T-cell stimulatory capacity was a result of dex-induced MDC death, MDC recovery and viability after incubation with dex and maturation with LPS were analysed. There were no significant differences in MDC recovery and viability, between control, predex and dex MDC (Fig. 2c and d). Moreover, when we recounted MDC before adding T cells, dex MDC also exhibited a lower allogeneic T-cell stimulatory capacity, indicating that dex only affects function and not survival of MDC (Fig. 2e).
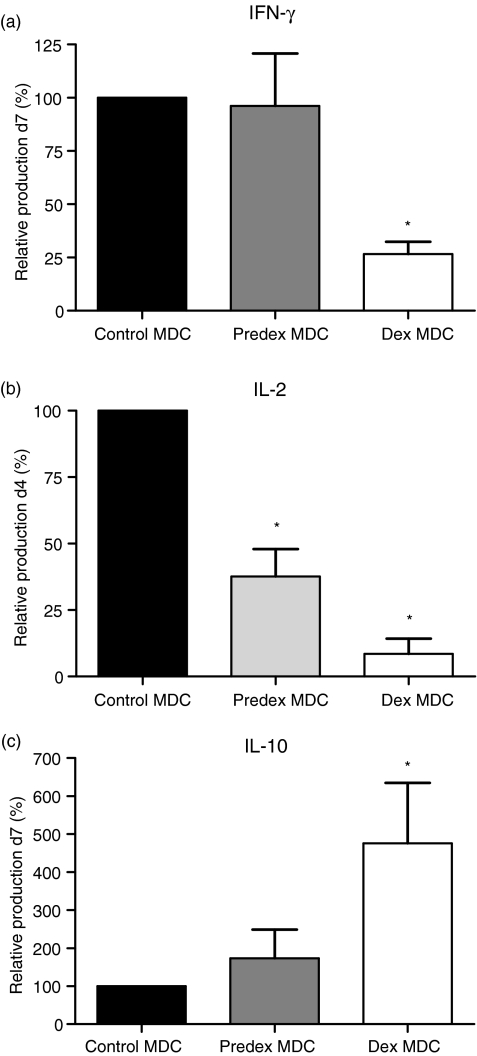
T cells activated with control MDC produced peak levels of IFN-γ on day 7 and of IL-2 on day 4 (data not shown). IFN-γ production was only significantly lowered in T cells stimulated by dex MDC, but not in T cells stimulated by predex MDC (Fig. 3a), whereas IL-2 production was reduced in T cells activated with predex and dex MDC (Fig. 3b). T cells activated with dex MDC produced peak levels of IL-10 on day 7, whereas little IL-10 production was observed when T-cells were stimulated with predex or control MDC (Fig. 3c). IL-4 was not detected in the MDC T-cell co-cultures (data not shown). These results show that continuous presence of dex during LPS stimulation inhibits maturation of primary human blood MDC to immunostimulatory MDC by reducing their capacity to stimulate proliferation and production of Th1 cytokines in T-cells and by priming production of the immunoregulatory cytokine IL-10 in T-cells. Therefore the properties of these dex MDC were studied in more detail in the following experiments.
Figure 3.
Dex MDC have reduced capacity to stimulate Th1 cytokine production but induce secretion of IL-10 by T-cells. (a) Relative IFN-γ production by predex and dex MDC compared to control-MDC on day 7. Supernatants of T cells stimulated by control MDC contained on average 456 ± 131 pg/ml IFN-γ. To exclude variations in absolute IFN-γ production between experiments, IFN-γ concentration in culture media of T cells stimulated with control MDC was normalized to 100%, thereby allowing easy pairwise comparison. Data represent mean with SEM of seven separate experiments. (b) Relative IL-2 production by control, predex and dex MDC on day 4. Supernatants of T cells stimulated by control MDC contained 260 ± 100 pg/ml IL-2. (c) Relative IL-10 production by control, predex and dex MDC on day 7. Supernatants of T cells stimulated by control MDC contained 10 ± 1·3 pg/ml IL-10. Data represent mean with SEM of six separate experiments. *P ≤ 0·03 for comparison of dex MDC versus control MDC.
Dex MDC do not induce hyporesponsive T cells in vitro
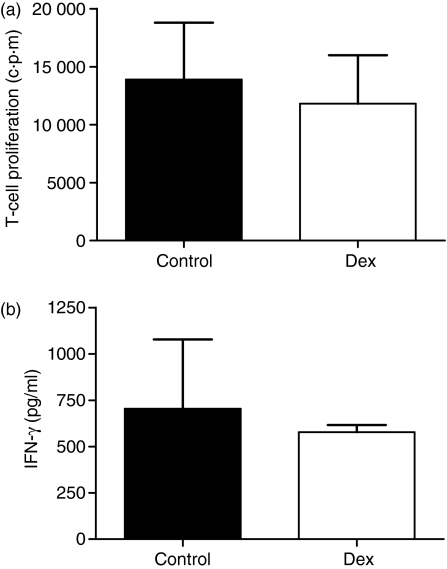
To investigate whether dex MDC induced a permanent hyporesponsiveness in allogeneic T cells, T cells were recovered from the wells of the primary MDC T-cell co-cultures and restimulated with mature Mo-MDC generated from the same donor as from which the control and dex MDC where isolated. T cells primed with either control or dex MDC displayed similar T-cell proliferation upon restimulation with mature Mo-MDC after 4 days of culture (Fig. 4a). Additionally these T cells also produced similar levels of IFN-γ (Fig. 4b) and IL-2 (data not shown) upon restimulation with mature Mo-MDC. IL-10 and IL-4 production were not detectable in restimulations of T cells with Mo-MDC. These data show that, although dex MDC have an impaired T-cell stimulatory capacity, they do not induce permanent hyporesponsiveness in T cells in vitro.
Figure 4.
Upon restimulation dex MDC-primed T cells proliferate and produce IFN-γ comparable to control MDC-primed T cells. 15 × 104 allogeneic T cells were primed with 20 × 103 control or dex MDC. At day 7 the T cells were rested for 2 days in refreshed medium. T cells (75 × 103) primed with control or dex MDC were restimulated with 75 × 102 mature mo-MDC originating from the same donor as the control- and dex MDC. (a) T-cell proliferation was assessed by [3H]-thymidine incorporation 4 days after restimulation of the control or dex MDC primed T cells. (b) IFN-γ production in the supernatant on day 4 of restimulation was determined by ELISA. Data represent mean with SEM of three separate experiments.
Dex MDC are functionally resistant to a second maturation stimulus
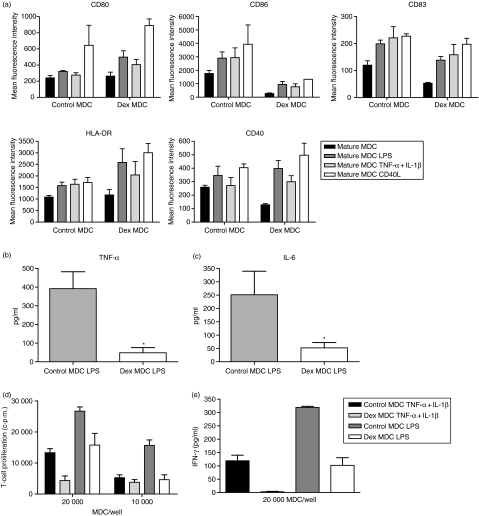
To assess whether dex MDC are resistant to a second maturation stimulus, it was first studied whether dex MDC retain a relatively immature phenotype upon activation with a second stimulus consisting of either LPS, TNF-α + IL-1β or CD40L. Figure 5a shows that both control MDC and dex MDC matured further upon stimulation with a second stimulus, but the expression of CD86 remained markedly lower on dex MDC compared to control MDC.
Figure 5.
Dex MDC are functionally resistant to a second maturation stimulus in the absence of dex. Control- and dex MDC were exposed to a second maturation stimulus in the absence of dex consisting of either LPS (100 pg/ml), TNF-α + IL-1β (50 pg/ml) or CD40L (5 × 103 000 J558 cells) for 24 hr. (a) The immunophenotypic characteristics of these MDC were determined by flow cytometry. Depicted is the mean fluorescence intensity of the various markers on control and dex MDC before (mature MDC) and after the second stimulus (mature MDC LPS, mature MDC TNF-α + IL-1β, mature MDC CD40L). Data represent mean with SEM of three separate experiments. (b) TNF-α levels in the culture supernatants of control and dex MDC 24 hr after restimulation with LPS. Data represent the mean with SEM of six separate experiments. *P = 0·03 for the comparison of dex MDC versus control MDC. (c) IL-6 levels in the culture supernatants of control or dex MDC 24 hr after restimulation with LPS. Data represent the mean with SEM of six separate experiments. *P = 0·03 for the comparison of dex MDC versus control MDC. (d) Allogeneic T-cell proliferation primed by control or dex MDC from the same donor that had been restimulated with LPS or TNF-α + IL-1β (one representative experiment out of three). (e) IFN-γ production in the supernatant on day 5 of the T cells stimulated by control or dex MDC that had been restimulated with LPS or TNF-α + IL-1β. Data represent mean with SEM of three separate experiments.
Functionally dex MDC produced very little TNF-α and IL-6 when activated with a second stimulus consisting of LPS, in contrast to control MDC (Fig. 5b and c). Likewise, IL-6 production was significantly lowered compared to control MDC when dex MDC were stimulated with TNF-α + IL-1β (data not shown). IL-10 and IL-12 production were not detectable in cultures of restimulated MDC. Furthermore, dex MDC activated with a second stimulus consisting of LPS or TNF-α + IL-1β did display a reduced capacity to stimulate allogeneic T-cell proliferation compared to control MDC (Fig. 5d). Also restimulated dex MDC hardly induced IFN-γ production by T cells (Fig. 5e). A similar difference in IL-2 production was detected when dex MDC and control MDC, upon restimulation with either LPS or TNFα + IL-1β, were co-cultured with allogeneic T-cells (data not shown). IL-10 and IL-4 production were not detectable in the MDC T-cell co-cultures. Collectively, these data show that regulatory dex MDC are functionally resistant to restimulation with different maturation stimuli.
Discussion
In this study we show that treatment of primary human blood MDC with the glucocorticoid dex in combination with the TLR4 ligand LPS transforms human blood MDC into tolerogenic MDC. These dex MDC poorly stimulated T-cell proliferation and production of Th1 cytokines (IFN-γ and IL-2), but primed production of the immunoregulatory cytokine IL-10 in T-cells. Moreover, these dex MDC did not convert into immunogenic MDC after subsequent exposure to different maturation stimuli.
On the contrary, pretreatment of primary human blood MDC with dex did not prevent the generation of immunostimulatory MDC upon subsequent activation with LPS in the absence of dex. Although dex pretreatment partially prevented immunophenotypic MDC maturation upon stimulation with LPS, as shown by a reduced expression of CD83 and CD86, it did not prevent functional maturation to immunogenic MDC. This is in agreement with data showing that immature mo-MDC treated with dex readily convert to immunogenic MDC after removal of glucocorticoids.15 In addition, we recently found that blood MDC from liver transplant recipients treated with glucocorticoids readily mature to immunostimulatory MDC when stimulated ex vivo in the absence of glucocorticoids.16 Apparently, corticosteroids alone do not imprint immature human blood MDC with a stable immunoregulatory function.
In contrast, treatment with dex and LPS simultaneously resulted in MDC that poorly stimulated allogeneic T-cell proliferation. This is in agreement with the reported effects of continuous corticosteroid treatment during maturation of human mo-MDC and murine MDC, that also inhibited immunophenotypic and functional maturation.15,17–20 One reason that treatment with dex alone did not reduce the T-cell stimulatory capacity of MDC may be that it stimulated upregulation of CD80 upon LPS-stimulation, while combined treatment with dex and LPS prevented this upregulation.
After restimulation MDC that had been treated with the combination of dex and LPS hardly upregulated CD86 at all. A durable effect of corticosteroids on CD86 expression on MDC was also observed in vivo in patients that were treated with high doses of glucocorticoids shortly after liver transplantation; when MDC from these patients were ex vivo stimulated with proinflammatory cytokines they showed impaired upregulation of CD86 expression.16 These data altogether show that the only lasting effect of glucocorticoids on the immunophenotype of primary human blood MDC is impairment of CD86 expression.
The secretion of MDC-derived immunoregulatory or immunostimulatory cytokines also plays a crucial role in the cascade of T-cell priming. In contrast to human mo-MDC,9,15,18,19 primary human blood MDC did not produce detectable amounts of IL-6, IL-10 or IL-12 upon LPS-stimulation. TNF-α production by human blood MDC stimulated by LPS was not suppressed by corticosteroid treatment. In this respect, primary human blood differ from human mo-MDC or murine bone marrow derived MDC in which cytokine production is inhibited by dex.15,18,19,21,22 However, we did observe a late effect of dex on cytokine production by human blood MDC; upon restimulation, MDC that had been treated with dex and LPS hardly produced any cytokines, in contrast to untreated MDC.
The key observation of our study is that upon combined treatment with dex and LPS primary human MDC are impaired in their capacity to stimulate production of Th1 cytokines, but acquire the capacity to prime IL-10 production in T cells. Most likely, dex-MDC can prime type 1 regulatory T cells, which produce IL-10.23,24 However, T-cells primed by dex-MDC also produced IFN-γ in low amounts, but no Th2 cytokines. Because the ratio of IL-10 to IFN-γ production by these T-cells was 1 : 2, we propose that these T cells represent a mixture of type 1 regulatory T cells and Th1 cells. MDC generated under the influence of LPS and dex from primary human blood MDC are similar to murine MDC that have been stimulated with LPS in the presence of dex or stimulated with cytokines only. These so-called ‘alternatively activated’, ‘semi-mature’, or ‘modified’ regulatory MDC similarly prime IL-10 production by T-cells in vitro20 and in vivo upon transfer into mice.21,22,25 Importantly, such tolerogenic murine MDC suppress Th1 responses in vivo and prevent allograft rejection,21,22 Graft-versus-host disease,26 and protect against experimentally induced autoimmune diseases25 upon transfer into mice.
Treatment of MDC with dex during LPS stimulation suppressed the upregulation of signal 2 (costimulatory molecules), but not of signal 1 (HLA-DR), suggesting that these MDC could induce T-cell anergy. Unfortunately, our in vitro data showed that these tolerogenic dex MDC did not induce a permanent state of hyporesponsiveness in T cells. However, this does not mean that hyporesponsiveness in T cells does not occur upon transfer of these MDC in vivo. Data from a murine study showed that alternatively activated MDC that did not induce hyporesponsiveness in vitro induced profound donor-specific hyporesponsiveness in T-cells, and prolonged transplant survival when applied in vivo.22
To use MDC in immunotherapy to induce tolerance to allografts, it is essential that the MDC do not convert into immunogenic MDC in vivo upon encountering a host environment rich in proinflammatory stimuli, especially in transplant recipients who are highly susceptible to infections. The tolerogenic MDC obtained after simultaneous treatment with dex and LPS fulfil this requirement: After restimulation with different maturation stimuli they become unable to produce proinflammatory cytokines, and almost incapable to induce proliferation and cytokine production in T cells.
Donor blood is readily available at time of transplantation, and it is technically feasible to isolate immature blood MDC rapidly with the method described in the present study, using clinical grade equipment. Therefore, in clinical transplantation alternatively activated donor blood MDC are an attractive option for cell-based immunotherapy to induce donor-specific tolerance. For this purpose the culture period must be shortened. We anticipate that the preincubation period with dex can be reduced considerably. In the case of liver transplantation an additional source of immature donor MDC is readily available, namely immature donor liver MDC obtained during the backtable perfusion of a liver graft.13 In addition, alternatively actived recipient blood MDC may be suitable for induction of tolerance in recipient T cells that recognize donor allo-antigens via the indirect pathway of antigen presentation. However, for this purpose effective means of loading MDC with donor HLA will have to be developed.
In conclusion, human blood MDC treated with dex and LPS are transformed in tolerogenic MDC that are strongly impaired in their capacity to stimulate allogeneic T-cell proliferation and Th1 secretion, but that prime T cells to produce the immunoregulatory cytokine IL-10. Moreover, these tolerogenic MDC were functionally unresponsive to maturation stimuli. These findings indicate alternatively activated primary blood MDC may be a promising option for cell-based immune modulatory therapy to induce donor-specific tolerance in transplant recipients.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86–) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198–208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today. 1997;18:418–24. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 7.Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60:563–72. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30:1807–12. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–81. [PubMed] [Google Scholar]
- 10.Woltman AM, van der Kooij SW, de Fijter JW, van Kooten C. Maturation-resistant dendritic cells induce hyporesponsiveness in alloreactive CD45RA+ and CD45RO+ T-cell populations. Am J Transplant. 2006;6:2580–91. doi: 10.1111/j.1600-6143.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 11.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–95. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–10. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 13.Bosma BM, Metselaar HJ, Mancham S, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 2006;12:384–93. doi: 10.1002/lt.20659. [DOI] [PubMed] [Google Scholar]
- 14.Heystek HC, den Drijver B, Kapsenberg ML, van Lier RA, de Jong EC. Type I IFNs differentially modulate IL-12p70 production by human dendritic cells depending on the maturation status of the cells and counteract IFN-gamma-mediated signaling. Clin Immunol. 2003;107:170–7. doi: 10.1016/s1521-6616(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 15.Rea D, van Kooten C, van Meijgaarden KE, Ottenhoff TH, Melief CJ, Offringa R. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood. 2000;95:3162–7. [PubMed] [Google Scholar]
- 16.Bosma BM, Metselaar HJ, Tra WM, Mancham S, Kuipers EJ, Tilanus HW, Kwekkeboom J. Impairment of circulating myeloid dendritic cells in immunosuppressed liver transplant recipients. Clin Exp Immunol. 2007;149:525–34. doi: 10.1111/j.1365-2249.2007.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 18.Vieira PL, Kalinski P, Wierenga EA, Kapsenberg ML, de Jong EC. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol. 1998;161:5245–51. [PubMed] [Google Scholar]
- 19.Vanderheyde N, Verhasselt V, Goldman M, Willems F. Inhibition of human dendritic cell functions by methylprednisolone. Transplantation. 1999;67:1342–7. doi: 10.1097/00007890-199905270-00009. [DOI] [PubMed] [Google Scholar]
- 20.Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30:1233–42. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Roelen DL, Schuurhuis DH, van den Boogaardt DE, et al. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells. Transplantation. 2003;76:1608–15. doi: 10.1097/01.TP.0000086340.30817.BA. [DOI] [PubMed] [Google Scholar]
- 22.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation. 2006;81:1451–9. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
- 23.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 24.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–9. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]