Abstract
A novel retinoid cycle has recently been identified in the cone-dominated chicken retina and this cone cycle accumulates 11-cis retinyl esters upon light adaptation. The purpose of this study is to investigate how 11-cis retinyl esters are formed in the retina. Primary cultures of chicken Muller cells and cell membrane were incubated with all-trans or 11-cis retinol to study retinyl ester synthesis. In Muller cells, esterification of 11-cis retinol was 4 times greater than esterification of all-trans retinol. In the presence of palmitoyl CoA and CRALBP, Muller cell membranes synthesized 11-cis retinyl ester from 11-cis retinol at a rate which was 20 fold higher than that of all-trans retinyl ester. In the absence of CRALBP, 11-cis retinyl ester synthesis was greatly reduced (by 7 fold). In the absence of palmitoyl CoA, retinyl ester synthesis was not observed. Muller cell membranes incubated with radiolabeled palmitoyl CoA resulted in the transfer of the labeled acyl group to retinol. This acyl transfer was greatly reduced in the presence of progesterone, a known ARAT inhibitor. 11-cis ARAT activity remained unchanged when assayed in the presence of all-trans retinol, suggesting a distinct catalytic activity from that of all-trans ARAT. Apparent kinetic rates for 11-cis ARAT were 0.135 nmol/min/mg (Vmax) and 11.25μM (KM); and for all-trans ARAT, 0.0065 nmol/min/mg (Vmax) and 28.88 μM (KM). Our data indicate that Muller cells in the chicken retina possess 11-cis ARAT activity, thus providing an explanation for the accumulation of 11-cis retinyl ester in the retina in the cone cycle.
The formation of a visual image is initiated by the photoisomerization of 11-cis retinaldehyde to the all-trans isomer in the photoreceptors. In order to sustain this visual function, all-trans retinaldehyde is re-isomerized to the 11-cis isomer for visual pigment regeneration. This retinoid recycling pathway is known as the visual or retinoid cycle, and it has been well described for rod photoreceptors [1]. The storage form of vitamin A in the eye is the ester form, and retinyl esters make up the largest pool of retinoids in the eye [2, 3]. It has been demonstrated that retinyl esters in the retinal pigment epithelium (RPE) are the substrate for the isomerase enzyme [4, 5], a key visual protein in the visual cycle, now identified as RPE65 [6-8].
Recently attention has been focused on a visual pathway which supplies vitamin A to cone photoreceptors. Cone pigment regeneration is several fold faster than rod pigment regeneration in light conditions [9]. Cones but not rods from isolated frog retina can spontaneously regenerate visual pigments [10, 11], and isolated cones from salamander can recover visual sensitivity from 11-cis retinol and 11-cis retinaldehyde while rods can only recover visual sensitivity from 11-cis retinaldehyde [12]. It has also been shown that retinas of cone-dominated species such as chicken and ground squirrel contain a larger amount of retinyl esters than the RPE, and that the majority of these retinyl esters are in the 11-cis conformation [13-15]. More recently, the accumulation and depletion of 11-cis retinyl esters, under light and dark conditions respectively, have been demonstrated in the cone-dominated chicken retina [16], and recently Mata demonstrated that the retinas of cone-dominated species possess retinoid processing enzymes, including a retinyl ester synthase [17].
Lecithin retinol acyl transferase (LRAT), which transfers the acyl group from the sn-1 position of phosphatidylcholine to all-trans retinol [18], is found in the liver and RPE [17]. Acyl retinol acyl transferase (ARAT)- like activity, in which the acyl group of acyl-CoA is used to esterify retinol [19], has also recently been localized in these tissues [20-22]. ARAT has not been purified or cloned but its activity can be inhibited with progesterone [21]. Furthermore, the retinyl ester synthase activities described by Mata et al. [14] in the retinas of cone-dominated species use palmitoyl CoA as an acyl donor.
Muller cells in culture have been reported to produce all-trans retinyl esters and 11-cis retinol from all-trans retinol [14]. It has also been suggested that Muller cells may participate in visual pigment regeneration [14, 15]. Vitamin A metabolism in support of visual chromophore regeneration in Muller cells is conceivable since Muller cells are the main glia in the neural retina [23] and are in close proximity to cones [24]. In the present study, we have examined the ability of Muller cells from the cone-dominated chicken retina to esterify retinol. We show that Muller cell membranes have the ability to esterify retinol, and that this esterification is 11-cis specific and it occurs through an ARAT activity. This is the first study to show an 11-cis ARAT activity in Muller cell specific for 11-cis retinol, and our results provide an explanation for 11-cis retinyl ester accumulation in the cone-dominated retina.
Materials and Methods
Primary Muller cell culture
Primary chicken Muller cell culture was established according to Das et al. 1992 with minor modifications. Freshly severed chicken heads were collected on ice (Tyson Foods, Inc., Seguin, Texas) and immediately transported to the laboratory. Eyes were enucleated within three hours post-mortem and sterilized with 10 % Wescodyne for 5 minutes. Eyes were sectioned at the level of the ora serrata, the vitreous removed and the retina was then dissected from the eye. After being rinsed in 1% antibiotic/antimycotic in Hanks balanced salt solution (HBSS), the retinas were incubated for 45 min. at 37° C in HBSS containing 5.0 mg/ml papain. The retinas were then rinsed three times in HBSS and tissue collected by centrifugation (1500 × g). Cells were then seeded in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) and 5 mg/ml of glucose and incubated at 37° C and 5% CO2. The media was changed 24hrs later and every 48 hrs thereafter until cultured cells were confluent. Cells from 2−3 retinas were seeded into each T75 flask. Cells were cultured until confluent between 14−19 days.
Immunochemistry
Primary Muller cells were prepared for culture as described above and seeded in Lab-Tek chamber slides. Cells were rinsed in phosphate buffered saline (PBS) and fixed in 10% paraformaldahyde for 15 minutes. Following fixation, the cells were rinsed once more. To remove background level fluorescence, the fixed cells were exposed to 600 mW of ultraviolet light using a Biorad GS gene linker. Muller cells were also collected and checked for endogenous retinoids by HPLC (10 T75 flasks at 3 × 106 cells/flask). The cells were permeabilized using PBST for 30 minutes at room temperature. Cells were incubated for one hour in PBS containing 1% fatty acid free BSA (Sigma Aldrich) to block nonspecific binding. Incubation with primary antibodies mouse anti-CRALBP (a gift from Dr. Jack Saari) and rabbit anti GFAP (obtained from Dakocytomation, Denmark) was performed overnight at a 1:1000 dilution at 4°C in PBS containing 1% BSA. After rinsing three times with PBS containing 0.2% Triton X-100 (PBST), the cells were incubated with FITC-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (Sigma) at a dilution of 1:200 for 60 minutes. Finally, the cells were rinsed with PBST three times and then mounted. As negative controls, some cells were processed without incubation with primary antibodies.
ARAT activity in primary Muller cell
All procedures with vitamin A were performed under dim red light. Primary Muller cells were cultured as described above, except they were cultured in T25 flasks. Upon reaching confluence, the cells were serum starved for 6 hours. The cultures were then incubated overnight in 5ml serum free media containing 10μM 11-cis retinol or 10μM all-trans retinol and 1% BSA. The cells were then harvested and glass-glass homogenized. Retinoids were extracted with hexane and analyzed by high performance liquid chromatography (HPLC). HPLC was performed using a Beckman System Gold with Agilents Zorbax RX-SIL 5μM 4.5 × 250 mm column, part number 880975−901; the mobile phase used was 0.2% HPLC grade dioxane/hexane at a flow rate of 2ml/min. In all procedures, retinyl esters were identified by comparison to authentic retinoid standards at absorbance wavelengths of 318 nm for 11-cis retinoids and 325 nm for all-trans retinoids and by on-line photodiode array absorption spectra. Retinoids were quantified by comparing experimental peak area to a retinoid standard curve.
Primary Muller cell membrane preparation
Whole cell membranes were prepared from primary chicken Muller cells, fresh rat liver and fresh rat muscle. All procedures were performed at 4°C. Muller cells were collected and glass-glass homogenized in buffer containing 10mM tris pH 7.5, 2mM CaCl2, 2mM MgCl2, and 1mM DTT. The homogenate was centrifuged at 250,000 × g for 30 minutes at 4° C and the pellet re-suspended in the same buffer.
Rat muscle and rat liver membranes were prepared according to Ross 1982 [18]. Briefly, fresh tissue was collected, and the rat liver was perfused overnight at 4° C in 250 mM sucrose and 10mM potassium phosphate solution. The tissues were then minced and glass-glass homogenized in buffer containing 150 mM potassium phosphate and 1 mM DTT pH 7.4. The liver homogenate was centrifuged at 725 × g for 10 minutes; the supernatant was then centrifuged at 13,000 × g after which floating fat was skimmed. To prepare microsomes, the supernatant was centrifuged at 250,000 × g for 30 minutes. Rat muscle tissue was prepared by glass-glass homogenization and then centrifuged at 250,000 × g for 30 minutes. The liver and muscle pellets were re-suspended and fast-frozen in 150 mM potassium phosphate and 1 mM DTT buffer (pH 7.4) using acetone and dry ice. The Bradford assay was used to determine protein concentrations.
ARAT assays
Membrane protein in increasing amounts was pre-incubated with palmitoyl CoA in 100 mM Tris pH 8, 2 mM CaCl2, 2 mM MgCl2, (reaction buffer) for 5 minutes at room temperature. The substrate, 11-cis retinol or all-trans retinol, was delivered in 2 μl of ethanol to the reaction buffer containing either cellular retinaldehyde binding protein (CRALBP), (a gift from Dr. Kris Palczewski), bovine serum albumin (BSA), or reaction buffer alone, and pre-incubated for 5 minutes at room temperature. The protein samples and retinol samples were then combined and incubated at 37° C for 30 minutes. The final reaction mixture was 100 μM palmitoyl CoA, 30 μM CRALBP or 1% BSA, 10 μM retinol and protein in increasing amounts (between 10 and 50 μg). Total reaction volume was 1ml. The reaction was stopped with 1ml ice cold ethanol. Retinoids were then extracted with hexane and analyzed by HPLC.
Transfer of labeled acyl group from 14C labeled palmitoyl CoA to retinol to form retinyl esters
To show direct transfer of the radiolabeled acyl group from palmitoyl CoA onto retinol to form retinyl palmitate, radiolabeled palmitoyl CoA 100μM, specific activity 4,442 DPM/nmol was pre-incubated with 30μg Muller cell membranes as described above. Retinol was delivered in ethanol as described above and pre-incubated with CRALBP. The protein sample and retinol sample were then combined and incubated at 37° C for 30 minutes and the reaction was stopped with 1ml of ice-cold ethanol. Retinoids were then extracted and analyzed by HPLC as described in the previous section, and by liquid scintillation counting (results in disintegration per minute, DPM at a counting efficiency of 100% for 14C). Radiolabeled authentic retinoid standards were used to determine elution time of experimental retinoid products.
Rat liver and leg muscle membranes were used as positive and negative controls, respectively. 30μg of protein were pre-incubated with 100μM radiolabeled palmitoyl CoA (specific activity 4,442 DPM/ nmol) in buffer containing 150mM K2PO4, 1mM DTT, and 1% BSA at pH 7.4 for 5 minutes at room temperature. 11-cis or all-trans retinol was then delivered to the mixture in 2 μl ethanol to a final concentration of 10 μM. A final volume of 1ml was then incubated, quenched, and analyzed as described above.
Inhibition of ARAT activity by progesterone
Muller cell membranes were pre-incubated with radiolabeled palmitoyl CoA (100 μM, specific activity 1,111 DPM/nmol) and progesterone (200μM) delivered in 4 μl of ethanol for 5 minutes at room temperature in reaction buffer. 11-cis retinol was delivered as described above. The reaction mixture was then incubated at 37° C for 30 min before being quenched with ice-cold ethanol.
For rat liver, membranes were pre-incubated with radiolabeled palmitoyl CoA (100 μM, specific activity 1,111 DPM/nmol) and progesterone (Sigma-Aldrich) (200μM) delivered in 4 μl of ethanol for 5 minutes at room temperature in buffer containing 150mM K2PO4, 1mM DTT and 1% BSA at pH 7.4. 10 μM all-trans retinol was then delivered to the reaction mixture and incubated at 37° C for 30 minutes. The reaction was then quenched with ice cold ethanol. Retinoids were extracted using hexane and analyzed using HPLC and scintillation counting.
Muller cell and rat liver membranes were also incubated with labeled palmitoyl CoA in the presence of 4 μl of ethanol as controls.
Kinetics of 11-cis and all-trans ARAT activity in membranes of primary chicken Muller cell
30 μg of Muller cell membrane protein was pre-incubated with 100μM palmitoyl CoA. 30 μM CRALBP was pre-incubated with 11-cis retinol in increasing concentrations (0−20μM). For all-trans ARAT activity, 1% BSA was preincubated with all-trans retinol. The protein and retinol samples were then combined and incubated at 37°C for 30 minutes. The reaction was quenched using ice-cold ethanol. Retinoids were extracted with hexane and analyzed by HPLC. The Eadie-Hofstee plot was constructed to determine kinetic parameters.
Results
Cell culture and Immunochemistry
Primary chicken Muller cell cultures were established according to the methods of Das, et al., 1992. Figure 1A shows the morphology of freshly explanted Muller cells 10 days after seeding at approximately 80% confluence in a T75 flask. 100% confluence was reached between 14 to 19 days with 3 × 106 cells per T75 flask. Confluent Muller cell cultures (10 T75 flasks at 3 × 106 cells/flask) showed no detectable retinoids when analyzed by HPLC (data not shown).
Figure 1.
Photomicrograph of a primary culture of chicken Muller cells. A. Muller cells freshly explanted from adult chicken eyes and cultured for 10 days (approximately 80% confluent; 4X). B. Muller cells stained with anti-CRALBP monoclonal antibody and visualized by FITC-conjugated secondary antibody (green). Propidium Iodide (red) was used to counter stain for cell nuclei (20X). C. Negative control without anti-CRALBP. D Muller cells stained with anti-GFAP polyclonal antibody, visualized with FITC (green) conjugated secondary antibody. Propidium Iodide (red) was used to counter stain for cell nuclei (10X). E. Negative control without anti-GFAP. Both primary antibodies were incubated at a 1: 1000 dilution overnight at 4° C.
To establish the identity of Muller cells in culture, we performed immunochemical studies with both Muller and glial cell markers. Figure 1B shows a primary Muller cell culture (40x) stained with anti-CRALBP antibody. CRALBP is expressed exclusively by Muller cells in the retina [25]. Figure 1D shows a primary Muller cell culture (20x) stained with anti-GFAP antibody. GFAP is commonly used as a glial cell marker and Muller cells are the main glia in the retina. Both anti-CRALBP and anti-GFAP staining were visualized using a FITC-conjugated secondary antibody.
Formation of retinyl esters by primary Muller cell cultures from exogenous retinol
Primary Muller cells cultured in T25 Flasks, about 106 cells/flask, were incubated overnight with either 10μM 11-cis retinol or 10μM all-trans retinol in 5 ml of media. Cells incubated with 11-cis retinol had 11-cis retinyl esters which were 8.0 fold the amount of all-trans retinyl esters produced by cells incubated with all-trans retinol (Table 1). No 11-cis ester synthesis was observed in cells incubated with all-trans retinol, and no all-trans esters were observed in cells incubated with 11-cis retinol. Controls for thermo isomerization and esterification were performed by incubating retinol with culture media in the absence of cells.
Table 1. Formation of retinyl esters from exogenous retinol by primary chicken Muller cells.
Primary Muller cells were cultured in T25 flasks. At confluence, each flask contained 106 cells. The cells were then incubated with either 10 μM 11-cis retinol or 10 μM all-trans retinol in 5 ml of media overnight. Cells were collected and retinoids extracted and analyzed by HPLC. Muller cells incubated with 11-cis retinol synthesized 11-cis retinyl esters 8.0 fold more than the amount of all-trans retinyl ester synthesized from added all-trans retinol. Statistical analysis (students T test) on the amount of 11-cis retinyl esters from (11-cis retinol) and the amount of all-trans retinyl esters from (all-trans retinol) yields a p value > .05 suggesting a significant difference between the synthesis of 11-cis and all-trans retinyl esters. Four separate experiments (n = 4; mean ± standard error) were conducted.
| Amount (nmol of retinyl esters) recovered from Muller cells incubated with retinol | ||
|---|---|---|
| 11 — cis retinyl ester nmol/106 cells | All-trans retinyl ester nmol/106 cells | |
| 11 - cis retinol | 0.18 ± .06 | 0.02 ± .02 |
| All - trans retinol | 0.0 ± 0.0 | 0.022 ± .02 |
Formation of retinyl esters from exogenous retinol by cultured chicken Muller cells membranes
Whole membrane preparations were made from primary chicken Muller cell and incubated with 11-cis retinol in the presence and absence of CRALBP and in the presence and absence of palmitoyl CoA. CRALBP is specific for 11-cis retinoids [26]; therefore, in these samples, we facilitated delivery of all-trans retinol to cell membranes with BSA in our reaction mixture. Figure 2 shows the data for experiments testing several conditions under which Muller cell membranes synthesized retinyl esters. In the presence of 11-cis retinol, palmitoyl CoA and CRALBP, (condition 1) Muller cell membranes catalyzed the synthesis of 11-cis retinyl ester, from 11-cis retinol at, a rate of 0.166 nmol/min/mg which was 20-fold higher than that for all-trans retinyl ester synthesis (0.007 nmol/min/mg), from of all-trans retinol, in the presence of palmitoyl CoA and BSA (condition 3). In the absence of CRALBP (condition 2), 11-cis retinyl ester synthesis was greatly reduced to 0.023 nmol/min/mg. In the absence of palmitoyl CoA, retinyl ester was not synthesized (data not shown). Similarly, all-trans retinyl ester was not synthesized from all-trans retinol in the presence or absence of CRALBP and absence of BSA (data not shown).
Figure 2.
Formation of retinyl esters from exogenous retinol by membranes from cultured chicken Muller cells. Whole membranes were prepared from primary cultures of chicken Muller cells. HPLC chromatograms of retinyl esters extracted from membranes incubated with: A. 11-cis retinol, CRALBP and palmitoyl CoA for 30 minutes (Condition 1) detected at 318nm; and B. without CRALBP (Condition2) detected at 318nm. C. HPLC chromatogram of retinyl esters extracted from membranes incubated with all-trans retinol, palmitoyl CoA and BSA for 30 minutes (Condition 3) detected at 325nm. Peak 1: 11-cis retinyl esters. Peak 2: all-trans retinyl esters D. Summary of production of retinyl esters by Muller cell membranes under conditions 1, 2, and 3. Insets are the spectra for 11-cis or all- trans retinyl esters in the HPLC chromatograms. In the absence of palmitoyl CoA no retinyl esters were formed (data not shown). Means and standard errors of duplicate determinations are indicated in the diagram. Two experiments were performed with similar results. • 11-cis retinyl esters, ▲ all-trans retinyl esters.
Transfer of labeled acyl group from C14 labeled palmitoyl CoA to retinol to form retinyl esters
In order to verify acyl transfer of ARAT activity in primary chicken Muller cell membranes, we performed an experiment to show direct transfer of a labeled acyl group from palmitoyl CoA onto retinol to form labeled retinyl esters. Table 2 shows that Muller cell membranes transferred the labeled acyl group to 11-cis retinol forming labeled 11-cis retinyl palmitate (2,800 DPM), while rat liver and rat muscle had relatively low activity. Muller cell membranes also transferred 3 times the amount of labeled acyl group to 11-cis retinol forming 11-cis retinyl palmitate, than to all-trans retinol to form all-trans retinyl palmitate (2,800 DPM vs. 842 DPM, respectively). Muller cell membranes and rat liver membranes synthesized similar amounts of labeled all-trans retinyl palmitate while muscle membranes exhibited relatively low level of acyl transfer activity.
Table 2. Transfer of labeled acyl group from C14 labeled palmitoyl CoA to retinol to form retinyl ester by primary chicken Muller cell membrane.
Whole membrane preparations were made from primary chicken Muller cell, rat liver, and rat leg muscle. 30 μg of each of the membranes were then incubated with 10 μM 11-cis retinol in the presence of CRALBP and 14C labeled palmitoyl CoA (specific activity = 4,442 DPM/nmol) for 30 minutes (total volume 1ml) to form 11-cis retinyl palmitate (baseline levels of DPM were observed indicating minimal all-trans retinyl palmitate synthesized from 11-cis retinol). These cell membranes were also incubated with 10 μM all-trans retinol in the presence of BSA and 14C label palmitoyl CoA (specific activity = 4,442 DPM/nmol) for 30 minutes to form all-trans retinyl palmitate (baseline levels of DPM were observed indicating minimal 11-cis retinyl palmitate synthesis from all-trans retinol). Reactions was quenched with ice cold ethanol and analyzed by HPLC and scintillation counting as described above. The formation of labeled 11-cis retinyl palmitate by Muller cell membrane indicates a significant level of acyl transfer. In contrast, low levels of labeled 11-cis retinyl palmitate associated with rat liver and muscle membranes suggest minimal acyl transfer activity in these cells. Muller cell membranes also synthesized 3 times the labeled 11-cis retinyl palmitate than all-trans retinyl palmitate. Muller cell membranes and rat liver membranes synthesized similar amounts of all- trans retinyl palmitate while the muscle membranes only produced baseline levels of DPM indicating minimal ester synthesis. Three experiments were conducted. Standard errors are indicated on the table.
| DPM on labeled retinyl esters | |||
|---|---|---|---|
| Radiolabeled retinyl esters | Muller cell membrane | Rat liver membrane | Rat Muscle membrane |
| 11-cis RP | 2800 ± 1290 | 72 ± 3 | 52 ± 17 |
| ATRP | 842 ± 409 | 713 ± 346 | 139 ± 65 |
Inhibition of ARAT activity in Muller cell Membranes
To further confirm the presence of ARAT activity, we included progesterone, a known ARAT inhibitor [19], in our reaction mixtures. The presence of progesterone reduced synthesis of labeled all-trans retinyl palmitate from all-trans retinol by rat liver membranes by 84% (Fig. 3D) bringing DPM levels down to baseline. In the presence of progesterone, all-trans retinyl palmitate synthesis was not completely abolished (Fig. 3C). This remaining synthesis is most likely due to LRAT which is the main retinol esterifying enzyme in liver. Synthesis of labeled 11-cis retinyl palmitate from 11-cis retinol in Muller cell membranes was reduced by 64% in the presence of progesterone (Fig. 3H), once again bringing DPM levels to base line. Synthesis of 11-cis retinyl palmitate was not completely abolished (Fig. 3G). It is possible that progesterone is a specific inhibitor for all-trans ARAT activity and may not function as well on 11-cis ARAT activity located in the Muller cell.
Figure 3.
Inhibition of retinyl ester synthesis by liver and Muller cell membranes using progesterone. Whole membranes were prepared from rat liver and from primary culture of chicken Muller cells. Membranes from liver and Muller cells (30 μg) were then incubated with 10μM all-trans retinol and 10μM 11-cis retinol, respectively, in the presence of 14C labeled palmitoyl CoA (specific activity 1,110 DPM/nmol) and progesterone (200μM) for 30 minutes. Retinyl esters were extracted with hexane and analyzed by HPLC and liquid scintillation counter for 14C label. A and C are HPLC chromatograms, detected at 325nm, of retinyl esters extracted from rat liver membranes incubated in the absence and presence of progesterone, respectively. B and D are DPM associated with retinyl esters produced by rat liver membranes incubated in the presence and absence of progesterone corresponding to A and C. E and G are HPLC chromatograms, detected at 318nm, of retinyl esters extracted from Muller cell membranes incubated in the absence and presence of progesterone respectively. F and H are DPM associated with retinyl esters produced by Muller cell membranes incubated in the absence and presence of progesterone corresponding to E and G respectively. Insets are the spectra for 11-cis or all- trans retinyl esters in the HPLC chromatograms. I. Summary of figures 3A-3H. Progesterone reduced the amount of labeled all-trans retinyl ester in rat liver by 84% and 11-cis retinyl esters in Muller cell membranes by 64 % bringing DPM levels to baseline. Two experiments were performed with duplicate determinations. Peak 1: all-trans retinyl ester. Peak 2: 11-cis retinyl ester.
Kinetics of ARAT activity in chicken Muller cell membrane
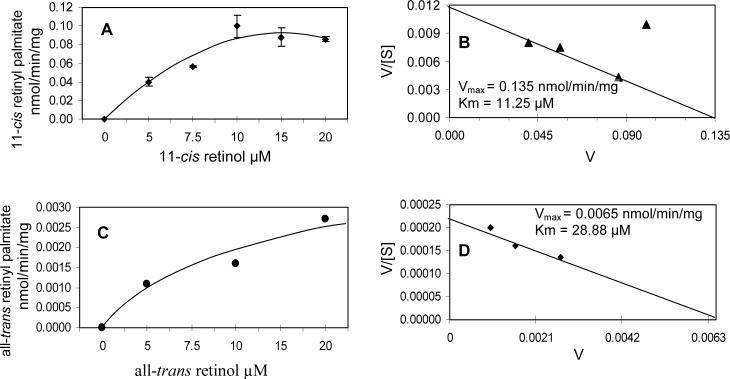
To determine the kinetics of 11-cis ARAT activity in primary Muller cell membranes, membranes were incubated with fixed concentrations of palmitoyl CoA and CRALBP; 11-cis retinol was added to the reaction mixture in increasing concentrations. For all-trans ARAT activity, Muller cell membranes were also incubated with fixed concentrations of palmitoyl CoA and BSA; all-trans retinol was added to reaction mixtures in increasing concentrations. We measured the initial rates of retinyl palmitate synthesis by HPLC. Eadie-Hofstee transformation of the data yielded apparent parameters for 11-cis ARAT activity: Vmax = 0.135 nmol/min/mg and Km = 11.25 μM and for all-trans ARAT: Vmax = 0.0065 nmol/min/mg and Km = 28.88 μM.
Discussion
Retinal Muller cells have been suggested to play an important role in vitamin A processing for visual chromophore regeneration [14, 15]. However, direct evidence in support of this hypothesis has not been available. In the present study, we investigated the ability of primary Muller cells to esterify retinol both in culture and in vitro. We first established primary Muller cell cultures from the cone-dominated chicken retina. Under light microscopy, our primary cells exhibited Muller cell morphology [13] and they stained positive for CRALBP, a marker for Muller cells in the neural retina [27], as well as for GFAP, a glia cell marker.
Incubation of Muller cells with 11-cis retinol(s) resulted in the production of more 11-cis retinyl esters than of all-trans retinyl esters (from all-trans retinol). This suggests that Muller cells in culture may selectively esterify 11-cis retinol over all-trans retinol. Retinyl esters in the RPE are found in the all-trans conformation and these all-trans retinyl esters are the substrate for the isomerase enzyme [4, 5]. Although the accumulation of 11-cis retinyl esters in the cone-dominated chicken retina has been described [16], it was not clear which cell type in the retina synthesizes 11-cis retinyl ester. As the Muller cells are the main glial cell in the retina, it is likely that they synthesized 11-cis retinyl esters for visual chromophore regeneration. This suggestion is further supported by the localization of CRALBP, which has a high affinity for 11-cis retinoids, in the Muller cell [26].
Results from the present study show that the production of retinyl esters by primary chicken Muller cell membranes was highest in the presence of CRALBP, palmitoyl CoA, and 11-cis retinol. The absence of CRALBP significantly reduced the synthesis of 11-cis retinyl esters. Since CRALBP binds 11-cis retinoids endogenously, it is a possibility that CRALBP delivered 11-cis retinol in the Muller cell membrane. Synthesis of all-trans retinyl esters was minimal and only occurred in the presence of palmitoyl CoA and BSA (Fig.2; BSA was used to deliver all-trans retinol to Muller cell membrane). The difference in the efficiency of 11-cis or all-trans retinyl ester synthesis by Muller cell membranes may have two explanations. One is that one ester synthase exists and that this ester synthase can catalyze esterification of both isomers of retinol, but is selective for 11-cis conformation. The second explanation is that two distinct enzymes exist in the Muller cells, one that esterifies 11-cis retinol and one that esterifies all-trans retinol. The higher 11-cis retinyl ester synthesis in Muller cells agrees with the recent discovery of a novel retinoid cycle in the retina of cone-dominated species in which 11-cis retinyl esters are accumulated in the retina while all-trans retinyl esters are accumulated in the RPE [16].
Since samples incubated in the absence of palmitoyl CoA did not produce any esterification products, it strongly suggests that the esterification reaction is acyl CoA dependent. An acyl-CoA dependent ester synthase activity has previously been shown in the cone-dominated chicken retina [15]. ARAT is an enzyme activity known to esterify retinol in an acyl CoA-dependent manner [19]. Recently, ARAT activity that can synthesize retinyl esters from both all-trans retinol and 11-cis retinol was reported in bovine RPE [20]. However, this is the first study to show that an ARAT activity specific for 11-cis retinol exists in Muller cells.
To fully establish ARAT enzyme activity, we have also demonstrated the acyl transfer reaction by directly transferring the radiolabeled acyl group from palmitoyl CoA onto retinol to form labeled retinyl esters. Data in Table 2 also show that Muller cell membranes transferred considerably more radioactive acyl groups to form labeled 11-cis retinyl esters than labeled all-trans retinyl esters, and further supports our results (using non-labeled substrates) on 11-cis ARAT activity. The acyl transfer activity of all-trans ARAT in Muller cell membranes was similar to that of liver membranes, suggesting that all-trans ARAT activity in the Muller cells may be homologous to the hepatic ARAT. When progesterone, a specific ARAT inhibitor [19] was added to our reaction mixture, a significant reduction of retinyl ester synthesis (Fig. 3) was noted in liver and Muller cell membrane. These data further verify the ARAT origin retinyl ester synthesis observed in Muller cell membranes.
To test the effect of all-trans retinol on 11-cis retinol esterification, we conducted a substrate competition assay. Results from this assay indicated that the 11-cis ARAT activity remained the same in the presence of (equi-molar of) all-trans retinol (at 5, 10 and 20μM of retinol; data not shown). This suggests that 11-cis ARAT activity in Muller cells membranes is distinct from the ARAT activity for all-trans retinol.
Km and Vmax are important enzyme kinetic parameters. The Km of an enzyme represents the substrate concentration that yields half the occupancy of catalytic sites. We determined the apparent Km for 11-cis retinol of the 11-cis ARAT activity in Muller cell membranes to be 11.25 μM. This value is well between the documented Km value for ARAT activity in RPE (4.1 μM) [20] and liver (30 μM) [21]. The Km value of the 11-cis ARAT activity in Muller cells is also comparable to the value recently described for 11-cis ARAT activity in RPE (8.7μM) [20]. We also determined an apparent Km value for all-trans ARAT activity in primary Muller cell membranes to be 28.88 μM, and twice as high than that for 11-cis retinol. This KM value is very comparable to liver all-trans ARAT activity, reported to be 30 μM [18]. The lower Km for 11-cis ARAT activity in Muller cell membranes further suggests the specificity of this enzyme activity.
The Vmax is representative of the activity of an enzyme at saturating substrate concentrations. Because Vmax is influenced by the purity of the enzyme, we determined the apparent Vmax value for 11-cis ARAT activity for 11-cis retinol and all-trans retinol to be 0.135 nmol/min/mg and 0.0065 nmol/min/mg in whole Muller cell membranes. The 11-cis ARAT activity from Muller cells membranes' apparent Vmax value is below the documented ARAT activities for liver (0.3 nmol/min/mg) [21] and for 11-cis ARAT in RPE (3.8 nmol/min/mg) [20]. Vmax of all-trans ARAT activity in the Muller cell membranes is also lower than the reported liver ARAT activity. This lower Vmax value for 11-cis and all-trans ARAT activities in our Muller cells samples is perhaps due to total membrane preparations from cell cultures used in the experiments rather that microsomal membrane preparation from fresh tissue which would yield an enzyme preparation of higher purity.
The enzyme responsible for ARAT activity remains unknown. ARAT activity has recently been reported in 293T cells transfected with a cDNA library from chicken retina. This activity may be the result of a nonspecific palmitoyl CoA dependent enzyme known as DGAT1[28] . Future work will certainly establish whether this DGAT1 is the retinol esterifying enzyme in the chicken retina.
Several lines of evidence show that the retina contains 11-cis retinyl ester pools [15, 16, 29]. However the cellular location of these esters within the retina is unknown. To assay for the possibility that the Muller cell may be a storage site for 11-cis retinyl esters, we have tested both confluent Muller cell cultures (approximately 15 day old cultures) and freshly explanted and cultured cells (within 48 hrs) for endogenous retinoids by HPLC. We found the primary Muller cell cultures to contain no detectable level of retinoids. Two explanations may apply to these findings. The first is that retinoids may have been lost during culture preparation, or second the Muller cell is able to synthesize retinyl esters and secrete them.
The presence of 11-cis specific ester synthase activity in the Muller cell suggests the presence of isomerase activity in this cell. Upon light exposure, all-trans retinol is released by the photoreceptors and transferred to Muller cells. This all-trans retinol would have to be isomerized, perhaps by a light-dependent isomerase, to the 11-cis conformation before being esterified by the 11-cis ARAT activity observed in the present study of Muller cells. This would explain the large amount of 11-cis retinyl ester recovered from cone-dominated retinas [29]. Since cones are known to recover visual sensitivity from both 11-cis retinol and 11-cis retinaldehyde [12], 11-cis retinyl esters may be transiently stored in the Muller cells to avoid retinol toxicity under high light illumination. Upon dark adaptation, esters are then hydrolyzed to 11-cis retinol and transferred to photoreceptor for pigment regeneration. Our results show no isomerase activity in the presence of all-trans retinol. It is a possibility that the Muller cells in culture lose expression or activity of the enzyme responsible for isomerizing retinoids. It is also possible that the isomerase is light-dependent and therefore not functional in our experimental conditions.
In summary, we have presented experimental evidence showing the presence of ARAT activity in the primary Muller cell culture from chicken retina. This activity is specific for 11-cis retinol and results in the formation of 11-cis retinyl ester. Our data provide the first experimental evidence to explain the accumulation of 11-cis retinyl ester in cone-dominated chicken retina. Additional experiments will be needed to study the expression of this ARAT and how it supports the function of the cone visual cycle in the retina.
Figure 4.
Kinetics of ARAT activity in chicken Muller cell membrane. Cell membranes were prepared from a primary culture of chicken Muller cells and incubated with increasing concentrations of 11-cis retinol in the presence of 100 μM palmitoyl CoA and 30 μM CRALBP for 30 minutes at 37° C. A. 11-cis retinol substrate saturation curve. B. Eadie-Hofstee plot of 11-cis retinol substrate saturation curve yielding an apparent Vmax of 0.135 nmol/min/mg and apparent KM value of 11.25μM. C. all-trans retinol substrate saturation curve. D. Eadie Hofstee plot of all-trans retinol substrate saturation curve yielding an apparent Vmax of .0065 nmol/min/mg and apparent KM value of 28.88 μM. For 11-cis ARAT kinetics data, means and standard errors of triplicate determinations are indicated in the diagram. Three experiments were performed with similar results. For all-trans ARAT kinetics data, two experiments was performed with single determinations.
Acknowledgements
We thank Dr. D. Allen, Dr. N. L. Mata, and Mr. Simon Trevino for their critical review of the manuscript. We also thank Dr. K. Palczewski for kindly donating CRALBP protein and Dr. J. Saari for kindly providing anti-CRALBP antibody. We thank Tyson Foods Co. for tissue donations, and Ms. Sarika Jahagirdar for technical assistance.
Financial aid institution: NIH grant: GM08194
Abbreviations
- CRALBP
cellular retinaldehyde binding protein
- RPE
retinal pigment epithelium
- LRAT
lecithin: retinol acyl-transferase
- ARAT
acyl CoA: retinol acyl-transferase
- HBSS
Hanks Balanced Salt Solution
- MEM
minimal essential medium
- PBS
phosphate buffer saline
- PBST
PBS containing 0.2% Triton X-100
- BSA
bovine serum albumin
- DPM
disintegrations per minute
- DGAT1
acyl CoA: diacylglycerol acyltransferase 1
References
- 1.McBee JK, et al. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20(4):469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 2.Bridges CD, Alvarez RA, Fong SL. Vitamin A in human eyes: amount, distribution, and composition. Invest Ophthalmol Vis Sci. 1982;22(6):706–14. [PubMed] [Google Scholar]
- 3.Flood MT, et al. Vitamin A utilization in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1983;24(9):1227–35. [PubMed] [Google Scholar]
- 4.Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42(19):5809–18. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- 5.Moiseyev G, et al. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42(7):2229–38. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 6.Moiseyev G, et al. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–8. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M, et al. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–59. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redmond TM, et al. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102(38):13658–63. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991;433:561–87. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein EB. Early receptor potential of the isolated frog (Rana pipiens) retina. Vision Res. 1967;7(11):837–45. doi: 10.1016/0042-6989(67)90004-1. [DOI] [PubMed] [Google Scholar]
- 11.Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13(10):1943–51. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- 12.Jones JG, Crouch K. Rosalie, Wiggert, Barbara, Cornwall M. Carter, Chader J. Gerald. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez KA, Tsin AT. Retinyl esters in the vertebrate neuroretina. Am J Physiol. 1989;256(1 Pt 2):R255–8. doi: 10.1152/ajpregu.1989.256.1.R255. [DOI] [PubMed] [Google Scholar]
- 14.Das SR, BHARDWAJ Nikhil, KJELDBYE Hild, Gouras Peter. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285:907–913. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mata NL, et al. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevino SG, et al. Retinoid cycles in the cone-dominated chicken retina. J Exp Biol. 2005;208(Pt 21):4151–7. doi: 10.1242/jeb.01881. [DOI] [PubMed] [Google Scholar]
- 17.Mata NL, et al. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44(35):11715–21. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264(15):8636–40. [PubMed] [Google Scholar]
- 19.Ross AC. Retinol esterification by rat liver microsomes. Evidence for a fatty acyl coenzyme A: retinol acyltransferase. J Biol Chem. 1982;257(5):2453–9. [PubMed] [Google Scholar]
- 20.Kaschula CH, et al. Acyl CoA:retinol acyltransferase (ARAT) activity is present in bovine retinal pigment epithelium. Exp Eye Res. 2006;82(1):111–21. doi: 10.1016/j.exer.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Randolph RK, Winkler KE, Ross AC. Fatty acyl CoA-dependent and -independent retinol esterification by rat liver and lactating mammary gland microsomes. Arch Biochem Biophys. 1991;288(2):500–8. doi: 10.1016/0003-9861(91)90227-a. [DOI] [PubMed] [Google Scholar]
- 22.Saari JC, Bredberg DL. CoA- and non-CoA-dependent retinol esterification in retinal pigment epithelium. J Biol Chem. 1988;263(17):8084–90. [PubMed] [Google Scholar]
- 23.V. Sarthy HR. In: The Retinal Muller Cell Structure and Function. Blakmore C, editor. Plenum Publishers; New York, N.Y.: 2001. p. 1. [Google Scholar]
- 24.Lee E, et al. An ultramicroscopic study on the distribution of Muller cell processes in the outer retinal layers of the zebrafish. Ann Anat. 2005;187(1):43–50. doi: 10.1016/j.aanat.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97(3):703–12. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saari JC, Bredberg L, Garwin GG. Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem. 1982;257(22):13329–33. [PubMed] [Google Scholar]
- 27.De Leeuw AM, et al. Immunolocalization of cellular retinol-, retinaldehyde- and retinoic acid-binding proteins in rat retina during pre- and postnatal development. J Neurocytol. 1990;19(2):253–64. doi: 10.1007/BF01217303. [DOI] [PubMed] [Google Scholar]
- 28.J.J. Kaylor MJ, Moghrabi W, Travis GH. IOVS. Ft. Lauderdale; Florida: 2006. Strategies for Cloning the Retinol Isomerase in Cone-Dominant Chicken Retinas. [Google Scholar]
- 29.Villazana-Espinoza ET, Hatch AL, Tsin AT. Effect of Light Exposure on the Accumulation and Depletion of Retinyl Ester in the Chicken Retina. Experimental Eye Research. 2006 doi: 10.1016/j.exer.2006.04.011. in press. [DOI] [PubMed] [Google Scholar]