Abstract
Iron is an essential element for almost all living organisms. The possible role of iron for growth, adherence and cytotoxicity of Entamoeba histolytica was evaluated in this study. The absence of iron from TYI-S-33 medium stopped amebic growth in vitro. However, iron concentrations in the culture media of 21.4-285.6 µM did not affect the growth of the amebae. Although growth was not retarded at these concentrations, the adhesive abilities of E. histolytica and their cytotoxicities to CHO cell monolayer were correlated with iron concentration. Amebic adhesion to CHO cell monolayers was significantly reduced by low-iron (24.6 ± 2.1%) compared with 62.7 ± 2.8 and 63.1 ± 1.4% of amebae grown in a normal-iron and high-iron media, respectively. E. histolytica cultured in the normal- and high-iron media destroyed 69.1 ± 4.3% and 72.6 ± 5.7% of cultured CHO cell monolayers, but amebae grown in the low-iron medium showed a significantly reduced level of cytotoxicity to CHO cells (2.8 ± 0.2%). Addition of divalent cations other than iron to amebic trophozoites grown in the low-iron medium failed to restore levels of the cytotoxicity. However, when E. histolytica grown in low-iron medium were transferred to normal-iron medium, the amebae showed completely restored cytotoxicity within 7 days. The result suggests that iron is an important factor in the adherence and cytotoxicity of E. histolytica to CHO cell monolayer.
Keywords: Entamoeba histolytica, Adherence, Cytotoxicity, Iron, Virulence
The intestinal protozoan parasite Entamoeba histolytica is the causative agent of amebiasis. In humans, E. histolytica can produce colitis characterized by ulceration and invasion of the intestinal wall. In advanced cases, trophozoites spread to distant organs [1]. The virulence of axenic laboratory strains of amebae is commonly assayed by their ability to disrupt monolayers of cultured mammalian cells [2]. Since cytolysis of the target cell is a major pathological feature of the disease, the factors contributing to cytolysis appear to be important in understanding the amebic invasion.
Iron is an essential element for the growth of almost all microorganisms [3], including E. histolytica. Synthesis of toxins and virulence determinants are regulated by the iron status of bacterial cells [4,5]. In Trichomonas vaginalis, cytoadherence [6], cysteine proteinase activity [7], and subcutaneous abscess formation [8] are also enhanced by growth in medium supplemented with iron. Despite extensive research on the control of bacterial and trichomonad virulence by iron, the role and significance of iron are poorly understood in E. histolytica. Nevertheless, it is likely that mechanisms of iron regulation are related to virulence expressions in pathogenic E. histolytica [9]. Amebic gene modulations of iron have been described [10]. Identification of virulence-related molecules of E. histolytica, which mediate adhesion and / or cytolysis of target cells is important for understanding the biology of the parasite as well as the amebic disease process. Adherence is a key step in mucosal invasion and it is a required step for target cell cytolysis [11,12]. In the present work, the possible role of iron in functions, such as growth, adherence, and cytotoxicity was evaluated.
Entamoeba histolytica (HM1 : IMSS) were axenically cultured in TYI-S-33 medium [13] supplemented with 10% calf serum at 37℃, and subcultured every 72 hr. The experimental media differed from the stock culture medium (TYI-S-33 without iron salt supplementation) by variations in the concentration of the added iron salt (21.4, 71.4 and 285.6 µM, low-, normal- and high-iron medium, respectively) or by substitution of other metal salts. Metal salts, which were substituted for the supplemented iron in experimental cultures, included copper, manganese, magnesium and aluminum. Chinese hamster ovary (CHO) cells were used for cytotoxicity and adherence assays, which were grown at pH 7.2 in Dulbecco's modified Eagle's medium (Gibco Laboratories, Life Technologies, Grand Island, New York, USA) supplemented with 10% fetal bovine serum in culture flasks maintained at 37℃ in a humid atmosphere containing 5% CO2.
Amebic growth patterns were examined by inoculating 5 × 104 trophozoites into 10 ml of experimental media. At various time points (0, 24, 48, 72 and 96 hr), amebae were detached by chilling on ice for 10 min, and collected by centrifugation at 500 g at 4℃. The numbers of amebae were then counted using a hemocytometer. Growth of the amebae did not differ among the 3 groups from low-iron (21.4 µM), normal iron (71.4 µM), and high-iron (285.6 µM) (Fig. 1). In all 3 groups, the viability of the amebae was higher than 87% during 72 hr of culture. These results indicate that iron is an essential element for multiplication of E. histolytica trophozoites, and growth was not affected among a wide range of iron concentrations.
Fig. 1.
The effect of iron on the growth of E. histolytica. Culture was initiated by inoculating 5 × 104 trophozoites into 10 ml of experimental media. As indicated, amebic growth was measured, which were grown in the medium supplemented with different concentrations of iron; TYI-S-33 without iron supplementation (◆), and supplemented with 21.4 µM (▲, low-iron), 71.4 µM (●, normal-iron), and 285.6 µM (■, high-iron).
To elucidate the effect of iron on the adherence and cytotoxicity to CHO cells by E. histolytica, adherence and cytotoxicity assays were carried out. E. histolytica trophozoites, grown in media of low-, normal- and high-iron concentrations and harvested from 48 hr cultures were washed in PBS and suspended in PBS. CHO cells, harvested by trypsinization, were washed and suspended in PBS. The amebic trophozoites (1 × 104) were mixed with 2 × 105 cultured CHO cells, and the mixture was centrifuged at 100 g for 5 min. The interaction was allowed to proceed for 2 hr at 37℃. Higher levels of adherence were reproducibly seen in amebic trophozoites grown in normal- and high-iron media, when compared with amebae grown in a low-iron concentration (Fig. 2). Adherence values were 62.7 ± 2.8% and 63.1 ± 1.4% for amebic trophozoites grown in a normal- and high-iron medium, respectively. Amebic adhesion to CHO cell monolayers was significantly reduced by the low iron concentration (24.6 ± 2.1%).
Fig. 2.
Comparison of the adherence (□) and cytotoxicity (■) of E. histolytica trophozoites cultivated in low-iron (21.4 µM), normal-iron (71.4 µM), and high-iron (285.6 µM) media to CHO cells. Results are shown as mean ± SD of 3 independent experiments.
The cytotoxicity assay was performed using CytoTox96® Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, Wisconsin, USA), as directed by the manufacturer. The CytoTox96® assay quantitatively measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis. CHO cells (target cells) were grown to confluency in a 96-well culture plate, seeded at a density of 5 × 104 / well. Effector cells (2.5 × 104 of E. histolytica) grown in experimental media were added to each well (CHO cell / ameba ratio of 1 : 5; experimental). The cytotoxicity assay plate was incubated for 4 hr in a humidified incubator at 37℃ under 5% CO2. Forty-five min prior to harvesting the supernatants, 10 µl of the lysis solution was added to the wells containing the target cell maximum LDH control. The plate was centrifuged at 250 g for 4 min and 50 µl aliquots were transferred to fresh 96-well enzymatic assay plates. Subsequently, 50 µl of the substrate mix was added to each well of the enzymatic assay plate and incubated for 30 min at room temperature. Finally, the absorbance was recorded at 490 nm after the addition of 50 µl of stop solution. Cytotoxicity was calculated as follows:
Cytotoxicity (%) = Experimental - Effector spontaneous - Target spontaneous / Target maximum - Target spontaneous × 100
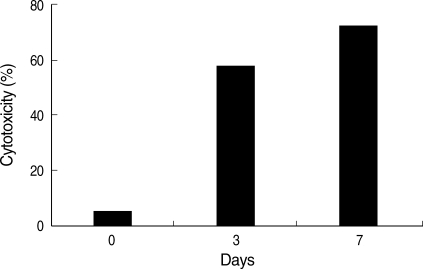
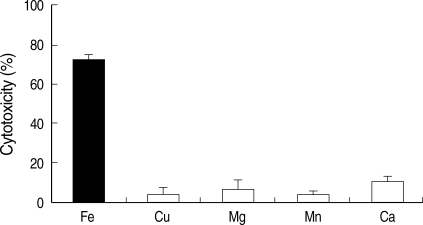
Amebic trophozoites cultured in the normal- and high-iron medium destroyed 69.1 ± 4.3% and 72.6 ± 5.7% of cultured CHO cell monolayers (Fig. 2). Cytotoxicity to CHO cells of amebic trophozoites grown in the low-iron medium showed significantly lower level (2.8 ± 0.2%). When E. histolytica grown in the low-iron medium were transferred to the normal-iron medium, the cytotoxicity of the amebae was completely restored within 7 days (Fig. 3). Microscopic examination of the amebae showed a similar appearance of cytotoxicity (data not shown). The specificity of iron modulation of cytotoxicity was tested, and the results from a typical experiment are presented in Fig. 4. Addition of cationic salts other than iron to amebic trophozoites grown in the low-iron medium failed to restore levels of cytotoxicity to those seen for parasites under the same conditions but after addition of iron. E. histolytica trophozoites were fully viable in these experiments. These observations indicated that these cationic salts were not toxic to the parasites and reaffirmed the specificity of iron in regulating cytotoxicity. Fig. 5 shows that only cysteine proteinase inhibitors, such as E64 and leupeptin abolish iron-induced cytotoxicity in both normal and high iron media. The cytotoxicities were decreased to 20-40% levels compared with that without a proteinase inhibitor. These results indicate that amebic cytolysis depends on cysteine proteinase activity.
Fig. 3.
Recovery of cytotoxicity after medium transfer. E. histolytica grown in low-iron medium (21.4 µM) were transferred to normal-iron medium (71.4 µM) and incubated for 3 days or 7 days. The amebae completely restored cytotoxicity in 7 days.
Fig. 4.
The specificity of iron-restored cytotoxicity was determined by measuring the level of cytotoxicity after additional growth in low-iron medium supplemented with 250 µM each cationic salt. Fe: ferric ammonium sulfate, Cu: cupric sulfate, Mg: magnesium chloride, Mn: manganese chloride, Ca: calcium chloride.
Fig. 5.
The effect of proteinase inhibitors on the iron-modulated cytotoxicity (A) and adherence (B). Each proteinase inhibitor was added to low-, normal- or high-iron grown E. histolytica trophozoites 5 min prior to cytotoxicity and adherence assay. E64 (0.2 mM); leupeptin (0.2 mM); PMSF, phenyl-methylsulfonyl fluoride (1 mM); pepstatin A (1 µM); aprotinin (3 µM).
Adherence of E. histolytica to colonic epithelial cells occurs by a mechanism similar to their adherence to CHO cells [14]. Our studies showed that the adherence and cytotoxicity of E. histolytica trophozoites to CHO cells are modulated by concentrations of iron (Fig. 2). These results confirm the importance of iron concentrations in increasing the cytoadherence and cytotoxicity of E. histolytica, and suggest that the extracellular iron concentration contributes to the virulence of this parasite. A study among Massai, African nomads with an unusual freedom from infection with E. histolytica, showed that the administration of iron to correct their dietary iron deficiency increased their susceptibility to amebiasis [9].
Several investigators have observed an association between the expression of cytopathic activity by E. histolytica and cysteine proteinase activity [15-18]. The results of the present study suggest that iron is an important factor in the adherence and cytotoxicity of E. histolytica, acting by modulation of gene expression of virulence factors.
ACKNOWLEDGEMENTS
This study received financial support from the Korea Research Foundation, Korea (Grant No. FP0080).
References
- 1.Martinez-Palomo A. The pathogenesis of amoebiasis. Parasitol Today. 1987;3:111–118. doi: 10.1016/0169-4758(87)90048-2. [DOI] [PubMed] [Google Scholar]
- 2.Mattern CF, Keister DB, Caspar PA. Experimental amebiasis. III. A rapid in vitro assay for virulence of Entamoeba histolytica. Am J Trop Med Hyg. 1978;27:882–887. [PubMed] [Google Scholar]
- 3.Wilson ME, Britigan BE. Iron acquisition by parasitic protozoa. Parasitol Today. 1998;14:348–353. doi: 10.1016/s0169-4758(98)01294-0. [DOI] [PubMed] [Google Scholar]
- 4.Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekalanos JJ. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehker MW, Arroyo R, Alderete JF. The regulation by iron of the synthesis of adhesions and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med. 1991;174:311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo R, Alderete JF. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch Med Res. 1995;26:279–285. [PubMed] [Google Scholar]
- 8.Ryu JS, Choi HK, Min DY, Ha SE, Ahn MH. Effect of iron on the virulence of Trichomonas vaginalis. J Parasitol. 2001;87:457–460. doi: 10.1645/0022-3395(2001)087[0457:EOIOTV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Murray MJ, Murray A, Murray CJ. The salutary effect of milk on amoebiasis and its reversal by iron. Br Med J. 1980;280:1351–1352. doi: 10.1136/bmj.280.6228.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SJ, Lee SM, Lee J, Yong TS. Differential gene expression by iron-limitation in Entamoeba histolytica. Mol Biochem Parasitol. 2001;114:257–260. doi: 10.1016/s0166-6851(01)00264-x. [DOI] [PubMed] [Google Scholar]
- 11.Ravdin JI. Entamoeba histolytica: from adherence to enteropathy. J Infect Dis. 1989;159:420–429. doi: 10.1093/infdis/159.3.420. [DOI] [PubMed] [Google Scholar]
- 12.Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanism of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoebae. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 14.Ravdin JI, John JE, Johnston LI, Innes DJ, Guerrant RL. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosa. Infect Immun. 1985;48:292–297. doi: 10.1128/iai.48.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan K, Deneke CF, Thorne GM, Gorbach SL. Entamoeba histolytica cytotoxin: purification, characterization, strain virulence, and protease activity. J Infect Dis. 1982;146:616–625. doi: 10.1093/infdis/146.5.616. [DOI] [PubMed] [Google Scholar]
- 16.Gadasi H, Kobiler D. Entamoeba histolytica: correlation between virulence and content of proteolytic enzymes. Exp Parasitol. 1983;55:105–110. doi: 10.1016/0014-4894(83)90003-6. [DOI] [PubMed] [Google Scholar]
- 17.Lushbaugh WB, Hofbauer AF, Pittman FE. Proteinase activities of Entamoeba histolytica cytotoxin. Gastroenterology. 1984;87:17–27. [PubMed] [Google Scholar]
- 18.Lushbaugh WB, Hofbauer AF, Pittman FE. Entamoeba histolytica: purification of cathepsin B. Exp Parasitol. 1985;59:328–336. doi: 10.1016/0014-4894(85)90088-8. [DOI] [PubMed] [Google Scholar]