Abstract
Weibel-Palade bodies within endothelial cells are secretory granules known to release von Willebrand Factor (VWF), P-selectin, chemokines, and other stored molecules following histamine exposure. Mice with a disrupted VWF gene (VWFKO) have endothelial cells that are deficient in Weibel-Palade bodies. These mice were used to evaluate the role of VWF and/or Weibel-Palade bodies in Bordetella pertussis toxin-induced hypersensitivity to histamine, a subphenotype of experimental allergic encephalomyelitis, the principal autoimmune model of multiple sclerosis. No significant differences in susceptibility to histamine between wild-type and VWFKO mice were detected after 3 days; however, histamine sensitivity persisted significantly longer in VWFKO mice. Correspondingly, encephalomyelitis onset was earlier, disease was more severe, and blood brain barrier (BBB) permeability was significantly increased in VWFKO mice, as compared with wild-type mice. Moreover, inflammation was selectively increased in the brains, but not spinal cords, of VWFKO mice as compared with wild-type mice. Early increases in BBB permeability in VWFKO mice were not due to increased encephalitogenic T-cell activity since BBB permeability did not differ in adjuvant-treated VWFKO mice as compared with littermates immunized with encephalitogenic peptide plus adjuvant. Taken together, these data indicate that VWF and/or Weibel-Palade bodies negatively regulate BBB permeability changes and autoimmune inflammatory lesion formation within the brain elicited by peripheral inflammatory stimuli.
The blood-brain barrier (BBB) is a physical and metabolic barrier between the central nervous system (CNS) and peripheral circulation that is critical for regulating and protecting the CNS microenvironment.1 Tight junctions formed by interactions between transmembrane proteins (claudins, occludin, and junction adhesion molecules) on adjacent cerebral microvascular endothelial cells (ECs) form the barrier, which, together with pericytes surrounded by basal lamina, astrocytic end-feet, and perivascular interneurons, comprise the neurovascular unit that is essential for the health and function of the CNS. Disruption of tight junctions leads to increased BBB permeability that is well documented in a variety of CNS diseases including multiple sclerosis (MS), an inflammatory demyelinating disorder of CNS of unknown etiology.1 Indeed, both demyelination and plaque formation are more pronounced in areas surrounding small vessels in MS patients.2 Dysregulation of the BBB and transendothelial migration of activated leukocytes across the BBB into the parenchymal perivascular space are essential and critical steps in triggering CNS inflammation and subsequent tissue injury.2 Therefore, understanding the mechanisms associated with BBB permeability changes leading to increased CNS infiltration may provide for newer therapeutic strategies to control disease progression.
Experimental allergic encephalomyelitis (EAE) is the principal autoimmune model of MS.3 Pertussis toxin (PTX) is used as an ancillary adjuvant in the induction of EAE.4 Intoxication with PTX elicits an array of physiological responses in vivo, including increased BBB permeability and sensitization of the vascular endothelium to vasoactive agents such as histamine (HA).5,6 Inbred strains of mice differ in susceptibility to challenge with vasoactive agents following sensitization with PTX in that genetically susceptible strains succumb to hypotensive and hypovolemic shock, while resistant strains do not.7 Additionally, the genetic control of susceptibility to lethal shock is agent specific.8 For example, PTX-induced vascular endothelial sensitization, controlled by Bordetella pertussis induced HA sensitization (Bphs), is detected by HA challenge but not by serotonin challenge. Bphs is an autosomal dominant locus that we recently identified as the histamine H1 receptor (Hrh1/H1R).9 Importantly, susceptibility to EAE is also controlled by Bphs/Hrh1,9,10 underscoring the role of genetic factors in regulating BBB permeability and susceptibility to inflammatory demyelinating diseases of the CNS.
The mechanism whereby PTX sensitizes the vascular endothelium to HA is unknown, but it is consistent with a two-step process: an induction phase, characterized by a 2- to 3-day latent period following intoxication, and an effector phase, manifest by rapid onset of lethal shock that usually occurs within 30 minutes of HA challenge.11,4 Bphs is also characterized by a protracted period of sensitivity that persists upwards of 30 days.4 The fact that sensitization of the vascular endothelium continues well beyond the likely half-life of the toxin in vivo suggests that the induction phase may be associated with the synthesis and storage of additional vasoactive factors within ECs that are released by exposure to HA during the effector phase. In this regard, it is known that following exposure to inflammatory mediators, vasoactive factors such as KC (IL-8 homologue), eotaxin-3, von Willebrand Factor (VWF), P-selectin, CD63/lamp3, angiopoietin 2, endothelin-1, endothelin converting enzyme, tissue-type plasminogen activator, factor XIIa, and/or α1,3-fucosyltransferase VI can be stored in EC Weibel-Palade bodies (WPBs),12 and that HA is a secretagogue for the release of these agents.13
Under this hypothetical two-step model, lethal shock would be caused by (1) the antecedent induction phase due to PTX exposure, and (2) the direct vasodilatory activity of HA combined with the effects of the stored products released from WPBs. In the absence of exposure to PTX, the ECs must be able to compensate for the effects of subsequently administered HA because most mice do not succumb following HA challenge alone. In contrast, PTX-exposed ECs are not able to compensate for the increase in synergistic second messenger signaling arising from exposure to both HA and PTX-induced vasoactive factors stored in WPBs. In this study, mice with a disrupted VWF gene (VWFKO) and a consequent deficiency in WPBs14 were used to directly test this hypothesis, and to evaluate the role of VWF and/or WPBs in regulating BBB permeability and susceptibility to EAE. We report that, contrary to this model, VWF and/or WPBs suppress both Bphs and adjuvant-induced alterations in BBB function associated with actively induced EAE.
Materials and Methods
Animals
B6.129S2-Vwf tm1Wgr (VWFKO) mice14 were maintained in the vivarium of the Given Medical Building at the University of Vermont (Burlington, VT). Wild-type (WT) C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were fed RMH 3000 Lab Diet Rodent Chow (Ralston-Purina, St. Louis, MO) and tap water ad libitum and maintained in accordance with the Animal Welfare Act and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. The experimental procedures used in this study were approved by the Animal Care and Use Committee of the University of Vermont.
Pertussis Toxin in Vivo Intoxication
Mice were injected intravenously (i.v.) with purified PTX (List Biological Laboratories, Inc., Campbell, CA) in 0.025 M Tris buffer containing 0.5 M NaCl and 0.017% Triton X-100, pH 7.6. Control animals received carrier.
HA Sensitivity Testing
HA sensitivity was determined by the i.v. injection of varying amounts of HA (mg/kg dry weight free base) (Sigma, St. Louis, MO) suspended in PBS. Deaths were recorded at 30 minutes postchallenge. The results are expressed as the number of deaths per the number of animals studied.
Proliferation Assay
Mice were immunized for the active induction of EAE and draining lymph nodes were harvested 10 days later. Single-cell suspensions were prepared and 5 × 105 draining lymph node cells/well were plated on standard 96-well flat-bottom tissue culture plates, for 72 hours at 37°C and 7% CO2 with and without myelin oligodendrocyte glycoprotein peptide35–55 (MOG35–55) (Beckman Institute, Paolo Alto, CA) and in the presence of 1.0 μCi 3H-thymidine during the last 18 hours. Cells were harvested onto glass fiber filters, and thymidine uptake was determined by liquid scintillation.
Cytokine/Chemokine Assays
Mice were immunized for the active induction of EAE and spleens were harvested 10 days later. Single-cell suspensions were prepared and 4 × 106 cells/ml were cultured in 24-well culture plates in media containing 50 μg/ml MOG35–55. Cell culture supernatants were recovered after 72 hours and frozen at −70°C until assayed. Interleukin (IL)-12, IL-10, IL-6, tumor necrosis factor-α, monocyte chemotactic protein-1 (MCP-1), and interferon-γ were detected by cytometric bead assay (Becton-Dickinson Bioscience, San Jose, CA). Fifty μl of cell culture supernatant was mixed with 50 μl of the mixed capture beads and 50 μl of the mouse phycoerythrin detection reagent. The tubes were incubated at room temperature for 2 hours in the dark, followed by a wash step. The samples were then resuspended in 400 μl of wash buffer before acquisition on the FACScan (Becton-Dickinson BioScience, San Jose, CA). The data were analyzed using cytometric bead assay software. Standard curves were generated for each cytokine using the mixed bead standard provided in the kit, and the concentration of cytokine in the cell supernatant was determined by interpolation from the appropriate standard curve.
For real-time RT-PCR analysis, total RNA was isolated using the RNeasy minikit protocol (Qiagen, Valencia, CA) and then converted to cDNA using oligo(dT), random hexamers, and Superscript RT II enzyme (Invitrogen, Grand Island, NY). Message levels were quantified using the ABI 7000 Real-Time PCR System (Applied Biosystems, Foster City, CA). Amplification was performed in a total volume of 25 μl for 40 cycles and products were detected using SYBR Green I dye (Molecular Probes, Eugene, OR). Samples were run in triplicate and relative expression level was determined by normalizing to L32 with the results presented as relative abundance. Primer sequences used have been described before.15
Induction and Evaluation of EAE
EAE was induced as previously described.16 Briefly, mice were injected subcutaneously in the flanks and neck with 0.1 ml of an emulsion containing 200 μg of MOG35–55 in saline and an equal volume of complete Freund’s adjuvant containing 200 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI). On the day of immunization, each mouse received 200 ng of PTX by i.v. injection. The mice were assessed daily for clinical signs of EAE using the following scale: 0, normal; 1, limp tail or mild hind limb weakness; 2, moderate hind limb weakness or mild ataxia; 3, moderately severe hind limb weakness; 4, severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5, paraplegia with no more than moderate forelimb weakness; 6, paraplegia with severe forelimb weakness or severe ataxia or moribund condition.
Brains and spinal cords were dissected from calvaria and vertebral columns, respectively, and fixed by immersion in 10% phosphate-buffered formalin (pH 7.2). Following adequate fixation, brains and spinal cords were trimmed and representative transverse section embedded in paraffin, sectioned at 5 μm, and mounted on glass slides. Sections were stained with H&E for routine evaluation and Luxol fast blue-periodic acid Schiff for demyelination. Sections from representative areas of the brain and spinal cord were scored in a semiquantitative fashion for the various histopathological parameters as previously described.15,16,17 Briefly, EAE pathology was scored for the overall severity of the lesions observed, the extent and degree of demyelination and tissue injury (swollen axon sheathes, swollen axons, and reactive gliosis), severity of the acute inflammatory response such as neutrophil infiltration, and the severity of the chronic inflammatory response (lymphocytes/monocytes).
BBB Permeability Determinations
BBB permeability was assessed as previously described.18 Briefly, a 50 μg/g dose of fluorescein isothiocyanate-labeled bovine serum albumin (Sigma, St. Louis, MO) was injected i.v. into WT and VWFKO mice on day 8, 10, or 12 post immunization with CFA+PTX+MOG35–55, CFA+PTX. Cerebrospinal fluid and blood were collected after 4 hours. Both cerebrospinal fluid and plasma samples, prepared by centrifugation at 3000 rpm for 15 minutes, were diluted in PBS, and the fluorescence intensity was measured with a microplate fluorescence reader (Flx-800-I, Bio-Tek Instruments Inc, Winooski, VT) using the software KC-4, with an excitation wavelength of 485 nm and an emission wavelength of 528 nm. The BBB permeability index is expressed as the ratio of the fluorescence intensity of the CSF divided by the fluorescence intensity of the plasma.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4 software (GraphPad software Inc, San Diego, CA). Significance of difference was assessed by two-way analysis of variance and/or regression analysis. For all analyses P ≤ 0.05 was considered significant.
Results
Bphs in VWFKO Mice
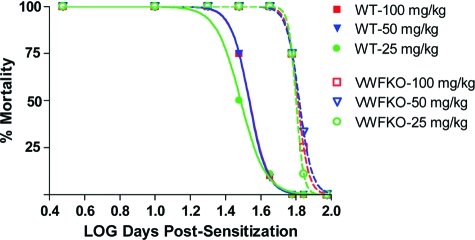
The role of VWF and/or WPBs in Bphs was evaluated by i.v. sensitization of WT and VWFKO mice with 200 ng PTX on day 0. Three days later, HA sensitivity was assessed in a dose-response fashion by i.v. challenge with HA and deaths were recorded at 30 minutes. A significant difference in the LD50 values between the two strains was not detected (WT = 1.65 ± 0.05 mg/kg versus VWFKO = 1.41 ± 0.08 mg/kg; F = 0.55, P = 0.46), indicating that neither VWF nor WPBs are required for Bphs susceptibility. Given the role of WPBs in vascular function, we nevertheless assessed their effect on the persistence of HA sensitivity. As compared with WT mice, HA sensitivity persisted longer in VWFKO mice at all challenge doses studied (F = 38.25; P < 0.0001) (Figure 1). The half-life of sensitization was 64.8 days in VWFKO mice compared 34.4 days in WT mice at 100 mg/kg HA challenge. Similarly, the sensitization half-lives were 66.0 and 63.3 days in VWFKO mice at 50 mg/kg and 25 mg/kg of HA, respectively while the corresponding sensitization half-lives in WT mice were 34.2 and 30.7 days. Taken together, these data demonstrate that VWF and/or WPBs ordinarily act to shorten the longevity of HA sensitivity elicited by in vivo intoxication with PTX.
Figure 1.
Assessment of Bphs in WT and VWFKO. Mice were sensitized with 200 ng purified PTX by i.v. injection on day 0. Mice (n = 4 to 11 per time point) were challenged with the indicated dose of HA (mg dry weight free base) by i.v. injection on the indicated day and deaths were recorded at 30 minutes post-challenge. The results are expressed as the number of animals dead over the number of animals studied (% mortality).
EAE in VWFKO Mice
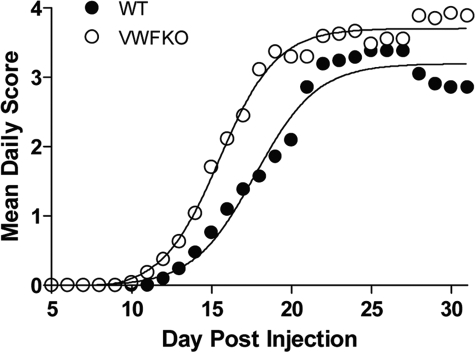
Because VWFKO mice exhibited significantly prolonged sensitivity to HA, and Bphs is an EAE susceptibility gene,9 we studied the role of VWF and/or WPBs in regulating EAE induced by immunization with MOG35–55+CFA+PTX. As compared with WT mice, VWFKO mice developed significantly more severe clinical signs of EAE (Figure 2). Clinical signs of EAE in VWFKO mice were notably enhanced during the acute-early phase (D7 through D18 postimmunization) as compared with the chronic late-phase of the disease (day 20 to 30).19 The mean day of disease onset in VWFKO mice was 14.1 ± 2.0 vs. 16.4 ± 3.2 (P = 0.01) in WT mice. The acute-early phase cumulative disease score in VWFKO mice was 22.6 ± 11.1 vs. 13.3 ± 11.9 (P = 0.008) in WT mice.
Figure 2.
Early onset and severe clinical course of EAE in VWFKO mice. EAE was induced in WT (n = 18) and VWFKO (n = 27) mice by immunization with MOG35–55+CFA+PTX. Regression analysis16 indicates that the disease course in both strains fits a variable slope sigmoidal curve and is significantly different between the two strains (F = 32.5; P < 0.0001).
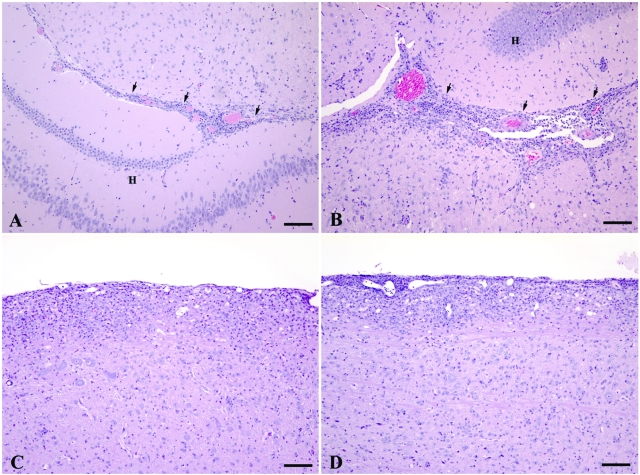
Histological analysis of CNS samples obtained during the acute-early phase of the disease revealed that VWFKO mice exhibited significantly greater pathology in the brain than did WT mice (Figure 3, A and B). This was reflected in VWFKO mice having a higher average overall pathology index with significantly greater lesion scores, more severe demyelination, more suppuration, and more extensive mononuclear cell infiltrates (Figure 4A). In contrast, however, no difference in the overall severity of the lesions between VWFKO and WT mice was observed in the spinal cord (Figure 3, C and D; Figure 4B). These results demonstrate that the absence of VWF and/or WPB selectively promotes lesion formation in the brain, compared to the spinal cord-dominant disease seen in mice with intact VWF and WPBs.
Figure 3.
Comparison of the histopathological lesions caused by EAE in the brains of WT (A) and VWFKO (B) mice and the spinal cords of WT (C) and VWFKO (D) mice. In the brains, the inflammatory response, consisting of an admixture of lymphocytes and monocytes with occasional neutrophils and rare eosinophils, was more severe in the VWFKO mice (B) than the response that occurred in the brains of the WT mice (A). In the brains of both strains of mice, the inflammatory response commonly occurred around capillaries and postcapillary venules in the interface area (arrows) between the brainstem and hippocampal formation (H). Mild demyelination occurred concurrently with inflammation in the VWFKO strain of mice; however, none was observed in the WT strain of mice. A and B: H&E stain, scale bar = 100 μm. In the spinal cords, the severity and character of the inflammatory response were similar between WT (C) and VWFKO (D) mice and again consisted of an admixture of lymphocytes and monocytes with occasional neutrophils and rare eosinophils. The inflammatory response commonly occurred around capillaries and post capillary venules in the pia-arachnoid and subjacent white matter. Mild demyelination occurred concurrently with inflammation in both strains of mice. C and D: H&E stain, scale bar = 100 μm.
Figure 4.
Quantification of lesion severity in WT and VWFKO mice. The histopathological lesions were scored in MOG35–55+CFA+PTX immunized WT (n = 18) and VWFKO (n = 25) mice in a semiquantitative manner as described in Material and Methods, which revealed that the lesions in the brains (A) but not in spinal cords (B) of VWFKO mice are more severe compared to WT controls. Significance of differences was determined using the Student’s t-test.
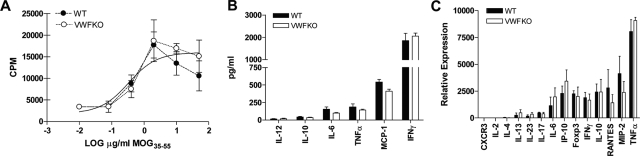
We examined a number of T cell parameters in WT and VWFKO mice following sensitization with MOG35–55+CFA+ PTX to evaluate the encephalitogenic T cell response elicited in each strain. No difference in the proliferative response of spleen cells to MOG35–55 at day 10 p.i. was observed between WT and VWFKO mice (Figure 5A). Similarly, no significant differences in cytokine and chemokine expression following ex vivo restimulation with MOG35–55, were detected between WT and VWFKO animals (Figure 5, B and C). Taken together, these results indicate that the more severe acute-early phase disease seen in VWFKO mice is unlikely to be due to a direct effect of the absence of VWF and/or WPBs on T cell effector responses.
Figure 5.
Normal T cell responses in EAE induced WT and VWFKO mice. (A) WT and VWFKO CD4 T cells (n = 6 to 8 mice/strain) have equivalent ex vivo proliferative responses to MOG35–55 10 days following immunization with MOG35–55+CFA+PTX. Mean cpm ± SD were calculated from triplicate wells. (B) Protein production and/or (C) mRNA expression of cytokines/chemokines by MOG35–55 stimulated splenocytes does not differ between WT and VWFKO mice immunized with MOG35–55+CFA+PTX 10 days earlier. Cytokine production was determined by cytometric bead assay and expression levels determined by real-time RT-PCR. Significance of differences between WT and VWFKO CD4 T-cells responses was determined using the Student’s t-test with a P value of 0.05 as the significance threshold.
Increased BBB Permeability in VWFKO Mice during EAE
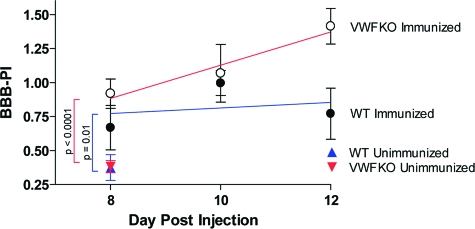
To delineate the mechanisms underlying the more severe acute-early phase EAE in VWFKO mice, we analyzed EC function by measuring BBB permeability. A BBB permeability index (BBB-PI) was determined by measuring the traverse of systemically injected fluorescein isothiocyanate-labeled bovine serum albumin into the cerebrospinal fluid of EAE-induced mice at 8, 10, and 12 days p.i. (Figure 6). BBB-PIs were not significantly different between unimmunized WT and VWFKO mice; however, BBB-PIs were significantly elevated in both mouse strains (P = 0.01 for WT and P < 0.0001 for VWFKO) following immunization with MOG35–55+CFA+PTX. Moreover, the test of the main effect of group (mouse strain) showed a significant difference (P = 0.02) between the two strains, with the BBB-PI being greater in VWFKO mice compared to WT mice.
Figure 6.
VWFKO mice exhibit increased BBB permeability compared to WT mice following injection with MOG35–55+CFA+PTX (n = 10 for each strain at each time point). The permeability indices were calculated by determining the fluorescence in the CSF and the plasma collected 4 hours after i.v. injection of fluorescein isothiocyanate-BSA and is the ratio of the fluorescence intensity of the CSF divided by the fluorescence intensity of the plasma. By two-way analysis of variance, a significant difference in the BBB permeability between the two strains was observed (F = 5.61; P = 0.02). No significant differences were observed in BBB permeability by time (F = 1.74; P = 0.2). Regression analysis (colored lines) also revealed that changes in BBB permeability differ significantly between the two strains (F = 5.32; P = 0.03), with the VWFKO mice being significantly greater than WT mice, but no differences over time (F = 1.43; P = 0.24) were observed.
Increased BBB Permeability in VWFKO Mice Is Encephalitogen-Independent
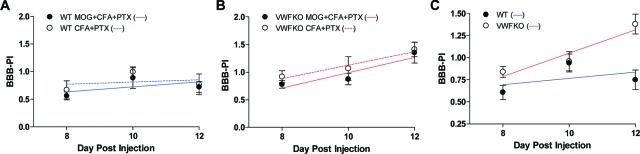
The significant difference in clinical signs and brain pathology between WT and VWFKO mice during the acute-early phase of the disease, despite the absence of detectable differences in encephalitogenic T cell effector responses, point to a potential role for VWF and/or WPBs in regulating the interface between the circulation and the brain. We therefore compared the integrity of the BBB in WT and VWFKO mice at various time points after injection with MOG35–55+CFA+PTX or CFA+PTX. Immunization with CFA+PTX alone lead to increased BBB permeability in WT mice (Figure 7A) to an extent that was not different from that seen following immunization with MOG35–55+CFA+PTX (P = 0.45). The change in BBB permeability over time was also not significant in WT mice (P = 0.08). Similarly, immunization with CFA+PTX alone significantly increased the BBB permeability in VWFKO mice but again, there was no difference in the BBB-PIs between the animals immunized with or without the encephalitogen (P = 0.27) (Figure 7B). This indicates that in both WT and VWFKO mice, antigen-specific encephalitogenic T cells are not responsible for eliciting increased BBB permeability across the time points studied. Because the BBB-PIs between the animals immunized with or without MOG35–55 were not different, they were pooled and reanalyzed. The results revealed that the BBB-PI in VWFKO mice was significantly greater than the BBB-PI in WT mice (P = 0.0005) (Figure 7C) when CFA and PTX are used as adjuvants, and that this difference is encephalitogen-independent. These data indicate that VWF and/or WPBs protect against the vascular permeability changes induced by these inflammatory agents.
Figure 7.
Increased BBB permeability in VWFKO mice is independent of encephalitogenic T cells. BBB permeability in CFA+PTX immunized WT and VWFKO mice was determined as described in Materials and Methods and was compared with BBB permeability in MOG35–55+CFA+PTX immunized WT (A), VWFKO mice (B) (n = 8 to 10 for each strain at each time point). Two-way analysis of variance revealed no difference in BBB permeability by treatment (F = 0.58; P = 0.45) or over time (F = 2.60; P = 0.08) in WT animals. Similarly, regression analysis also indicated that there is no significant difference in BBB permeability by treatment (F = 0.56; P = 0.46) or over time (F = 0.12; P = 0.73) in WT mice. In VWFKO mice, while two-way analysis of variance suggested a significant difference in BBB permeability over time (F = 6.97; P = 0.003) but not by treatment (F = 1.27; P = 0.27), regression analysis revealed no significant difference by treatment (F = 0.05; P = 0.83) or over time (F = 1.52; P = 0.23). Since there was no difference in BBB permeability between MOG35–55+CFA+PTX and CFA+PTX immunized animals, the data were pooled and re-analyzed (C). By two-way analysis of variance, changes in BBB permeability differ significantly between WT and VWFKO mice (F = 13.15; P = 0.0005) and over time (F = 5.98; P = 0.004). Regression analysis also revealed that the BBB permeability is significantly higher in VWFKO mice (F = 10.08; P = 0.001) but do not differ over time (F = 3.45; P = 0.07).
Discussion
In this study we show that the absence of VWF and/or WPBs leads to increased BBB permeability and, in appropriately immunized mice, concomitantly more severe EAE. The disruption of BBB integrity is due to adjuvants alone; and although the mechanism(s) by which CFA and PTX act to increase BBB permeability is unknown, these processes are independent of encephalitogenic T cell responses. This is in agreement with previous studies looking at the effects of inflammatory pain elicited by formalin, CFA and λ-carrageenan on BBB permeability.20,21 BBB permeability changes elicited by these stimuli were associated with unique alterations in the temporal expression of tight junctional proteins as well as disruption of the interaction between tight junctional complexes and the cytoskeleton. It is unclear what the mechanism(s) for tight junctional alterations following peripheral inflammation might be, but both CFA and PTX are known to lead to increased levels of IL-1β, tumor necrosis factor-α, and/or IL-6 within the periphery and CNS rapidly after exposure.22,23,24,25,26,27 Within the CNS the increase in IL-1β expression is associated with widespread changes in neuronal activity, including Cox-2 expression in CNS neurons leading to elevated prostaglandin E2 levels in the CSF.28 Consequently, alterations in BBB permeability might be subject to modulation via both peripheral and centrally mediated responses to inflammatory stimuli. In fact, we recently demonstrated that neurogenic control of BBB permeability is negatively regulated by central histamine H3 receptor signaling.15
Alterations in BBB permeability can occur in a variety of different situations,20 some of which relate directly to MS. There is an increasing body of evidence in both EAE29,30 and MS31,32 that subtle, progressive alterations in BBB integrity precede the formation of inflammatory lesions. These changes are detected in all MS disease subtypes, suggesting that a common abnormality in BBB function exists in the normal-appearing white matter of MS patients and may be a predisposing factor in initiating and propagating new inflammatory foci. Importantly, in our studies the VWF and/or WPB-related changes in BBB permeability also do not derive from T cell activity indicating that VWF and/or WPBs play an important role in regulating BBB permeability in response to peripheral inflammatory stimuli. In this setting, understanding the mechanism of Bphs as a genetic model of BBB dysregulation is important.
The effector phase of Bphs is characterized by death due to hypotensive and hypovolemic shock within minutes of HA challenge.11 This time frame suggests that the effector phase may be associated with a sudden and rapid release of preformed factors generated during the sensitization phase rather than the induction of new gene expression by HA receptor signaling. Because inflammatory signals such as PTX induce the synthesis and storage of the vasoactive factors in the WPBs33,34 and HA is a secretagogue for the release of these agents,13 the shock following PTX sensitization and HA challenge could be due to the combined direct vasodilatory effects of HA and autocrine activity of the released stored products from WPBs. However, our results in VWFKO mice demonstrate that the stored vasoactive factors in WPBs are not responsible for the sensitization and that VWF and/or other WPB components are instead protective in this model.
This observation was surprising given the fact that WPBs store several factors that are good candidates for the observed trait, the sudden release of which could change endothelial function in an autocrine fashion. WPBs are dynamic granules with very random movements in resting cells.35 WPBs are released from ECs in response to a large number of secretagogues, which can be divided into two distinct groups: those that act by elevating intracellular calcium levels such as thrombin and HA and those that act by raising cAMP levels such as epinephrine and vasopressin.12 Calcium-raising agonists induce a periphery-directed movement of WPBs while cAMP-mediated agonists induce a transport-directed redistribution toward the center of the cell, leading to a star-like cluster at the perinuclear region.36 WPB clustering is believed to prevent excessive release of WPB constituents, and may also lead to a selective exclusion of subsets of WPBs from exocytosis. Clustering is induced by thrombin stimulation in human aortic EC37 but not in HUVECs.38 PTX can inhibit both VWF and tPA release from WPBs in HUVECs in response to thrombin, most likely due to inhibition of calcium influx and PTX inhibits HA-induced calcium release in HUVECs.39 PTX causes ADP ribosylation of Gi/o family of G proteins that are negative regulators of Gαs protein and its subsequent activation of adenylate cyclase. By this mechanism, PTX treatment leads to increased intracellular cAMP concentration.40,41,42,43 Hence, it is possible that the suppression of Bphs observed in mice that have VWF and WPBs could be due to PTX-induced, cAMP-mediated clustering of WPBs and sequestration of their contents in WT mice and not in VWFKO mice.
Several studies have provided evidence in support of the existence of different subsets of WPBs that apart from VWF do not contain the same set of additional constituents.33,34,44,45 It has also been reported that different stimuli induce different WPB responses, depending on the physiological need. In the case of vascular damage, thrombin induces a rapid, local response leading to exocytosis of the WPBs while epinephrine induces a gradual release of WPBs.36 Moreover, epinephrine induces the exocytosis of only the peripheral WPBs, whereas thrombin stimulates the exocytosis of peripheral, as well as central WPBs.36 This difference in release pattern and the fact that different stimuli, such as thrombin and epinephrine, induce WPB exocytosis via distinct mechanisms enables the cell to regulate the exocytosis of WPBs, and possibly the release of specific WPB constituents, in such a way that it meets the pathophysiological requirements induced by distinct triggers.46 Therefore in an inflammatory disease as complex as EAE, stored factors in WPBs may regulate a fine balance between pro-inflammatory and anti-inflammatory responses while in animals that lack WPBs this regulation is lost, which may explain increased susceptibility of VWFKO mice to EAE. In addition, VWF is an adhesion molecule for leukocytes47 that may be involved in their recruitment (Wagner DD, unpublished data). VWF has also been reported to mediate clearance of metastatic tumor cells in lungs through a mechanism still not known.48 Therefore, it is possible that VWF may be promoting the clearance of pathogenic autoimmune cells or pro-inflammatory debris in the CNS of WT mice leading to lesser CNS inflammation than in VWF deficient mice.
The mechanism of BBB disruption in MS and EAE is unknown. Currently it is believed that neuroantigen-specific T cells within the systemic circulation interacting with ECs bring about the changes that lead to the formation of inflammatory foci and BBB permeability changes.49 Our results, however, indicate that disruption of the BBB in active EAE is independent of the encephalitogenic T cell responses and that this is caused by the interaction of EC with peripheral inflammatory stimuli such as CFA and PTX, and may also include neuropathic and inflammatory pain,20,50 which can be a major component of most forms of MS including benign disease.51 Overall, our study demonstrates that the interaction of EC with environmental agents other than those that lead to pathogenic T cell responses also influence the development of autoimmune disease by modifying BBB permeability. Taken together our findings underscore the potential importance of co-infection and/or non-autoimmune related gene-by-environment interactions in the etiology of MS.
Footnotes
Address reprint requests Dr. Cory Teuscher, Immunobiology Program, C317 Given Medical Building, University of Vermont, Burlington, VT 05405. E-mail: c.teuscher@uvm.edu.
Supported by the National Institutes of Health (grants NS36526, AI45105, AI41747, HL041002 and AI45666) and the National Multiple Sclerosis Society (grant RG-3575).
References
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Correale J, Villa A. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40:148–160. doi: 10.1080/08916930601183522. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- Munoz JJ, Arai H, Bergman RK, Sadowski PL. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981;33:820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C. Molecular aspects of Bordetella pertussis pathogenesis. Int Microbiol. 1999;2:137–144. [PubMed] [Google Scholar]
- Munoz JJ. Sekura RD, Moss J, Vaughn M, editors. Orlando FL: Academic Press; Biological activities of pertussigen (pertussis toxin) 1985:p 1–18. [Google Scholar]
- Wardlaw AC. Inheritance of responsiveness to pertussis HSF in mice. Int Arch Allergy Appl Immunol. 1970;38:573–589. doi: 10.1159/000230313. [DOI] [PubMed] [Google Scholar]
- Gao JF, Call SB, Fillmore PD, Watanabe T, Meeker ND, Teuscher C. Analysis of the role of Bphs/Hrh1 in the genetic control of responsiveness to pertussis toxin. Infect Immun. 2003;71:1281–1287. doi: 10.1128/IAI.71.3.1281-1287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- Linthicum DS, Frelinger JA. Acute autoimmune encephalomyelitis in mice. II. Susceptibility is controlled by the combination of H-2 and histamine sensitization genes, J Exp Med. 1982;156:31–40. doi: 10.1084/jem.156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Bergman RK. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol Rev. 1968;32:103–126. doi: 10.1128/br.32.2.103-126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis, Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF, Blankenhorn EP. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS, Proc Natl Acad Sci USA. 2007;104:10146–10151. doi: 10.1073/pnas.0702291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice, Proc Natl Acad Sci USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- Tang T, Frenette PS, Hynes RO, Wagner DD, Mayadas TN. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J Clin Invest. 1996;97:2485–2490. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M, Yellayi S, Zamora A, Subramanian S, Tovey M, Vandenbark AA, Offner H, Zachary JF, Fillmore PD, Blankenhorn EP, Gustafsson JA, Teuscher C. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am J Pathol. 2004;164:1915–1924. doi: 10.1016/s0002-9440(10)63752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Brooks TA, Ocheltree SM, Seelbach MJ, Charles RA, Nametz N, Egleton RD, Davis TP. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 2006;1120:172–182. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher CE, Donnelly S, Mills KH, Lynch MA. Interleukin-1beta-dependent changes in the hippocampus following parenteral immunization with a whole cell pertussis vaccine. J Neuroimmunol. 2000;111:68–76. doi: 10.1016/s0165-5728(00)00366-0. [DOI] [PubMed] [Google Scholar]
- Loscher CE, Donnelly S, Lynch MA, Mills KH. Induction of inflammatory cytokines in the brain following respiratory infection with Bordetella pertussis. J Neuroimmunol. 2000;102:172–181. doi: 10.1016/s0165-5728(99)00177-0. [DOI] [PubMed] [Google Scholar]
- Donnelly S, Loscher CE, Lynch MA, Mills KH. Whole-cell but not acellular pertussis vaccines induce convulsive activity in mice: evidence of a role for toxin-induced interleukin-1beta in a new murine model for analysis of neuronal side effects of vaccination. Infect Immun. 2001;69:4217–4223. doi: 10.1128/IAI.69.7.4217-4223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong ME, Loscher CE, Lynch MA, Mills KH. IL-1beta-dependent neurological effects of the whole cell pertussis vaccine: a role for IL-1-associated signalling components in vaccine reactogenicity. J Neuroimmunol. 2003;136:25–33. doi: 10.1016/s0165-5728(02)00468-x. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- Tonra JR. Cerebellar susceptibility to experimental autoimmune encephalomyelitis in SJL/J mice: potential interaction of immunology with vascular anatomy. Cerebellum. 2002;1:57–68. doi: 10.1080/147342202753203096. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Reiseter BS, Kolbeck R, Nagashima K, Robertson R, Keyt B, Lindsay RM. Comparison of the timing of acute blood-brain barrier breakdown to rabbit immunoglobulin G in the cerebellum and spinal cord of mice with experimental autoimmune encephalomyelitis. J Comp Neurol. 2001;430:131–144. [PubMed] [Google Scholar]
- Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology. 1993;43:2484–2490. doi: 10.1212/wnl.43.12.2484. [DOI] [PubMed] [Google Scholar]
- Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res. 2006;28:230–235. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- Wolff B, Burns AR, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med. 1998;188:1757–1762. doi: 10.1084/jem.188.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani de Wit T, Rondaij MG, Hordijk PL, Voorberg J, van Mourik JA. Real-time imaging of the dynamics and secretory behavior of Weibel-Palade bodies. Arterioscler Thromb Vasc Biol. 2003;23:755–761. doi: 10.1161/01.ATV.0000069847.72001.E8. [DOI] [PubMed] [Google Scholar]
- Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:883–891. doi: 10.1161/01.atv.20.3.883. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Roudnik VE, Bystrevskaya VB, Smirnov VN. Centrosome-directed translocation of Weibel-Palade bodies is rapidly induced by thrombin, calyculin A, or cytochalasin B in human aortic endothelial cells. Cell Motil Cytoskeleton. 2000;47:141–153. doi: 10.1002/1097-0169(200010)47:2<141::AID-CM5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, Kragt A, Gijzen KA, Sellink E, van Mourik JA, Fernandez-Borja M, Voorberg J. Dynein-dynactin complex mediates protein kinase A-dependent clustering of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:49–55. doi: 10.1161/01.ATV.0000191639.08082.04. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen Y, Atsma DE, Lupu F, de Vries RE, Kooistra T, Emeis JJ. Involvement of calcium and G proteins in the acute release of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:2177–2187. doi: 10.1161/01.atv.17.10.2177. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Mittra S, Bourreau JP. Gs and Gi coupling of adrenomedullin in adult rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H1842–H1847. doi: 10.1152/ajpheart.00388.2005. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Rosiak J, Nowak JZ. Pertussis toxin-sensitive G protein modulates the ability of histamine to stimulate cAMP production in the chick pineal gland. Pol J Pharmacol. 2004;56:407–413. [PubMed] [Google Scholar]
- Sugden D, Davidson K, Hough KA, Teh MT. Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell Res. 2004;17:454–460. doi: 10.1111/j.1600-0749.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Pendu R, Terraube V, Christophe OD, Gahmberg CG, de Groot PG, Lenting PJ, Denis CV. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108:3746–3752. doi: 10.1182/blood-2006-03-010322. [DOI] [PubMed] [Google Scholar]
- Terraube V, Pendu R, Baruch D, Gebbink MF, Meyer D, Lenting PJ, Denis CV. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J Thromb Haemost. 2006;4:519–526. doi: 10.1111/j.1538-7836.2005.01770.x. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lucchinetti CF, Noseworthy JH. Multiple sclerosis: current pathophysiological concepts. Lab Invest. 2001;81:263–281. doi: 10.1038/labinvest.3780235. [DOI] [PubMed] [Google Scholar]
- Inoue K. ATP receptors of microglia involved in pain, Novartis Found Symp. 2006;276:263–272. discussion 273–281. [PubMed] [Google Scholar]
- Glad S, Nyland H, Myhr KM. Benign multiple sclerosis. Acta Neurol Scand Suppl. 2006;183:55–57. doi: 10.1111/j.1600-0404.2006.00617.x. [DOI] [PubMed] [Google Scholar]