Abstract
The Asthma Control Test (ACT) is a patient-completed questionnaire developed to assess asthma control. Health-related quality of life (HRQL) in asthmatics has shown relatively low correlations with parameters of asthma control and the relationship between the ACT and HRQL in asthmatics is yet unclear. Because revalidations of translated versions of questionnaires are critical for its utilization, we first sought to validate the Korean version of ACT and then to evaluate the relationship between the ACT and HRQL. Patients (n=117) completed the ACT and asthma-related quality of life questionnaire (AQLQ) at 3 physician visits. Pulmonary function was measured and an asthma specialist rated asthma control. The Korean version of ACT was found to be reliable, valid, and responsive to changes in asthma control over time up to three consecutive visits. ACT scores correlated significantly (p=0.001) with symptoms domain (r=0.72), activity domain (r=0.65), emotional domain (r=0.69), and environmental domain (r=0.67) of AQLQ. In conclusion, the Korean version of the ACT was found to be a reliable and valid tool for measuring asthma control, and to correlate well with AQLQ scores. Moreover, the ACT was responsive to changes in AQLQ scores over time.
Keywords: Asthma, Quality of Life, Questionnaires, Validation Studies, Longitudinal Studies
INTRODUCTION
Asthma is an important chronic airway disease worldwide, and its prevalence is increasing in all regions including Korea (1). The goal of asthma management, according to the GINA guidelines, is to achieve optimum disease control (2). However, because of the multifaceted nature of asthma, different measures such as clinical assessment (symptoms and quality of life), functional parameters (spirometry), and biomarkers of inflammation are used to evaluate asthma control.
Several questionnaires have been developed to measure asthma control, such as, the Asthma Control Test (ACT™; ACT is a trademark of QualiMetric, Lincoln RI) (3), the Asthma Control Questionnaire (ACQ) (4), the Asthma Therapy Assessment Questionnaire (ATAQ) (5), and the Asthma Control Scoring System (ACSS) (6). These tools have been validated for various applications, including use by health care providers to assess the state of control of asthma and by patients for self-assessment purposes as part of a written personal asthma action plan. These tests have the potential to improve assessments of asthma control and provide reproducible objective measures that can be charted over time (2).
It has been assumed that if an objective clinical measure improves that a patient's symptoms and quality of life must also improve. However, asthma may affect health-related quality of life (HRQL) in ways that objective clinical measures cannot predict, and they may be unable to fully assess whether a patient feels or functions better (physically, socially, and emotionally) in everyday life (7, 8). Moreover, it is difficult to establish a clear correlation between objective and subjective parameters, as they appear to measure particular aspects of the disease and to be partially independent. This has been demonstrated by poor correlations between symptoms and lung function (4, 9, 10), or quality of life (7).
We considered that the ACT may directly correlate with HRQL because it includes questions on symptoms, daily activities, and uniquely, a self-assessment of asthma control, which may all affect HRQL. However, the correlation between the ACT and HRQL in asthmatics is still unclear.
Because the translation of questionnaires into other languages and their utilizations in different cultural backgrounds may affect results, revalidations of translated versions of questionnaires are critical (11, 12). The aims of this study were to validate the Korean version of the ACT and to evaluate its relationship with HRQL.
MATERIALS AND METHODS
Study design
We conducted a longitudinal study in an adult outpatient population at our Asthma and Allergy Clinic. At each of the three clinic visits, separated by 4 to 12 weeks, spirometry was conducted and the self-administered ACT and asthma-related quality-of-life questionnaire (AQLQ) were completed. Ratings of asthma severity were done as previously described (3, 13). However, the "completely controlled" category was omitted because differentiating "completely controlled" from "well controlled" was not at issue in the present study. Asthma specialists blinded to the results of ACT and AQLQ surveys rated asthma control on a 4-point scale: 1) "not controlled at all"; 2) "poorly controlled"; 3) "somewhat controlled"; and 4) "well controlled". Specialists were instructed to base their ratings on how well the GINA-defined goals of asthma were being met (14), as determined on the basis of patient history, a physical examination, and FEV1 measurement.
No accepted system of defining control in relation to these goals has been articulated during the study period, which started before the revised version of GINA guidelines with 3 control categories was published in late 2006. In the absence of specific criteria, we believed that the best approach was to use the summary judgment of experienced specialists aware of both the GINA goals of therapy and all relevant aspects of patient clinical status. Moreover, to maintain the same design as the original study, we decided to allow the well experienced asthma specialist to assess asthma control without specific written criteria (3, 13).
The study protocol was approved by the institutional review board of Seoul National University Bundang Hospital.
Subjects
From January 2006 through December 2006, 117 subjects under the care of an asthma specialist were recruited from among outpatients visiting the Asthma and Allergy Clinic of Seoul National University Bundang Hospital. Patients 15 yr of age or older were eligible if they had; 1) current physician-diagnosed asthma (diagnosed within the previous 3 yr) without other respiratory comorbidities, 2) documented evidence of β2-agonist reversibility of forced expiratory volume in one second (FEV1) of more than both 20% and 200 mL (15), 3) had normal chest radiograph, 4) were literate in Korean, and 5) not participating in other clinical studies at the time of enrollment. Patients and parents or guardians, or both, provided written informed consent.
Asthma control test
The ACT, a patient-completed questionnaire, contains 5 questions, which require responses on a 1 to 5 scale, where higher scores reflecting better asthma control. The ACT assesses the effect of asthma on daily functioning, daytime asthma symptoms (shortness of breath), nocturnal symptoms, use of rescue medications, and self assessment of asthma control. Thus, the higher total response scores to the 5 questions (range 5 to 25) correspond to better control. In the original ACT survey study, ACT scores of 19 or less have been associated with uncontrolled asthma and scores of 15 or less have been associated with poorly controlled or uncontrolled asthma (3, 13).
The use of the Korean version of ACT survey was permitted by QualiMetric Incorporated, U.S.A. (16).
Health-Related quality of life measures
The asthma-related quality-of-life was evaluated using the Korean modification of the Juniper asthma quality-oflife questionnaire (AQLQ), named Quality of Life Questionnaire in Adult Korean Asthmatics, which has been previously described (8, 17, 18). Answers to each question were scored on a 5-point scale, with a score of 1 representing greatest impairment and a score of 5 representing no impairment. Items were weighted equally and are reported as mean scores for each domain (activity limitations, emotions, symptoms, and exposure to environmental stimuli) along with the overall mean score.
Spirometry
Spirometry was performed using a SensorMedics 2130 (SensorMedics Corporation, CA, U.S.A.). Forced vital capacity (FVC) and forced expiratory volume at one second (FEV1) were recorded according to ATS recommendations (19). The higher of two values for FEV1 (reproducible within 100 mL) was recorded and percentages of predicted FEV1 values were calculated (20).
Statistical analysis
To assess the measurement properties of the Korean version of the ACT, the following aspects were analyzed: 1) internal consistency reliability; 2) test-retest reliability; 3) criterion validity; and 4) discriminant validity.
Cronbach's α-coefficient values were estimated from ACT item responses at baseline and follow-up visits. This coefficient was calculated based on the mean correlation of each item in the scale with the sum total of the scale, and on the total number of items (21). Test-retest reliabilities were assessed by calculating intraclass correlations between ACT scores during the three clinic visits. Test-retest reliability analysis was limited to a subset of patients whose asthma control was stable, as determined by the same specialist's ratings of asthma control at both baseline and follow-up visits.
Criterion validities of the Korean version of ACT survey were evaluated by computing correlations between ACT scores and specialist's assessments of asthma control and between ACT scores and percentage of predicted FEV1 values at baseline visits.
Discriminant validity of the ACT was also evaluated based on clinical tests using the known-group validity method and data from baseline visits. In this study, 3 such measures were used. The first was the asthma specialist's rating of asthma control, which consisted of the 4 categories mentioned above, and the second measure consisted of percent predicted FEV1 values. Patients were categorized into 4 groups based on their percent predicted FEV1 values, i.e., 1) less than 60%; 2) 60% to 79%; 3) 80% to 99%; and 4) greater than 100%. This stratification of percent predicted FEV1 values was roughly based on the FEV1 values mentioned in the four levels of asthma severity in the GINA guidelines (14). The third measure consisted of the asthma specialist's prescription of oral corticosteroids for asthma exacerbation. This was used to categorize patients into 2 groups: 1) the group with oral corticosteroid rescue and 2) the group without oral corticosteroid rescue. For each of the above measures, mean ACT scores were computed and compared across patient groups. One-way ANOVA was used to test the significances of differences between mean ACT scores across groups of patients who differed in terms of specialist ratings of asthma control, percent predicted FEV1 values, and oral corticosteroid prescriptions.
The responsiveness of ACT scores was evaluated using Pearson's correlation and ANOVA. First, changes in ACT scores from baseline to follow-up visits were correlated with changes in the specialist's rating of asthma control and changes in lung function as measured by the change in FEV1 values. Second, ANOVA was used to compare mean changes in ACT scores across groups of patients who differed in terms of changes in the specialist's rating of asthma control and changes in percent predicted FEV1 values.
Changes in specialist control ratings were derived by simply subtracting baseline ratings from follow-up ratings; these rating involved 4 levels, as mentioned above. Patients were grouped into 5 categories of control rating changes: 1) a poorer rating by two or more levels; 2) a poorer rating by one level; 3) the same rating; 4) an improved rating by one level; and 5) an improved rating by two levels.
Changes in percent predicted FEV1 values were derived by subtracting baseline percent predicted FEV1 values from follow-up percent predicted FEV1 values and then dividing by the baseline percent predicted FEV1 value. Patients were categorized into 3 groups based on the stratification method used in the original study (3, 13), where a 10% change in percent predicted FEV1 value from baseline was considered clinically significant: 1) improved by 10% or more from baseline; 2) improved by less than 10% from baseline; and 3) decreased versus baseline.
Correlations between changes in the ACT and AQLQ scores were evaluated at each visit. Mean ACT scores were compared in different AQLQ groups. We categorized the subjects into 4 groups by dividing the 5-point scale: 1) 1 to 1.9; 2) 2 to 2.0; 3) 3 to 3.9; 4) 4 to 5. When using 7-point scale response options in the original Juniper's Asthma Quality of Life Questionnaire, a within subject change in score of 0.5 represents the minimal important difference in quality of life and advocates that score change of 0.5 is generalizable to all areas of health-related quality of life in assessing minimal important change (22). Because our modified AQLQ uses 5-point scale response, although it has not been validated, it can be assumed that score lesser than 0.5 could represent minimal important change in quality of life. Thus, the groups differing in mean AQLQ score of 1, which is twice the score of minimal important change mentioned above, should represent groups differing in quality of life. Additionally, changes in mean ACT scores were evaluated in groups of patients differing in changes in AQLQ scores of 0.5 measured at each visit.
Concurrent validity between the ACT and AQLQ was examined using Pearson's correlation coefficients (r) for relevant domains in order to compare the questionnaires. Validity measurements were performed using ACT scores and scores of the activity, symptoms, emotions, and environment domains of the AQLQ. A correlation coefficient of greater than 0.8 indicates an excellent correlation, between 0.5 and 0.8 a moderate correlation, between 0.3 and 0.5 a weak correlation, and of less than 0.3 a lack of correlation (23). Variables were analyzed using SPSS 12.0 for Windows.
RESULTS
Sample
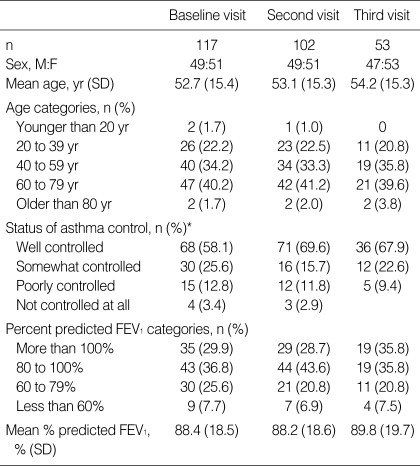
A total of 117 patients participated in this study and these patients made 272 visits. Of the 117 patients, 102 patients visited the office twice and 53 patients visited three times. Patients enrolled early in the study were able to make up to 3 visits and patients enrolled toward the end of the study period made 2 visits (Table 1). There was a wide range of ages among the subjects from minimum age of 15 to maximum age of 87. More than two thirds of the asthmatic subjects were well controlled. The lowest percent predicted FEV1 value was 27% and the highest percent predicted FEV1 value was 143%.
Table 1.
Sample characteristics
*Asthma specialists rated asthma control on a 4-point scale based on how well the GINA-defined goals of asthma were met as determined based on patient history, physical examination, and FEV1 measurements. This rating method was based on the original study of the ACT survey (3, 13).
FEV1, forced expiratory volume in one second.
Reliability of the ACT
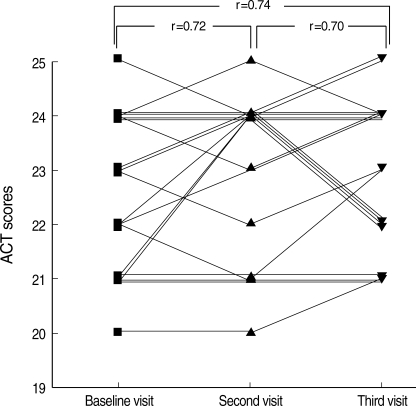
The internal consistency reliability of the ACT survey, as determined by Cronbach's α-coefficient, was 0.71 at baseline visits (n=117) and the test-retest reliability among the 61 subjects who had the same specialist ratings for asthma control over two visits, had an intraclass correlation coefficient (ICC) of 0.83 (n=61) between baseline and second visits. To determine whether ACT is reliable for multiple consecutive visits, it was calculated in 19 patients, who had the same specialist asthma control rating for three consecutive visits. The test-retest reliability shown by ICC in these 19 patients was 0.72, between baseline and second visits, 0.70 between second and third visits, and 0.74 between baseline and third visits (Fig. 1).
Fig. 1.
Test-retest reliability of the Asthma Control Test in 19 patients with the same control rating levels at 3 consecutive visits. Intraclass correlation coefficients (ICC) were evaluated between baseline and second visits, second and third visits, and between baseline and third visits.
Criterion validity of the ACT
Statistically significant correlations were observed between ACT scores and specialist's ratings of asthma control at baseline visits (r=0.87, p=0.001). However, ACT scores were not correlated with baseline percent predicted FEV1 values (r=0.28, p=0.004).
Discriminant validity of the ACT
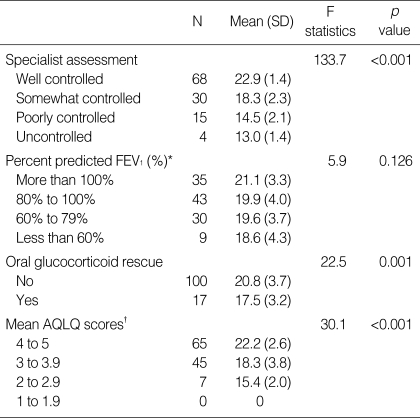
Significant differences in mean scores across groups of patients who differed in terms of clinical measures related to asthma control may reflect the discriminant validity of the ACT scores (Table 2). Mean ACT scores were significantly lower among patients with poorer control, as judged by the specialist, than among patients with more favorable control ratings (p=0.001). Likewise, patients prescribed oral glucocorticoid rescue treatment scored significantly lower on the ACT than patients not prescribed this treatment (p=0.001). However, no differences in mean ACT scores were observed among patients with different percentages of predicted FEV1 values (p=0.126).
Table 2.
Discriminant validity tests on mean ACT scores at baseline visits (n=117)
*This stratification of percentage of predicted FEV1 values was roughly based on the FEV1 values mentioned in the four steps of asthma severity in the GINA guidelines (2); †This stratification method of AQLQ scores was done simply by dividing the 5-scale point scores into 4 groups. Although this stratification method has not been validated, it was based on the fact that change in score of 0.5 in 7-point scale represents minimal important change of quality of life (22) and that score difference of 1 which is twice the score of minimal important change mentioned above would stratify groups differing in quality of life.
FEV1, forced expiratory volume in one second; AQLQ, asthma quality of life questionnaire.
Responsiveness of the ACT
The responsiveness of ACT scores to changes in asthma control were evident from correlations between changes in ACT scores and changes in specialist control ratings or changes in percentage predicted FEV1 values. Changes in the ACT scores were found to be correlated significantly with changes in control levels as assessed by the specialist at second visits (r=0.81, p=0.001) and at third visits (r=0.80, p=0.001). Changes in ACT scores correlated weakly with changes in percent predicted FEV1 values measured at second visits (r=0.43, p=0.001) and at third visits (r=0.31, p=0.022).
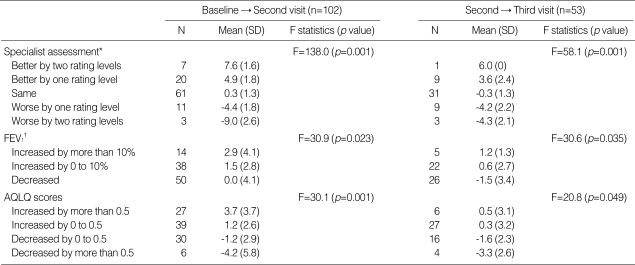
The responsiveness of the ACT was also demonstrated by evaluating mean changes in ACT scores across groups of patients who differed in level of change according to the specialist's rating of asthma control and percent predicted FEV1 values (Table 3). Mean changes in ACT scores differed significantly across groups of patients differing in level of change according to the specialist's control ratings measured at second and third visits (p=0.001). Mean changes in ACT scores differed in groups of patients with different changes in percent predicted FEV1 values at second visits (p=0.023) and third visits (p=0.035).
Table 3.
Mean changes in ACT scores in different groups of patients
*Differences in asthma control level on a 4-point scale assessed by the asthma specialist; †Stratified based on the method previously reported where a 10% change in percentage predicted FEV1 from baseline was considered clinically significant (3, 13).
FEV1, forced expiratory volume in one second; AQLQ, asthma quality of life questionnaire.
Relationship between the ACT and AQLQ
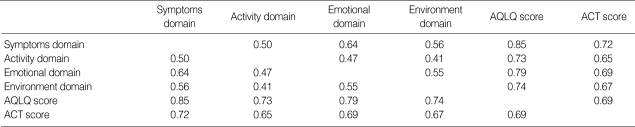
To evaluate the concurrent validity of the ACT versus the AQLQ, correlations between ACT and each of the four domains of AQLQ were evaluated over all total visits (Table 4). ACT scores correlated significantly with the symptoms domain (r=0.72, p=0.001), the activity domain (r=0.65, p=0.001), the emotional domain (r=0.69, p=0.001), and the environmental domain (r=0.67, p=0.001) on the AQLQ. Total AQLQ scores also correlated with total ACT scores (r=0.69, p=0.001).
Table 4.
Concurrent validity between ACT and AQLQ (n=272)*,†
*n=total visits; †p<0.001 for all values shown as Pearson's correlation coefficients.
AQLQ, asthma quality of life questionnaire; ACT, Asthma Control Test; FEV1, forced expiratory volume in one second.
Statistically significant correlations were observed between ACT scores and AQLQ scores at baseline visits (r=0.70, p=0.001). Mean ACT scores were significantly lower for patients with lower AQLQ scores than for patients with higher AQLQ scores (p=0.001) (Table 2). Changes in ACT and AQLQ scores correlated with each other at second (r=0.62, p=0.001) and at third visits (r=0.64, p=0.016). Changes in mean ACT scores differed significantly in groups of patients differing in changes in AQLQ scores measured at second visits (p=0.001) and at third visits (p=0.049) (Table 3).
DISCUSSION
The results of this study demonstrated that the Korean version of ACT is reliable, valid, and responsive to changes in asthma control over time in a sample of Korean asthmatic outpatients under the care of an asthma specialist. Based on this tool, we found that the ACT correlates significantly with the AQLQ and with each of the four specific domains (activity, emotions, symptoms, and environment) in the AQLQ.
Because the translation of questionnaires into other languages and different cultural backgrounds may affect the results of questionnaires, and in order to facilitate international multicentric studies, cross-cultural adaptation and revalidation of each translated version of a questionnaire are crucial for its utilization (11, 12). We successfully validated the officially translated version of the Korean ACT, which is available on a public website (16). Thus, this study indicates that the Korean version of ACT is as valid and reliable as the original, and that it can be used in international multi-center studies.
There is no universal gold standard for asthma control measurement because of its multifaceted nature. Thus, as was done during the original study (3, 13), we decided to use an experienced asthma specialist's assessment of asthma control based on the GINA goals of asthma control as the "gold standard" (3). Although there are no specific criteria for assessing asthma control, as is stated in the newly revised version of GINA guidelines (2), we considered that the best approach was to use the summary judgment of experienced specialists, who were aware of both the GINA goals of therapy and all relevant aspects of patient clinical status. We also used AQLQ, a valid and reproducible tool to assess quality of life in Korean asthma patients (17, 18), to allow comparisons of ACT scores with the multidimensional effects of asthma on daily life.
The original ACT survey was evaluated over two clinic visits (3, 13). In our study, we deepened the value of the longitudinal study by evaluating test-retest reliability, discriminant validity, and responsiveness to changes in asthma control over 3 consecutive clinic visits. Test-retest reliability of 19 patients with same control rating levels at 3 consecutive visits showed a consistent value of intraclass correlation coefficient, indicating that the Korean version of the ACT is reproducible over serial applications in stable adult asthma patients. It has been shown that assessments of short-term symptom burdens underestimate the long-term functional burden of asthma (24). Thus, the long-term functional effect of the disease is best captured by serial applications of a standard questionnaire over time (25), and the present study shows that ACT may be useful in such serial applications.
In the present study, we correlated ACT scores and AQLQ scores. Concurrent validity was evaluated by demonstrating that each domain of the AQLQ is significantly correlated with total ACT scores. ACT scores were found to consistently correlate well with AQLQ scores over multiple visits with highest correlation in symptoms domain and showed good discriminant validity in terms of assessing different levels asthma-related quality of life (Table 2). Moreover, the ACT was found to be responsive to changes in asthma-related quality of life over time.
No correlations were found between ACT scores and FEV1 values or between AQLQ scores and FEV1 values. Because the ACT and the AQLQ ask to what extent asthma has been like over several weeks, measurement of lung function at a single time may not correlate well with either ACT or AQLQ scores. This observation concurs with those made in other studies, where either no significant correlation or a relatively low correlation was found between asthma symptoms and FEV1 (4, 9, 10). However, changes in ACT scores were found to correlate weakly with changes in percent predicted FEV1 values, and mean changes in ACT scores differed in groups of patients with different changes in percentage predicted FEV1 values at different visits. These results suggested that changes in lung function over time may have a more important clinical values than lung function value per se in terms of assessing asthma control.
In summary, this is the first validation study of the Korean version of the ACT survey. The Korean version of ACT was found to provide an objective assessment of asthma control, and to be reliable, valid, and responsive to changes in asthma control over time. Moreover, the ACT correlated well with AQLQ and was also responsive to changes in AQLQ over time. Because the ACT reflects multiple dimensions of quality of life as well as clinical symptoms, it may be the most useful single measure in busy clinics and clinical studies, where multiple aspects of asthma control should be considered in a relatively short time.
ACKNOWLEDGMENTS
This work was supported by the Clinical Research Center for Chronic Obstructive Airway Disease Grant 0412-CR03-0704-0001 from the Korean Ministry of Health and Welfare. We extent our thanks to So-Jeong You and Ji-Hyun Hong, clinical research nurses, for their efforts and support.
Footnotes
This work was supported by the Clinical Research Center for Chronic Obstructive Airway Disease Grant 0412-CR03-0704-0001 from the Korean Ministry of Health and Welfare.
References
- 1.Cho SH, Park HW, Rosenberg DM. The current status of asthma in Korea. J Korean Med Sci. 2006;21:181–187. doi: 10.3346/jkms.2006.21.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Heart Lung and Blood Institute. Global initiative for asthma: global strategy for asthma management and prevention. Rev. 2006. ed. Bethesda, MD: U.S.: Dept. of Health and Human Services, Public Health Service; 2006. [Google Scholar]
- 3.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 5.Vollmer WM, Markson LE, O'Connor E, Sanocki LL, Fitterman L, Berger M, Buist AS. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160:1647–1652. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- 6.Boulet LP, Boulet V, Milot J. How should we quantify asthma control? A proposal. Chest. 2002;122:2217–2223. doi: 10.1378/chest.122.6.2217. [DOI] [PubMed] [Google Scholar]
- 7.Carranza Rosenzweig, JR, Edwards L, Lincourt W, Dorinsky P, ZuWallack RL. The relationship between health-related quality of life, lung function and daily symptoms in patients with persistent asthma. Respir Med. 2004;98:1157–1165. doi: 10.1016/j.rmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147:832–838. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 9.Teeter JG, Bleecker ER. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest. 1998;113:272–277. doi: 10.1378/chest.113.2.272. [DOI] [PubMed] [Google Scholar]
- 10.Moy ML, Israel E, Weiss ST, Juniper EF, Dube L, Drazen JM. Clinical predictors of health-related quality of life depend on asthma severity. Am J Respir Crit Care Med. 2001;163:924–929. doi: 10.1164/ajrccm.163.4.2008014. [DOI] [PubMed] [Google Scholar]
- 11.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–1432. doi: 10.1016/0895-4356(93)90142-n. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SM, Alonso J, Bucquet D, Niero M, Wiklund I, McKenna S. Cross-cultural adaptation of health measures. European Group for Health Management and Quality of Life Assessment. Health Policy. 1991;19:33–44. doi: 10.1016/0168-8510(91)90072-6. [DOI] [PubMed] [Google Scholar]
- 13.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.National Heart Lung and Blood Institute. Global initiative for asthma: global strategy for asthma management and prevention. Rev. 2002. ed. Bethesda, MD: U.S.: Dept. of Health and Human Services, Public Health Service; 2002. [Google Scholar]
- 15.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 16.GlaxoSmithKline: Asthma Control Test. [Accessed January 7, 2006]. Available at: http://www.asthmacontroltest.com.
- 17.Jang AS, Lee JH, Park SW, Lee YM, Uh ST, Kim YH, Park CS. Factors influencing the responsiveness to inhaled glucocorticoids of patients with moderate-to-severe asthma. Chest. 2005;128:1140–1145. doi: 10.1378/chest.128.3.1140. [DOI] [PubMed] [Google Scholar]
- 18.Park JW, Cho YS, Lee SY, Nahm DH, Kim YK, Kim DK, Sohn JW, Park JK, Jee YK, Cho YJ, Yoon HJ, Kim MK, Park HS, Choi BH, Choi IS, Park CS, Min KU, Moon HB, Park SH, Lee YK, Kim NS, Hong CS. Multi-center study for the utilization of quality of life questionnaire for adult Korean asthmatics (QLQAKA) Korean J Asthma Allergy Clin Immunol. 2000;20:467–479. [Google Scholar]
- 19.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 21.Nunnally JC. Assessment of reliability. 2nd ed. New York: McGraw-Hill; 1978. [Google Scholar]
- 22.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 23.Indrayan A, Sarmukaddam SB. Medical biostatistics. Boca Raton: Taylor & Francis; 2001. [Google Scholar]
- 24.Fuhlbrigge AL, Adams RJ, Guilbert TW, Grant E, Lozano P, Janson SL, Martinez F, Weiss KB, Weiss ST. The burden of asthma in the United States: level and distribution are dependent on interpretation of the national asthma education and prevention program guidelines. Am J Respir Crit Care Med. 2002;166:1044–1049. doi: 10.1164/rccm.2107057. [DOI] [PubMed] [Google Scholar]
- 25.Stoloff SW, Boushey HA. Severity, control, and responsiveness in asthma. J Allergy Clin Immunol. 2006;117:544–548. doi: 10.1016/j.jaci.2006.01.005. [DOI] [PubMed] [Google Scholar]