Abstract
Merozoite surface protein-1 (MSP-1) and merozoite surface protein-2 (MSP-2) were used to develop vaccines and to investigate the genetic diversity in Plasmodium falciparum malaria in Iran. Nested polymerase chain reaction amplification was used to determine polymorphisms of block 2 of the MSP-1 and the central domain of MSP-2 genes. A total of 67 microscopically positive P. falciparum infected individuals from a major endemic region, southeast Iran, were included in this trial. Nine alleles of MSP-1 and 11 alleles of MSP-2 were identified. The results showed that amplified product from these surface antigen genes varied in size and there was specific pattern for each isolate. Besides, regarding this pattern, 23 multiple infections with at least 2 alleles were observed. While the endemic regions of malaria in Iran is classified in low to moderate group, but extensive polymorphism was observed for each marker and the MSP-2 central repeat was the most diverse that could be considered in designing malaria vaccine.
Keywords: Plasmodium falciparum, merozoite surface protein-1 (MSP-1), MSP-2, malaria, Iran
Malaria is a major public health problem and is associated with 300-500 million clinical cases worldwide as well as 0.5-3 million deaths annually, almost all of them are caused by P. falciparum (Ferreira et al., 2004; Phillips, 2000). Genetic diversity presented by P. falciparum field isolates, the occurrence of variant forms of the parasite in different geographic areas, and occultation of multiple genotypes during a single mosquito, constitute one of the main obstacles to the design of a malaria vaccine (Raj et al., 2004; Moor et al., 2002).
Merozoite surface protein (MSP)-1 and merozoite surface protein-2 (MSP-2) are 2 proteins causing immune responses in humans (Taylor et al., 1995; Aubouy et al., 2003) and are important candidates for development of blood stage malaria vaccines (Moorthy and Hill., 2002). The block 2 MSP-1 is particularly polymorphic and 3 distinct allelic families have been described as Mad 20, K1 and Ro33 (Contamin et al., 1996). The polymorphic central domain of the gene encoding MSP-2 belongs to 2 distinct families; Ic and Fc27 (Sallenave et al., 2000). Allelic forms of these antigen genes have been reported from different parts of the world (Babiker and Waliker, 1997; Jordan et al., 2001) and further characterization of the degree of polymorphism in these antigens will be of interest for appropriate design of malaria vaccine.
The tribulations encountered in Sistan and Baluchistan Provinces, Iran, are resistance of P. falciparum to drugs (Edrissian et al., 1993; Eskandarian et al., 2002; Jafari et al., 2003), and that of vectors to insecticides, likewise importation of malaria mostly of P. falciparum originating from Afghani and to some lesser extent, from Pakistani immigrants. Hence, designing efficient malaria vaccine is useful for control of falciparum malaria in this area. We investigated the polymorphic nature of these vaccine candidate antigen genes and complexity of P. falciparum infection among field isolates in an endemic area of Iran. In this study, nested polymerase chain reaction (PCR) was used for genotyping P. falciparum isolates.
Iran is located in the Eastern Mediterranean Region, and grouped as low-moderate endemic region (Rakhshani, 2003). Sistan and Baluchistan Province, southeast Iran, is the endemic area of falciparum malaria and is considered as the oriental eco-epidemiological region of malaria (Sadrizadeh, 1999). It is bordered by Pakistan and Afghanistan. Prevalence of malaria has been 1,382 cases in Iran in 2004 (WHO, 2005).
This study involved 67 resident subjects aged from 2 to 45 years. Sample collection was carried out in 2004. P. falciparum malaria patients attending randomly local malaria clinics and health centers were enrolled in this study. Residence in the regions for over 6 mo, no history of anti-malarial treatment for the last month, and written informed consent were required for inclusion in this study. This study was approved by the Ethical Review Committee of Research in Tehran University of Medical Sciences, Iran.
Diagnosis of P. falciparum was confirmed by light microscopy on thick blood smears. Origin, clinical signs, age, sex and parasitemia (number of asexual parasites per ?l of blood) of each patient were recorded. Blood was collected in tubes containing anti- coagulant solution and stored at -20℃ until used. Isolation of DNA and genotyping were performed in the Institute of Tropical Medicine of Berlin, Germany, in 2005.
DNA was extracted from the blood sample by QIAamp DNA mini Kit (Quigen kit, Germany). PCR was performed following a 2-step amplification scheme, in which the product of the first reaction (outer PCR) was used as the template for the second reaction (nested PCR) for both MSP-1 and MSP-2, the primers used in the first amplification reaction were conserved among all isolates. Allelic family-specific primers were used in second amplification reaction for block 2 of MSP-1 corresponding to Mad 20, K1 and Ro33 allelic families, and Fc27 and Ic for the central region of MSP-2. The sequences of the primers are listed elsewhere (Arez et al., 1999). For amplification, 2 µl DNA was used in outer PCR in total reaction volume of 26 µl containing 50 mM KCl; 1.5 mM MgCl2; 125 µM of each dNTP (Invitrogen); 1 unit of Taq and pair primers (160 nM each). This reaction was amplified for 35 cycles at 94℃ for 1 min, 58℃ for 2 min and 72℃ for 2 min. Two µL of DNA product from the outer PCR reaction was used in nested PCR in a total reaction volume of 50 µl containing 50 mM KCl; 1.5 mM MgCl2 175 µM of each dNTP; 1.5 unit of Taq and the 3 and 2 pairs of primer (160 nM each) separately for MSP-1 and MSP-2, respectively.
Samples were amplified for 30 cycles at 94℃ for 1 min, 61℃ for 2 min and 72℃ for 2 min. The PCR amplified gene fragments of MSP-1 and MSP-2 were electrophoresed on 3% agarose gels, stained with ethidium bromide, and visualized under ultraviolet light. Chi-square test was applied to analyze the results.
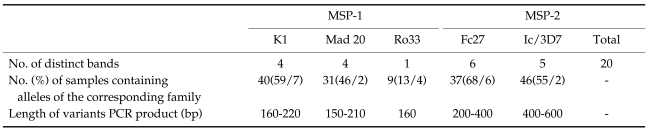
Sixty percent of studied subjects were males. Parasitemia in patients ranged from 500 to 30,000 parasites/mm3 (Mean 11,700). All samples were analyzed for polymorphisms on MSP-1 and MSP-2 genes. Among the fragment of 67 samples at the MSP-1 locus 40, 31 and 9 fragments belonged to K1, Mad 20 and Ro33 families, respectively. For the MSP-2 locus, 37 and 46 fragments were identified as FC27 and IC families, respectively (Table 1). However, the variant 160 bp of Ro33, 180 bp of K1 and 190 bp of Mad 20 allelic family MSP-1, and variant 400 bp of Fc27 and 470 bp of Ic for MSP-2 demonstrated the most frequency. Thirteen (19.4%) samples of MSP-1 and 15 (44.6%) of MSP-2 contained multiclonal infection at least with 2 clones and in total 23 samples (34.3%) illustrated multiclonal infection. There was no significant association between sex, age, parasitemia, and origin of patients with alleles for the 2 mentioned genes.
Table 1.
Distribution of different allele types of merozoite surface protein (MSP)-1 and MSP-2 genes of Plasmodium falciparum in Southeast of Iran
This study illustrated 9 and 11 different variants at MSP-1 and MSP-2 loci, respectively, which showed higher rates than that of a similar report in a hypoendemic region in Colombia (Montoya et al., 2003), where 1 allele of MSP-1 and 3 alleles of MSP-2 were detected. A common factor of this work and the present study is the low malaria endemicity compared to Thailand or African countries. In Thailand (Snounou et al., 1999), 10 alleles of MSP-1 and 17 alleles of MSP-2 were observed, whereas in Gabon (Aubouy et al., 2003), 25 alleles of MSP-1 and 19 alleles of MSP-2, and in West Uganda (Peyerl et al., 2001), 22 alleles of MSP-1 and 12 alleles for MSP-2 were reported. However, the Ro33 family of MSP-1 did not show any polymorphism, with only 1 variant (160 bp) detected. This result differs from that of Gabon and West Uganda, where the Ro33 family was polymorphic with 3 and 4 fragments, respectively (Aubouy et al., 2003, Peyerl et al., 2001), but was close to that in Senegal (Zwetyena et al., 1998) and Brazil (Sallenave et al., 2000), where the Ro33 family was poorly polymorphic. At the MSP-1 locus alleles belonging to the K1 family were more frequent, whereas at the MSP-2 locus alleles belonging to the IC alleles were more frequent; our results demonstrated discrepancy with a previous study (Zakeri et al., 2005), in which all patients referred to a clinic were selected. Disparity in the number of clinics and geographical area may justify this finding. Although Iran, in general, is a low endemic country for malaria (Rakhshani et al., 2003), our findings showed high polymorphisms in a major falciparum malaria endemic region. It seems that movement and migrating of people between the mentioned region and neighboring countries (especially Afghanistan) may introduce different alleles of P. falciparum into this area of Iran. The coexistence of 2 or more clonal population from 1 gene within a host has been constantly reported (Virikyakoso et al., 1995). Finding of 34.3% of patients with more than 1 gene type in Sistan and Baluchistan Provinces showed lower degree of multi-strain infection in comparison to isolates from previous study in this area that showed a 88% multiinfection. It seems that the decrease of immigration from neighboring countries that occurred in recent years is a reason for this finding.
The presence of more than 1 parasitic gene type in a single human host may lead to cross fertilization, meiotic recombination and generation of new strains during the developmental stage in the mosquito vector (Raj et al., 2004; Snounou et al., 1999). This might be a reason that Iranian isolates showed high polymorphisms in each gene.
In this study, nested-PCR showed a suitable method to study MSP-1 and MSP-2 polymorphisms. The present study reported variations in the selected vaccine candidate antigens in Iranian isolates of P. falciparum that could be taken into accounts in developing malaria vaccines. Further population-based studies of sequences of MSP-1 and MSP-2 and genotyping of other candidate antigen genes of P. falciparum will provide more information in this area for designing of malaria vaccines.
ACKNOWLEDGMENTS
We would like to thank individuals from malaria endemic regions of Iran, who kindly contributed to this study. We are grateful to Mr. Akbarzadeh and Mr. Seidzadeh for their assistance in sampling, and also thank Dr. Farhood, Dr. Edrissian and all staff of Institute of Tropical Medicine of Berlin.
Footnotes
This study was supported financially by the Tehran University of Medical Sciences and the Institute of Tropical Medicine, Berlin, Germany.
References
- 1.Arez AP, Snounou G, Pinto J, Sousa CA, Modiano D, Ribeiro H, Franko AS, Aleves J, do Rosario VE. A clonal Plasmodium falciparum population in an isolated outbreak of malaria in the Republic of Cabo Verde. Parasitology. 1999;118:347–355. doi: 10.1017/s0031182099003972. [DOI] [PubMed] [Google Scholar]
- 2.Aubouy A, Migot-Nabias F, Deleron P. Polymorphism in two merozoite surface proteins of Plasmodium falciparum isolates from Gabon. Malar J. 2003;2:12–12. doi: 10.1186/1475-2875-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babiker HA, Walliker D. Current views on the population structure of Plasmodium faciparum: implication for control. Parasitol Today. 1997;13:262–267. doi: 10.1016/s0169-4758(97)01075-2. [DOI] [PubMed] [Google Scholar]
- 4.Contamin H, Fandeur T, Rogier C, Bonnefoy S, Konate L, Trape JF, Mercereau-puijalon O. Different genetic characteristics of Plasmodium falciparum isolates collected during successive clinical malaria episodes in Senegalese children. Am J Trop Med Hyg. 1996;54:632–643. doi: 10.4269/ajtmh.1996.54.632. [DOI] [PubMed] [Google Scholar]
- 5.Edrissian GH, Afshar A, Sayedzadeh A, Mohsseni G, Satvat MT. Assessment of the response in vivo and in vitro of Plasmodium falciparum to sulphadoxine-pyrimethamine in the malarious areas of Iran. J Trop Med Hyg. 1993;96:237–240. [PubMed] [Google Scholar]
- 6.Eskandarian AA, Keshavarz H, Basco LK, Mahboudi F. Do mutations in Plasmodium falciparum dihydropteroate synthase and dihydrofolate reductase confer resistance to sulfadoxine-pyrimethamine in Iran? Trans R Soc Trop Med Hyg. 2002;96:96–98. doi: 10.1016/s0035-9203(02)90254-3. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira MU, da Silva Nunes M, Wunderlich G. Antigenic diversity and immune evasion by malaria parasites. Clin Diagn Lab Immunol. 2004;11:987–995. doi: 10.1128/CDLI.11.6.987-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafari S, LeBars J, Asmar M, Durand R. Molecular survey of Plasmodium falciparum resistance in south-eastern Iran. Ann Trop Med Parasitol. 2003;97:119–24. doi: 10.1179/000349803235001552. [DOI] [PubMed] [Google Scholar]
- 9.Jordan S, Jelinek T, Aida AO, Peyerl-Hoffmann G, Heuschkel C, Valey AO, Christophel EM. Population structure of Plasmodium falciparum isolates during an epidemic in southern Mauritania. Trop Med Int Health. 2001;6:761–766. doi: 10.1046/j.1365-3156.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 10.Montoya l, Maestre A, Carmona J, Lopes D, Do Rosario V, Blair S. Plasmodium falciparum: Diversity studies of isolates from two Colombian regions with different endemicity. Exp Parasitol. 2003;104:14–19. doi: 10.1016/s0014-4894(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 11.Moore SA, Surgey EG, Cadwgan AM. Malaria vaccines: where are we and where are we going? Lancet Infect Dis. 2002;2:737–743. doi: 10.1016/s1473-3099(02)00451-6. [DOI] [PubMed] [Google Scholar]
- 12.Moorthy VS, Hill AV. Malaria vaccines. Br Med Bull. 2002;62:59–72. doi: 10.1093/bmb/62.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Pauel Rel, Hackford I, Brokman A, Muller-Graf C, Price R, Luxemburger C, White NG, Peyerl-Hoffmann G, Jelinek T, Kilian A, Kabagambe G, Metzger WG, von Sonnenburg F. Genetic diversity of Plasmodium falciparum and its relationship to parasite density in an area with different malaria endemicities in west Uganda. Trop Med Int Health. 2001;6:607–613. doi: 10.1046/j.1365-3156.2001.00761.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RS. Current status of malaria and potential for control. Clin Microbiol Rev. 2000;14:208–226. doi: 10.1128/CMR.14.1.208-226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raj DK, Das BR, Dash AP, Supakar PC. Genetic diversity in the msp1 gene of Plasmodium falciparum in different malaria endemic localities. Am J Trop Med Hyg. 2004;71:285–289. [PubMed] [Google Scholar]
- 16.Rakhsani F, Ansari Moghadam AR, Alemi R, Moradi A. Knowledge, perceptions and prevention of malaria among women in Sistan Va Baluchestan, Islamic Republic of Iran. East Mediter Health J. 2003;9:248–256. [PubMed] [Google Scholar]
- 17.Sadrizadeh B. Malaria in the world, in the eastern Mediterranean region and in Iran: Review article. Arch Iranian Med. 1999;2 [Google Scholar]
- 18.Sallenave-Sales S, Daubersies P, Mercereau-Puijalono O, rahimalala L, Contamin H, Druilhe P, Daniel-Ribeiro CT, Ferreira-da-Cruz MF. Plasmodium falciparum: A comparative analysis of the genetic diversity in malaria-mesoendemic area of Brazil and Madagascar. Parasitol Res. 2000;86:692–698. doi: 10.1007/pl00008554. [DOI] [PubMed] [Google Scholar]
- 19.Snounou G, Zhu X, Spiripoon N, Jarra W, Thaithong S, Brown Kn, Viriyakosol S. Biased distribution of msp1 and msp2 allelic variant in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999;93:369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 20.Taylor RR, Smith DB, Robinson VJ, McBride JS, Riley EM. Human antibody response to Plasmodium falciparum Merozoite surface protein 2 is serogroup specific and predominantly of the immuneoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virikyakoso N, Petcharapirat C, Petcharapirat P, Jarra W, Thaithong S, Brown KN, Snounou G. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull World Health Organ. 1995;73:85–95. [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Annual report of regional director. WHO/ Regional office for the Eastern Mediterranean; 2005. the work of WHO in the Eastern Mediterranean region. [Google Scholar]
- 23.Zakeri S, Bereczky S, Naimi P, Pedro Gil J, Djadid ND, Farnet A, Snounou G, Bjorkman A. Multiple genotypes of the merozoite surface proteins 1 and 2 in Plasmodium falciparum infections in a hypoendemic region of Iran. Trop Med Int Health. 2005;10:1060–1064. doi: 10.1111/j.1365-3156.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 24.Zwetyenga J, Rogier C. No influence of age on infection complexity allelic distribution in Plasmodium falciparum infection in Ndiop, a Sengales village with seasonal, mesoendemic malaria. Am J Trop Med Hyg. 1998;59:726–735. doi: 10.4269/ajtmh.1998.59.726. [DOI] [PubMed] [Google Scholar]