Abstract
Delirium occurs in 23% of sepsis patients, in which pro-inflammatory cytokines and nitric oxide are suggested to be involved. However, in animal experiments, even a subseptic dose of lipopolysaccharide (LPS) injection induces both pro-inflammatory cytokines and inducible nitric oxide synthase in the brain, suggesting that the brain oxidative reaction can be induced in the subseptic condition. Then, we evaluated the changes of heme oxygenase-1 (HO-1), a sensitive oxidative marker, as well as interleukin (IL)-1β, IL-6, and inductible nitric oxide synthase (iNOS) mRNA in the hypothalamus and hippocampus of rats using real-time PCR after peripheral injection of LPS (2.0 mg/kg). As a result, these four kinds of mRNAs were induced significantly in both areas after LPS injection. These results suggest that peripheral inflammation induces an oxidative reaction in the brain, even if the inflammation is not lethal. It is also considered that several pathways are involved in brain HO-1 induction.
Keywords: heme oxygenase-1, interleukin-1β, interleukin-6, lipopolysaccharide, hypothalamus, hippocampus
Introduction
Delirium occurs in 23% of sepsis patients (Sprung et al 1990), and the term sepsis-associated delirium (SAD) has been proposed (Ebersoldt et al 2007). Using magnetic resonance imaging, multiple ischemic strokes and white matter lesions were observed in the brain of a SAD patient (Sharshar et al 2007). As part of the mechanism underlying SAD, pro-inflammatory cytokines and inducible nitric oxide synthase (iNOS) are considered to play important roles in brain damage (Sharshar et al 2003). Since antioxidants have been reported to provide effective treatment in a sepsis model (Abd El-Gawad and Khalifa 2001) and NO induces free radicals leading to apoptosis in neuronal cells, the oxidative reaction is considered to be one of the foci of SAD.
In a sepsis model, more than 10 mg/kg of lipopolysaccharide (LPS) is administered by intraperitoneal or intravenous injection (Fujiwara et al 1999; Birnbaum et al 2006). However, after peripheral injection of even a subseptic dose (2.0 mg/kg or less) of LPS, interleukin-1β (IL-1β), IL-6, and iNOS mRNA are induced in the brain (Vallieres and Rivest 1997; Tonelli et al 2003; Singh and Jiang 2004). So far, it is unclear whether oxidative reactions are caused in the brain after peripheral injection of a subseptic dose of LPS. Thus, we investigated further. Brain heme-oxygenase-1 (HO-1) mRNA levels were measured quantitatively using real-time PCR because HO-1 responds sensitively to many kinds of oxidative stress (Ryter and Choi 2002).
The hypothalamus and hippocampus were chosen for analysis because the hypothalamus is known to be sensitive to ip injection of LPS (Tonelli et al 2003), and the hippocampus is denatured in neurodegenerative diseases, which has been suggested to involve an interaction between HO-1 and inflammatory cytokines (Mattson and Magnus 2006).
Materials and methods
Animals and treatment
Male Sprague-Dawley rats were obtained from Shimizu Laboratory Supplies (Kyoto, Japan) at five weeks of age. The animals were housed in groups of two or three per cage at a constant temperature of 25 °C with standard food pellets and water available ad libitum. All animals were maintained on a 12:12 L/D cycle (lights on at 0700 h). Between 0800 h and 1000 h the animals were injected intraperitoneally with 2.0 mg/kg LPS (e-coli 055 B5) (Sigma, St. Louis, MO), according to a previous study (Eriksson et al 2000; Tonelli et al 2003), in which an immune reaction was shown to be induced in the brain. Animals were decapitated 3, 6, and 12 hr after the injection. Just before decapitation, the animals were perfused with normal saline solution to avoid contamination of blood under anesthesia by pentobarbital. As a control, rats with no treatment were killed in the same manner. The hypothalamus and hippocampus were dissected on an ice-cooled glass plate with an established method (Glowinski and Iversen 1966). Both parts were immersed in RNA later (Qiagen, Hilden, Germany), and kept at −20 °C. Treatment of the rats was approved by the Okayama University Dental School Animal Care and Use Committee.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from the brain samples using Trisol (Invitrogen, Carlsbad, CA), and residual genomic DNA was removed by incubating the RNA sample with RNase-free DNaseI (Takara, Tokyo, Japan). Then, total RNA was extracted with phenol, followed by ethanol precipitation. Total RNA was reverse-transcribed to cDNA by AMV reverse transcriptase (Roche Diagnostics, Basel, Switzerland), according to the manufacturer’s instructions, followed by 20 times’ dilution with ultra-pure water.
Quantitative real-time PCR analysis
Forward (F) and reverse (R) primers used to amplify genes are listed in Table 1. Quantitative real-time PCR for HO-1 was performed using a LightCycler (Roche Diagnostics, Basel, Switzerland). Reactions were performed in a 10 μl volume with 0.5 μM primers using LightCycle-DNA Master SYBR Green I mix (Roche Diagnistics, Basel, Switzerland). The reactions were incubated at 95 °C for 10 min, followed by a cycling protocol consisting of three stages: 20 s at 95 °C for denaturation, 20 s at 69 °C for annealing, and 20 s at 72 °C for extension. IL-1β, IL-6, and iNOS mRNAs were analyzed with Miniopticon (Bio-rad, Hercules, CA). Reactions were performed in a 20 μl volume with 0.5 μM primers using iQ SYBR Green Supermix (Bio-rad, Hercules, CA). After incubation at 95 °C for 10 min, the cycling protocol was performed. The cycling protocol for IL-1β was as follows: 10 s at 95 °C for denaturation, 20 s at 61 °C for annealing, and 10 s at 72 °C for extension. For the reaction of IL-6 and iNOS mRNA, the annealing temperature was set at 64 °C and 59 °C, respectively. The fluorescence signal was detected at the end of the extension period. After the final cycle of PCR, melting curve analysis was performed routinely.
Table 1.
Primers used for the amplification of cDNAs
| cNA (accession No.) | Sequence (5’-3’) in cDNA | Location |
|---|---|---|
| HO-1 | F: CGTGCAGAGAATTCTGAGTTC | 195–215 |
| (NM 012580) | R: AGACGCTTTACGTAGTGCTG | 469–450 |
| IL-1 beta | F: ATGCCTCGTGCTGTCTGACC | 472–491 |
| (M98820) | R: CCATCTTTAGGAAGACACGGGTT | 889–867 |
| IL-6 | F: AGCGATGATGCACTGTCAGA | 299–318 |
| (M26744) | R: GGTTTGCCGAGTAGACCTCA | 697–678 |
| iNOS | F: GCAGGTTGAGGATTACTTCTTCCA | 3505–3528 |
| (NM 012611) | R: GCCCTTTTTTGCTCCATAGGAAA | 3597–3576 |
| beta actin | F: CCTGTATGCCTCTGGTCGTA | 504–523 |
| (NM 031144) | R: CCATCTCTTGCTCGAAGTCT | 763–744 |
The specificity of amplification was also confirmed by agarose gel electrophoresis. The starting cDNA copy number of each gene of interest in the cDNA sample was calculated by comparison with the corresponding standard curve, and the target cDNA copy number was normalized to the cDNA copy number of β-actin amplified from the same cDNA sample. For standard curves, specific plasmids were made for each sequence, and they were diluted to six concentrations as standards. Data were analyzed using ANOVA followed by Dunnet’s multiple comparison test.
Results
After all PCR reactions, only one peak was observed for each set of primers in melting curve analysis, and only one band was detected at the expected size by agarose gel electrophoresis.
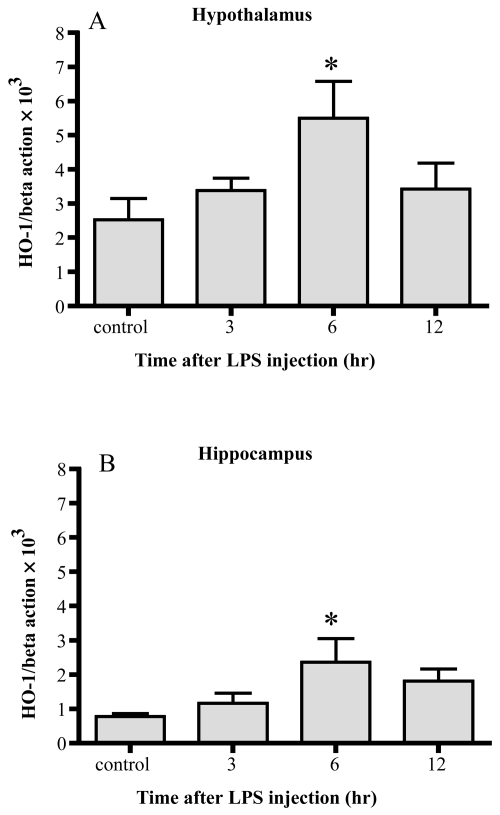
Changes of HO-1 mRNA (Figure 1)
Figure 1.
Changes in HO-1 mRNA. HO-1 mRNA increased 6 hrs after LPS injection significantly in both the hypothalamus (A) and hippocampus (B). Data represent the mean ±SE. (n = 4–6, *:p < 0.05).
In both the hypothalamus and hippocampus, HO-1 mRNA levels were significantly increased 6 hr after the injection of LPS. In both areas, the levels decreased 12 hr after the injection, becoming comparable to the control levels. By 6 hrs, HO-1 mRNA was enhanced up to 2-fold in the hypothalamus, and 3-fold in the hippocampus.
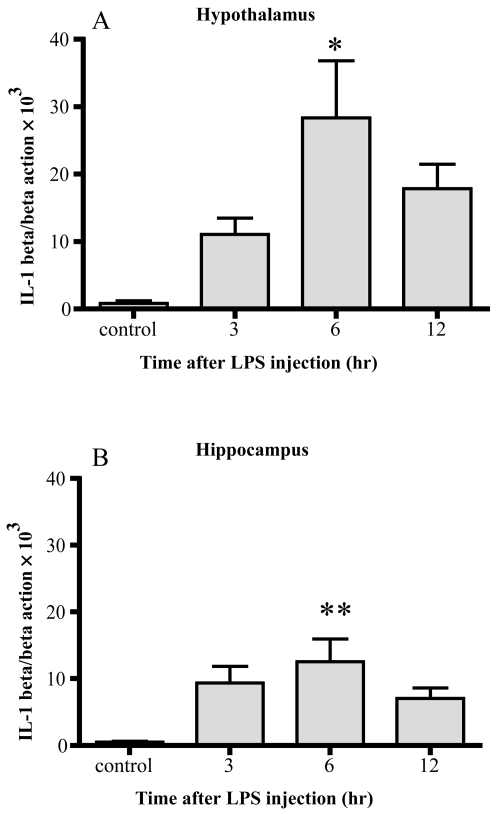
Changes of IL-1β mRNA (Figure 2)
Figure 2.
Changes in IL-1β mRNA. IL-1β mRNA markedly increased 6 hrs after LPS injection in both the hypothalamus (A) and hippocampus (B). Data represent the mean ± SE. (n = 4–6, **:p < 0.01, *:p < 0.05).
IL-1β mRNA was increased up to 33-fold 6 hrs after LPS injection in the hypothalamus and 24-fold 6 hr after injection in the hippocampus. Significant differences were observed 6 hr after injection in both areas compared with the control.
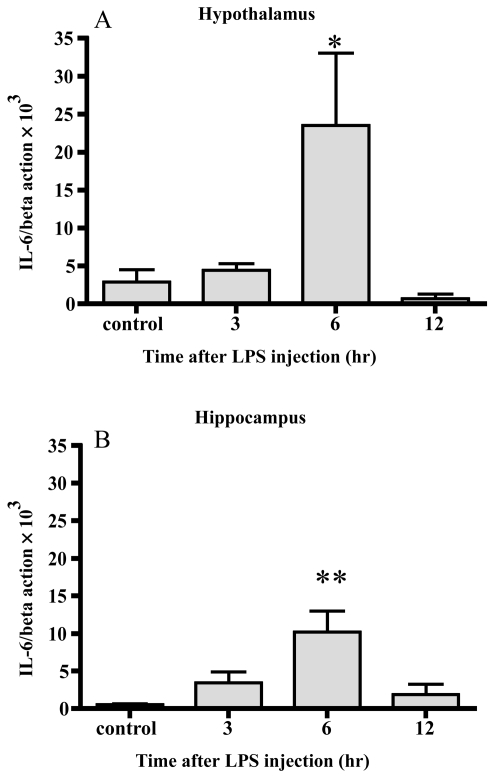
Changes of IL-6 mRNA (Figure 3)
Figure 3.
Changes in IL-6 mRNA. IL-6 mRNA increased 6 hrs after LPS injection in both the hypothalamus (A) and hippocampus (B). Data represent the mean ± SE. (n = 4–6, **:p < 0.01, *:p < 0.05).
After 6 hrs, the level of IL-6 mRNA was increased up to 8-fold and 19-fold in the hypothalamus and hippocampus, respectively. Significant differences were observed 6 hr after injection in both areas.
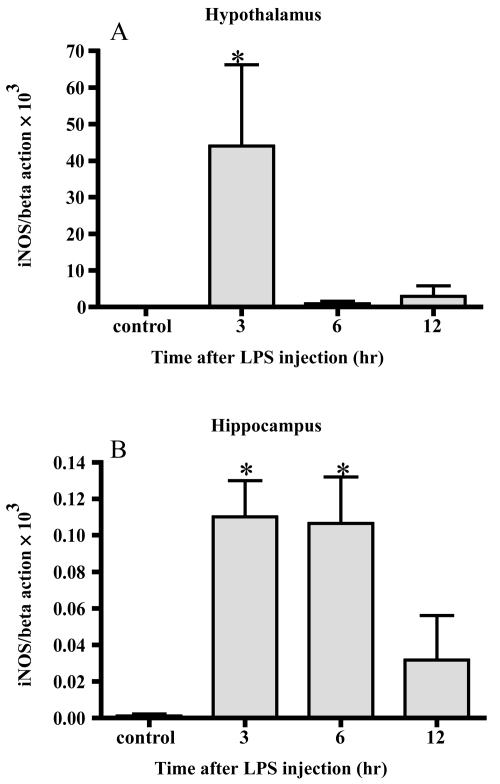
Changes of iNOS mRNA (Figure 4)
Figure 4.
Changes in iNOS mRNA. iNOS mRNA markedly increased 3 hrs after LPS injection in the hypothalamus (A) In the hippocampus, the level of iNOS mRNA increased 3 and 6 hrs after LPS injection (B) Data represent the mean ± SE. (n = 4–6, *:p < 0.05).
In the hypothalamus, iNOS mRNA was increased up to 6000-fold 3 hrs after LPS injection compared with the control. The average levels 6 and 12 hrs after injection were higher than the control but there was no significant difference. In the hippocampus, the level of iNOS mRNA increased up to more than 80-fold 3 and 6 hrs after LPS injection. The average value 12 hrs after injection was higher than the control but there was no significance.
Discussion
Brain HO-1 mRNA responded to peripheral injection of LPS in association with IL-1β, IL-6, and iNOS. Although inductions of IL-1β, IL-6, and iNOS were demonstrated after peripheral LPS injection in previous reports (Vallieres and Rivest 1997; Satta et al 1998; Tonelli et al 2003), this is the first study showing that HO-1, IL-1β, IL-6, and iNOS mRNA were induced in the same brain after peripheral injection of LPS.
The response of iNOS mRNA was quick and very strong in this experiment. NO, generated by iNOS, leads to the induction of free radicals (Moncada and Bolanos 2006), and NO is reported to contribute to HO-1 induction in the brain (Kitamura et al 1998). Therefore, it is considered that brain HO-1 induction is mediated with iNOS in this experiment. Peripheral LPS injection induces brain I-κBα (Quan et al 1997), and NF-κB, released from I-κBα, initiates the transcription of HO-1 (Lavrovsky et al 1994; Wijayanti et al 2004). This pathway is considered to be involved in this study. In addition, as IL-6 induces HO-1 mRNA through the JAK/stat pathway in the liver (Tron et al 2006), this mechanism may also work in the brain. Peripheral acute inflammation caused by LPS induces HO-1 mRNA, and this reaction is considered to be involved in several mechanisms.
In the hypothalamus, HO-1 mRNA could not be detected after LPS injection with classical RT-PCR (Jacobs et al 1997). However, real-time PCR is sufficiently sensitive to quantify all three constitutive types of HO genes in each part of the brain (Scapagnini et al 2002). In previous studies using in situ hybridization, after peripheral injection of LPS, IL-1β, IL-6, and iNOS were shown to be induced in circumventricular organs (CVOs) and the paraventricular nucleus, included in the hypothalamus in this study (Wong et al 1996; Vallieres and Rivest 1997; Tonelli et al 2003). Since oxidative stress is considered to arise in these areas due to cytokines and NO, HO-1 mRNA may be induced there, resulting in a signifi-cant increase in HO-1 mRNA in the hypothalamus shown in this study. In the hippocampus, signals of these cytokines or iNOS have never been detected by in situ hybridization (Wong et al 1996; Vallieres and Rivest 1997; Tonelli et al 2003). In this study, HO-1 mRNA increased along with the other three genes, suggesting that the hippocampus reacts to LPS injection with overall sensitivity, but not at a sensitive area like CVOs.
A subseptic dose of LPS injection caused HO-1 reaction in the brain, suggesting that acute peripheral inflammation causes oxidative stress in the brain even if the inflammation is not lethal. Several kinds of pathways are considered to be involved in the mechanism of brain HO-1 induction. In a future study, other oxidative changes should be evaluated. Regarding the clinical situation, in a person with peripheral inflammation, such as patients after surgery, brain oxidative changes may be induced.
Acknowledgments
We are grateful to Dr. T. Sugimoto for reviewing our manuscript.
Footnotes
Disclosure This study is supported by a Grant-in-Aid for Scientific Research (18592178) from the Ministry of Education, Science and Culture of Japan. The authors report no conflicts of interest.
References
- Abd El-Gawad HM, Khalifa AE. Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res. 2001;43:257–63. doi: 10.1006/phrs.2000.0781. [DOI] [PubMed] [Google Scholar]
- Birnbaum J, Klotz E, Spies CD, et al. Effects of dopexamine on the intestinal microvascular blood flow and leukocyte activation in a sepsis model in rats. Crit Care. 2006;10:R117. doi: 10.1186/cc5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33:941–50. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Nobel S, Winblad B, et al. Expression of interleukin 1 alpha and beta, and interleukin 1 receptor antagonist mRNA in the rat central nervous system after peripheral administration of lipopolysaccharides. Cytokine. 2000;12:423–31. doi: 10.1006/cyto.1999.0582. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Takahashi T, Suzuki T, et al. Differential induction of brain heme oxygenase-1 and heat shock protein 70 mRNA in sepsis. Res Commun Mol Pathol Pharmacol. 1999;105:55–66. [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Satta MA, Dahia PL, et al. Induction of nitric oxide synthase and interleukin-1beta, but not heme oxygenase, messenger RNA in rat brain following peripheral administration of endotoxin. Brain Res Mol Brain Res. 1997;49:238–46. doi: 10.1016/s0169-328x(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Furukawa M, Matsuoka Y, et al. In vitro and in vivo induction of heme oxygenase-1 in rat glial cells: possible involvement of nitric oxide production from inducible nitric oxide synthase. Glia. 1998;22:138–48. [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, et al. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA. 1994;91:5987–91. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–89. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Kim L, et al. Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc Natl Acad Sci USA. 1997;94:10985–90. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal. 2002;4:625–32. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- Satta MA, Jacobs RA, Kaltsas GA, et al. Endotoxin induces interleukin-1beta and nitric oxide synthase mRNA in rat hypothalamus and pituitary. Neuroendocrinology. 1998;67:109–16. doi: 10.1159/000054305. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, D’Agata V, Calabrese V, et al. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–9. doi: 10.1016/s0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Gray F, Lorin de la Grandmaison G, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–805. doi: 10.1016/s0140-6736(03)14899-4. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–6. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Maeda S, Rapp KL, et al. Differential induction of interleukin-I beta mRNA in the brain parenchyma of Lewis and Fischer rats after peripheral injection of lipopolysaccharides. J Neuroimmunol. 2003;140:126–36. doi: 10.1016/s0165-5728(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Tron K, Samoylenko A, Musikowski G, et al. Regulation of rat heme oxygenase-1 expression by interleukin-6 via the Jak/STAT pathway in hepatocytes. J Hepatol. 2006;45:72–80. doi: 10.1016/j.jhep.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69:1668–83. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- Wijayanti N, Huber S, Samoylenko A, et al. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–10. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- Wong ML, Rettori V, al-Shekhlee A, et al. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med. 1996;2:581–4. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]