Abstract
Rats develop strong resistance to re-infection and super-infection by Clonorchis sinensis. The present study investigated the antibodies present in the sera and bile juice of rats that were primary infected and re-infected with C. sinensis. The serum level of specific IgG antibodies, which were elevated 2 wk of the primary infection, peaked at 4 wk and subsequently remained unchanged even during re-infection. The total IgE level in serum increased slowly from 388 ng / ml to 3,426 ng / ml beginning 2 wk after the primary infection, and remained high up to 8 wk but dropped to a normal level (259 ng / ml) after treatment. In resistant re-infected rats, the serum IgE level increased rapidly and peaked within 1 wk, whereas no increase was observed in immunosuppressed rats. The serum level of specific IgA antibodies was elevated beginning 1 wk after infection, and decreased 4 wk after treatment. The total bile IgA level unchanged during the primary infection but increased in treated and re-infected rats. The elevated levels of serum IgE and bile IgA indicate that these immunoglobulins may be correlated with the development of resistance to re-infection by C. sinensis in rats.
Keywords: Clonorchis sinensis, Serum, Bile juice, IgA, IgE, Rat, Re-infection
INTRODUCTION
Clonorchis sinensis, which is a liver fluke living in the bile ducts of humans and mammals, is endemic in East Asia, including Korea, China, Russia, and Vietnam, where it is estimated that about 35 million people are infected [1]. Clonorchiasis, i.e., human infection by C. sinensis, leads to cholangitis with secondary bacterial infection, bile duct obstruction, and cholangiofibrosis in the liver [2]. Clonorchiasis also causes the serious complication of cholangiocarcinoma in humans [3-5].
Humans with clonorchiasis are usually re-infected or have a super-imposed infection (super-infection) [6]. This finding suggests that previous infection affords poor protection against re-infection or super-infection in humans. However, rats are known to develop near-complete resistance to re-infection or super-infection by C. sinensis [7,8]. Although the mechanism of resistance to re-infection is unknown, it has been suggested to involve an immune-related host response [8,9]. It was observed that rats immunized with crude antigens of C. sinensis had high serum IgG levels but exhibited no protection against infection [10]. Nude and immunosuppressed rats lose resistance to C. sinensis but splenectomized rats do not. Furthermore, immunization of rats with C. sinensis antigens induces no resistance to re-infection [8]. There have been several vaccine trials using DNA vaccines that encode fatty acid-binding or a cysteine proteinase and a tegument protein vaccine, although these vaccines were found to provide only partial protection [11-13]. These findings suggest that the resistance of rats to re-infection by C. sinensis is linked mainly to local immune responses in the bile duct [8].
Antibodies are an important host defense response to infectious disease. In this context, we analyzed the antibodies present in the sera and bile juices of rats that were primary and re-infected with C. sinensis in order to evaluate their roles in the development of resistance.
MATERIALS AND METHODS
Preparation of parasites and experimental animals
Metacercariae of C. sinensis were collected from naturally infected freshwater fish and kept in storage solution at 4℃, as previously described [14]. Male Sprague-Dawley rats were divided into several groups of 5 animals each according to the duration of primary infection (from 3 days to 8 wk), treatment, and time after re-infection (1-4 wk) (Table 1). Immunosuppressed rats, which were subcutaneously injected with methyl-prednisolone for 3 wk [8,9], were compared with the normal rats after re-infection. The rats were maintained in the animal facilities of the Seoul National University College of Medicine and provided with commercial pellet food and tap-water. All procedures of the animal experiment followed the guideline for the animal experiment of the Seoul National University.
Table 1.
Worm recovery rates in rats infected by Clonorchis sinensis
aMean of recovery rates of 5 rats.
Re-infection procedure
The procedure used for re-infection was the same as that used in our previous studies [8,9]. For the primary infection, 100 fresh metacercariae were introduced into the stomach of each rat through a gavage needle. Four weeks later, the rats were treated with 100 mg / kg praziquantel (Distocide® Shinpoong Pharmaceutical Co., Seoul, Korea) for 3 days. The treatment was subsequently confirmed by examination of stool samples for the presence of eggs. Four weeks after treatment, each egg-negative rat was re-infected with 100 metacercariae of C. sinensis.
Collection of serum, bile juice, and adult worms
Sera and bile juice were collected from the rats before infection (Weeks 0), 3 days after infection (Weeks 0.5), 1, 2, 4, and 8 wk after infection, and 4 wk after treatment. Serum and bile juice samples were also collected from the immunosuppressed rats before and after re-infection (at 3 days and 1, 2, and 4 wk). Bile juice was collected from the bile duct through a polyethylene cannulation tube (external diameter, 0.8 mm; internal diameter, 0.4 mm), as previously described [15]. One end of the tube was inserted into the bile duct, while the other end was exteriorized through an opening in the right flank by an 18 G needle, allowing the bile to be collected into a tube. All the serum and bile juice samples were kept at -20℃ until used. Adult and juvenile worms were recovered from the liver and bile duct of the rats as described previously [9].
Preparation of the excretory-secretory protein (ESP)antigen of C. sinensis
ESP antigen of C. sinensis was prepared from cultured adult worms as previously described [16], and stored at -70℃ until used. The protein concentration was determined using a BCA protein assay reagent kit (Pierce, Rockford, Illinois, USA).
Serum and bile antibody detection
The total serum and bile IgE and IgA levels were determined using ELISA quantitation kits (Bethyl Laboratories, Montgomery, Texas, USA) following the manufacturer's instruction. The reaction conditions were as follows: 96-well plates were coated with anti-rat IgE or IgA capture antibodies diluted 1 : 250 for IgE and 1 : 100 for IgA. For the IgE assay, the serum and bile samples were diluted 1 : 200 and 1 : 20, respectively, and for the IgA assay, the samples were diluted 1 : 4,000. The HRP conjugates for IgE and IgA were used at dilution of 1 : 500 and 1 : 40,000, respectively. Each reaction was carried out at room temperature for 60 min. Color development using TMB as the substrate was allowed to proceed for 30 min in the dark, and then stopped by addition of 4N H2SO4. The absorbance was subsequently read at 450 nm using an ELISA reader (Emax® Precision Microplate Reader, Molecular Devices Corp., Sunnyvale, California, USA). The antibody concentrations were calculated from standard curves prepared using reference sera in the same plate. The listed concentration of each sample is the mean of duplicate tests.
The level of specific antibodies in the serum and bile samples was assessed by indirect ELISA using the ESP antigen of C. sinensis. In brief, 96-well plates were coated with 0.2 µg of ESP antigen per well and blocked with blocking solution. The sera were diluted 1 : 400 for specific IgG and 1 : 20 for specific IgA while the bile juices were diluted 1 : 20 for specific IgA. The HRP conjugates were diluted 1 : 30,000 for serum IgG, and 1 : 20,000 and 1 : 500 for serum IgA and bile IgA, respectively. Each reaction was run at 37℃ for 2 hr. Color development using TMB was allowed to proceed for 10 min in the dark, and then stopped by the addition of 4N H2SO4. The absorbance was subsequently read at 450 nm using an ELISA reader.
Data analysis
All statistical analyses were performed using SPSS for Windows version 10.0 (SPSS Inc., Chicago, Illinois, USA). The statistical significance of the mean values for the worm recovery rates between the groups was evaluated by one-way ANOVA with a threshold P-value of 0.05.
RESULTS
Worm recovery
The recovery rates for adult C. sinensis worms were 13.7% at 3 days after the primary infection and 32.8% at 1 wk after the primary infection. The recovery rates were greater than 50% between 2 and 8 wk after the primary infection ( Table 1). In contrast, the recovery rates after re-infection were less than 5% for all the time-points studied. However, in the immunosuppressed re-infected rats, the rates were similar to those of the primary infected rats.
Serum antibodies
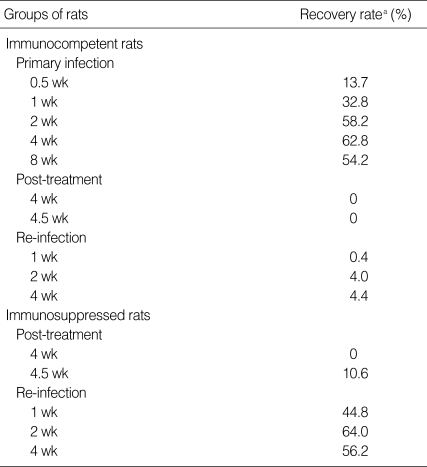
The serum level of C. sinensis specific IgG antibodies was elevated 2 wk after primary infection to absorbance 0.22 ± 0.071 from 0.076 ± 0.022 pre-infection, and peaked at absorbance 1.393 ± 0.057 after 4 wk (Fig. 1). The specific IgG levels showed little change up to 4 wk post-treatment and after re-infection. The specific IgG level in the sera of the immunosuppressed rats was similar to that in the sera of the immunocompetent re-infected rats. The total serum IgE level was 388.8 ± 237.5 ng / ml before infection, but increased slowly after primary infection to 3,426.7 ± 2,000 ng / ml from Week 2, and maintained high levels at Week 4 and 8. Upon re-infection, the total IgE level in the normal rats increased rapidly and peaked to 4,973.7 ± 3,507.5 ng / ml after 1 wk, whereas no IgE response was observed in the immunosuppressed rats. The total serum IgA level peaked at a relatively low level, 326.4 ± 208.5 µg / ml 1 wk after the primary infection; however, no peak was observed following re-infection. The specific serum IgA level increased beginning 1 wk after infection but decreased at 4 wk after treatment.
Fig. 1.
Antibody levels in the sera of rats infected with Clonorchis sinensis. Each dot represents mean values of 5 rats.
P, primary infection, sera were collected at 0, 0.5, 1, 2, 4 and 8 wk after primary infection; R, re-infection in immunocompetent rats; IsR, re-infection in immunosuppressed rats. Sera were collected at 0, 0.5, 1, 2 and 4 wk after re-infection.
*Significantly different from that of 0-wk, P = 0.0098. †Significantly different from that of 0-wk, P = 0.021.
Bile juice antibodies
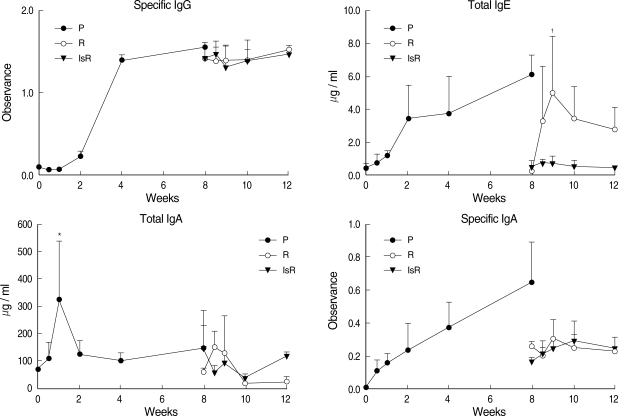
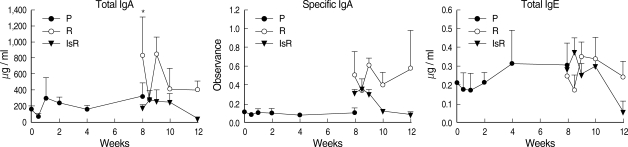
The total bile IgA level was unchanged during the primary infection but increased in the treated rats, 830.4 ± 480.9 µg / ml (Fig. 2), reaching high levels up to 838.9 ± 226.4 µg / ml during re-infection. In the immunosuppressed rats, the total bile IgA level was low both before and during re-infection. The changing pattern of specific IgA in the bile was similar to that of the total IgA. The bile IgE level was lower than the serum level of IgE and remained relatively constant during primary infection and re-infection.
Fig. 2.
Antibody levels in the bile of rats infected with Clonorchis sinensis. P: primary infection, R: re-infection in immunocompetent rats, IsR: re-infection in immunosuppressed rats. *Significantly different from that of 0-wk, P = 0.015.
DISCUSSION
Rats develop almost complete resistance to re-infection by C. sinensis. However, this resistance is compromised by methyl-prednisolone-induced immunosuppression or in nude rats [8, 9]. The present results confirm again the development and disappearance of resistance in rats to re-infection by C. sinensis.
In the present study, we observed the changing patterns of serum and bile antibodies in rats during primary infection, treatment, and re-infection with C. sinensis. The increase in antibody concentration during primary infection was similar to that reported previously [10,17]. The serum level of specific IgG antibodies began to increase 2 wk after the primary infection and reached a high plateau at 4 wk, after which it showed little change, even at 4 wk post-treatment and during re-infection. The serum IgG antibody level was also stable during re-infection in the immunosuppressed rats. The serum IgG level was increased by immunization with adult worm antigen although protective immunity was not afforded [8,10]. Although the serum level of specific IgG antibody production was high, this appeared to have little influence on the development of resistance to re-infection.
The total serum IgA level peaked 1 wk after the primary infection and decreased to the normal level. The serum specific IgA level also increased little during the primary infection, but it peaked at a high level 1 wk after treatment and dropped to normal at 4 wk post-treatment. In contrast, the total or specific serum IgA response to re-infection was very weak (Fig. 1). The serum IgA levels differed little between immune-competent and suppressed rats.
Unlike the serum IgA levels, the total and specific bile IgA levels changed significantly upon re-infection. The bile IgA revealed no peak during the primary infection, instead, it increased to a concentration peak in the treated rats (Fig. 2). The bile IgA level was consistently high in the re-infected rats, whereas the bile and serum IgA levels remained low in the immunosuppressed rats, even during re-infection. The IgA antibody response to C. sinensis infection has been reported previously [18,19], with antibodies being secreted from the biliary mucosal tissues in response to stimulation by infected juvenile flukes. In addition, a protective role for IgA antibodies has been proposed in response to infection by nematodes at the intestinal phase [20]. Rats infected with Heligmosomoides polygyrus rapidly expel the intestinal nematodes, and there is a negative correlation between the IgA level and worm burden [20]. The levels of circulating IgG1 and intestinal IgA are associated with the elimination of Strongyloides ratti from infected rats [21]. One study has confirmed the presence of specific IgA antibodies in the feces of rats that were orally immunized with a recombinant tegumental protein of C. sinensis (TP20.8) [13]. The finding suggests that intestinal IgA antibodies may inhibit the migration of excysted C. sinensis larvae into the bile duct [13]. However, the IgA antibodies in bile should play a more important role than those in the intestine in terms of the development of resistance to re-infection by C. sinensis, given that the bile antibodies are in more intimate contact with the worms. The present study confirms the increase in IgA antibodies in the bile juice during re-infection. The C. sinensis-specific IgA antibodies in bile may possibly impede the migration of newly invading juvenile worms into the intrahepatic bile duct, although the exact mechanism remains to be elucidated.
It is well-known that IgE antibodies are produced in response to infection by C. sinensis [19,22,23]. The total and specific IgE levels in serum are increased in clonorchiasis patients, and the worm burden is inversely correlated with the serum IgE level [22]. Our present results show that the total serum IgE level increased slowly upon primary infection and then decreased to the normal level 4 wk after treatment. However, upon re-infection, the total IgE level increased rapidly to a peak within 1 wk in immunocompetent rats, but not in immunosuppressed rats. This pattern of increase is the same as that reported in a previous study [24], in which resistance to C. sinensis was observed in rats following infection with irradiated metacercariae. In endemic areas of Schistosoma japonicum in China, high serum IgE levels against adult worms or soluble egg antigens have been reported in cured schistosomiasis patients who are resistant to re-infection [25]. The rapid increase in IgE antibodies during the first week of re-infection in the present study correlates well with the timing of resistance development in rats. The resistance to newly invading worms develops during the first week of re-infection or super-infection [8].
In conclusion, levels of bile IgA antibodies and serum IgE antibodies increase significantly in resistant re-infected rats. The bile IgA and serum IgE antibodies may be involved in the development of resistance to re-infection by C. sinensis in rats.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Research Foundation, KRF-2003-042-E00034.
References
- 1.Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 2.Rim HJ. Clonorchiasis: an update. J Helminthol. 2005;79:269–281. doi: 10.1079/joh2005300. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Lim JH, Hong ST. Relation of cholangiocarcinomas to clonorchiasis and bile duct stones. Abdom Imaging. 2004;29:590–597. doi: 10.1007/s00261-004-0193-4. [DOI] [PubMed] [Google Scholar]
- 4.Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ, Hwang SS, Park SK, Cho SI, Sohn WM, Kim DI, Yoo KY, Hong ST, Shin HR. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93–96. [PubMed] [Google Scholar]
- 5.Rana SS, Bhasin DK, Nanda M, Singh K. Parasitic infestations of the biliary tract. Curr Gastroenterol Rep. 2007;9:156–164. doi: 10.1007/s11894-007-0011-6. [DOI] [PubMed] [Google Scholar]
- 6.Hong ST. Clonorchis sinensis. In: Miliotis MD, Bier JW, editors. International Handbook of Foodborne Pathogens. New York, USA: Marcel Dekker; 2003. pp. 581–592. [Google Scholar]
- 7.Sohn WM, Zhang H, Choi MH, Hong ST. Susceptibility of experimental animals to reinfection with Clonorchis sinensis. Korean J Parasitol. 2006;44:163–166. doi: 10.3347/kjp.2006.44.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Chung BS, Li S, Choi MH, Hong ST. Factors in the resistance of rats to re-infection and super-infection by Clonorchis sinensis. Parasitol Res. 2008 doi: 10.1007/s00436-008-0878-7. Jan 19 (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Chung BS, Zhang H, Choi MH, Jeon DK, Li S, Lee MJ, Hong ST. Development of resistance to reinfection by Clonorchis sinensis in rats. Korean J Parasitol. 2004;42:19–26. doi: 10.3347/kjp.2004.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi D, Hong ST, Chung BS, Lim JH, Lee SH. Bile duct changes in rats reinfected with Clonorchis sinensis. Korean J Parasitol. 2004;42:7–17. doi: 10.3347/kjp.2004.42.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JS, Kim IS, Sohn WM, Lee J, Yong TS. A DNA vaccine encoding a fatty acid-binding protein of Clonorchis sinensis induces protective immune response in Sprague-Dawley rats. Scand J Immunol. 2006;63:169–176. doi: 10.1111/j.1365-3083.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Kim IS, Sohn WM, Lee J, Yong TS. Vaccination with DNA encoding cysteine proteinase confers protective immune response to rats infected with Clonorchis sinensis. Vaccine. 2006;24:2358–2366. doi: 10.1016/j.vaccine.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Xia H, Hu X, Huang Y, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X. Immunogenicity of recombinant Bacillus subtilis spores expressing Clonorchis sinensis tegumental protein. Parasitol Res. 2008;102:293–297. doi: 10.1007/s00436-007-0762-x. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Kang HW, Choi MH, Hong ST. Long-term storage of Clonorchis sinensis metacercariae in vitro. Parasitol Res. 2006;100:25–29. doi: 10.1007/s00436-006-0242-8. [DOI] [PubMed] [Google Scholar]
- 15.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923–927. doi: 10.3748/wjg.v8.i5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi MH, Park IC, Li S, Hong ST. Excretory-secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA . Korean J Parasitol. 2003;41:35–39. doi: 10.3347/kjp.2003.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan FS, Lee HJ, Chung MS, Lee JS, Rim HJ, Joo KH. Chemotherapeutic efficacy of praziquantel in rats with protective immunity to Clonorchis sinensis infection. Chinese J Parasitol Parasit Dis. 2000;18:98–102. [PubMed] [Google Scholar]
- 18.Lin YL, Chen ER, Yen CM. Antibodies in serum of patients with clonorchiasis before and after treatment. Southeast Asian J Trop Med Public Health. 1995;26:114–119. [PubMed] [Google Scholar]
- 19.Hong ST, Kho WG, Lee M, Lee JS, Lee SH. Immunoblot patterns of clonorchiasis. Korean J Parasitol. 1997;35:87–93. doi: 10.3347/kjp.1997.35.2.87. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Smith A, Wahid FN, Lammas DA, Behnke JM. The relationship between circulating and intestinal Heligmosomoides polygyrus-specific IgG1 and IgA and resistance to primary infection. Parasite Immunol. 1999;21:383–395. doi: 10.1046/j.1365-3024.1999.00236.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilkes CP, Bleay C, Paterson S, Viney ME. The immune response during a Strongyloides ratti infection of rats. Parasite Immunol. 2007;29:339–346. doi: 10.1111/j.1365-3024.2007.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min DY, Soh CT. Evaluation of specific IgE antibody in Clonorchis sinensis infection. Korean J Parasitol. 1983;21:27–31. doi: 10.3347/kjp.1983.21.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Yong TS, Kim DS, Lee SY, Im KI, Lee KY. Detection of specific serum IgE in clonorchiasis cases and analysis of Clonorchis sinensis allergens. Yonsei Med J. 1993;34:248–257. doi: 10.3349/ymj.1993.34.3.248. [DOI] [PubMed] [Google Scholar]
- 24.Quan FS, Lee JB, Bae JS, Ohwatari N, Min YK, Yang HM. Resistance to reinfection in rats induced by irradiated metacercariae of Clonorchis sinensis. Mem Inst Oswaldo Cruz. 2005;100:549–554. doi: 10.1590/s0074-02762005000500016. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Sleigh AC, Ross AG, Li Y, Zhang X, Williams GM, Yu X, Tanner M, McManus DP. Human susceptibility to Schistosoma japonicum in China correlates with antibody isotypes to native antigens. Trans R Soc Trop Med Hyg. 2001;95:441–448. doi: 10.1016/s0035-9203(01)90210-x. [DOI] [PubMed] [Google Scholar]