Abstract
This phase II study evaluated the efficacy and safety of combination chemotherapy with paclitaxel, cisplatin, and 5-fluorouracil (5-FU) in advanced gastric cancer. Patients with histologically confirmed gastric adenocarcinoma were eligible for the study. Paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) were given as a 1-hr intravenous infusion on day 1, followed by 5-FU (750 mg/m2) as a 24-hr continuous infusion for 5 days. This cycle was repeated every 3 weeks. Forty-five eligible patients (median age, 56 yr) were treated in this way. Of the 41 patients in whom efficacy was evaluable, an objective response rate (ORR) was seen in 51.2% (95% CI, 0.35-0.67), a complete response in two, and a partial response in 19 patients. The median progression free survival was 6.9 months (95% CI, 5.86-7.94 months), and the median overall survival was 12.7 months (95% CI, 9.9-15.5). The main hematological toxicity was neutropenia and greater than grade 3 neutropenia was observed in twelve patients (54%). Febrile neutropenia developed in three patients (6.8%). The major non-hematological toxicities were asthenia and peripheral neuropathy, but most of patients showed grade 1 or 2. In conclusion, combination chemotherapy with paclitaxel, cisplatin, and 5-FU is a promising regimen, and was well tolerated in patients with advanced gastric cancer.
Keywords: Stomach Neoplasms, Paclitaxel, Cisplatin, Fluorouracil
INTRODUCTION
While the incidence and the mortality of gastric cancer in the Western world have decreased, gastric cancer is the most common form, and the second leading cause of death in Korea. With most cases being diagnosed at an advanced stage, the prognosis for this disease is extremely poor (1). Although localized advanced gastric cancer can be cured by surgery, metastatic or recurrent gastric cancer remains incurable, and these patients have a median survival of 6 to 9 months. The efficacy of palliative chemotherapy is now widely accepted based on many clinical trials (2-4), although no single or combination chemotherapeutic regimen has been accepted as the standard treatment.
The 5-Fluorouracil (5-FU) is one of the most effective agents in advanced gastric cancer commonly used with cisplatin or anthracycline. Especially, 5-FU and cisplatin (CF) combination chemotherapy has been widely accepted as a standard treatment with a significant higher response rate and prolongation of progression free survival (PFS) compared to single 5-FU or combination with adrimamycin/mitomycin (5-7). However, the meaningful prolongation of overall survival (OS) is dim yet. To overcome these problems, many studies have been reported and are under progress.
Taxanes (docetaxel and paclitaxel) are a class of anticancer agents that bind to microtubules and induce hyperstabilization, causing cell cycle arrest and apoptosis (8, 9). They have demonstrated promising activity in gastric cancer, and are currently being tested in many clinical trials as a combination regimen (10-13). In particular, the contribution of docetaxel in advanced gastric cancer has been reported in large scale of clinical trials. Ajani et al. showed the docetaxel addition to CF (DCF) produced a higher response rate than DC (14). Based on this result, V325 study has been going on to compare of DCF in experimental arm to CF in reference arm. It showed that DCF arm improved the time-to-progression (TTP), response rate, but also OS than CF arm (15). However, hematological toxicity was significant, with grade 3 or 4 neutropenia being over 80%. In view of palliative aim in advanced gastric cancer, these toxicities, sometimes related with fatal infection, could be attenuated the benefit of chemotherapy.
Therefore, we conducted the study to show the efficacy and decrease the toxicity of taxane-based chemotherapy. The toxicities between paclitaxel and docetaxel are somewhat different, especially in neurotoxicity and neutropenia. In ovarian cancer, two taxanes showed an equivalent therapeutic efficacy, but neutropenia was more common with docetaxel-based regimen, up to 3 times than paclitaxel-based regimen (16, 17). Also, Park et al. (18) compared the combination of palictaxel plus 5-FU (PF) with docetaxel plus 5-FU (DF) in gastric cancer. There were no significance differences in therapeutic efficacy. In DF group, dose reduction was more common than in PF group and grade 3 or 4 neutropenia was slightly frequent in DF (21%) than PF (18%). There were four and eight patients with febrile neutropenia in the PF and DF, respectively. Taken together, docetaxel could be replaced by paclitaxel to reduce the neutropenia without diminish the efficacy. Thus, we performed this study to evaluate the efficacy and safety of combination chemotherapy with paclitaxel addition to CF in advanced gastric cancer as a three-week regimen.
MATERIALS AND METHODS
Patients
Patients had histologically confirmed metastatic gastric adenocarcinoma. Eligibility criteria included at least one measurable lesion; Eastern Cooperative Oncology Group performance status ≤2; age >18 yr, life expectancy ≥3 months, no other malignancy; and adequate hematological, renal, and hepatic function (white blood cell count ≥3,000/µL, granulocytes ≥1,500/µL, platelets ≥100,000/µL, total bilirubin <1.3 mg/dL, and creatinine clearance >60 mL/min). No prior palliative chemotherapy was permitted in patients with advanced disease. Adjuvant chemotherapy was allowed if >12 months had elapsed between the end of therapy and registration.
Exclusion criteria were as follows: preexisting neurotoxicity greater than or equal to grade 2 of the National Cancer Institute Common Toxicity Criteria (NCI-CTC, version 2.0), concurrent cancer, central nervous system metastasis, active infection, other serious underlying medical conditions that would impair the ability of the patient to receive the planned treatment, or inadequate calorie and fluid intake. Participants gave written informed consent before they entered the study, which was approved by the Chonnam National University Hospital ethical committee.
Treatment schedule and dose modification
Paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) were given as a 1-hr intravenous infusion on day 1, followed by 5-FU (750 mg/m2) as a 24-hr continuous infusion for 5 days. Cycles were repeated every 3 weeks. Thirty minutes prior to the paclitaxel infusion, each patient received 20 mg dexamethasone, 50 mg ranitidine, and 5 mg chlorpheniramine maleate intravenously to prevent hypersensitivity reactions. After prehydration with at least 1 L normal saline, the calculated dose of paclitaxel, diluted in 300 mL normal saline, was infused over 1 hr. The calculated dose of cisplatin, diluted in 500 mL normal saline, was then administered over 1 hr, followed by posthydration with 3 L normal saline over 24 hr. Soon after finishing the cisplatin infusion, 5-FU was infused continuously for 5 days. Ondansetron (8 mg, i.v) was routinely given. Patients received further cycles of chemotherapy only when the absolute neutrophil count was ≥1,000/µL and platelets were ≥150,000/µL. Toxicity was graded according to NCI-CTC version 2.0. Dose modifications were determined by hematological or non-hematological toxicities. The cisplatin dose was reduced to 75% of the original dose in subsequent cycles if one of the following occurred: grade 3 neutropenia with infection, grade 4 neutropenia, grade 3 thrombocytopenia with bleeding that required platelet transfusion and greater than grade 3 sensory neurotoxicity, or grade 2 or greater nephrotoxicity. In case of subsequent toxicities after these dose reductions, the paclitaxel dose was reduced to 75% of the original dose. In cases of asthenia above grade 3, treatment was postponed for 1 week and restarted when the patient had recovered to below grade 2. If the toxicity did not improve to below grade 1 after 4 weeks, the patients was removed from the study. Also, the paclitaxel dose was reduced to 75% of the original dose if grade 2 sensory neurotoxicity was sustained subsequent 2 cycles despite the medication such as amitryptiline. For patients with grade 3 diarrhea that lasted for more than 7 days despite the administration of loperamide or mucositis grade 3 lasting for more than 5 days or grade 4 mucositis, a 25% reduction in the daily dose of 5-FU was required. To avoid the delay of chemotherapy, G-CSF was given if the absolute neutrophil count was lower than 1,000/µL seven to ten days after chemotherapy. Patients showing disease regression or stable disease received additional cycles of chemotherapy, up to a maximum of nine cycles, unless they experienced disease progression or unacceptable toxicity, or if the patients refused or the physician decided against further treatment.
Evaluation
The antitumor activity was evaluated according to the World Health Organization (WHO) criteria. Computed tomography (CT) scans of measurable lesions were carried out within 4 weeks before the start of the treatment and were repeated after every three cycles. Complete response (CR) was defined as the complete disappearance of all evaluable disease, persisting for >4 weeks. Partial response (PR) was defined as a ≥50% reduction in the sum of the products of the largest perpendicular diameters in all measurable lesions for ≥4 weeks, without evidence of progression or the development of new lesions. Progressive disease (PD) was defined as an increase in a previous lesion by >25%, or the development of any new lesion, and stable disease (SD) as any change in a previous lesion that did not fit into either the PR or PD categories. The patients were consulted in assessing the response if they had early disease progression or had received at least two cycles of treatment.
Data analysis
The primary endpoint was ORR, and secondary endpoints were PFS, OS and toxicity. This study was conducted using a Simon two-stage design. The optimal number of patients was determined assuming a target level of interest, p1=0.6, and a lower activity level, p0=0.4. Initially we planned to enroll 34 patients in first stage. If 18 or more responses were observed, we planned to continue second stage for a total of 39 evaluable patients. This design provides for a probability of ≤0.05 of accepting drugs worse than p0, and a probability of ≤0.20 of rejecting drugs better than p1. If we assume the dropout rate to be 15%, the total number of enrolled patients will be 45. All enrolled patients were included in the intent-to-treat analysis. The Kaplan-Meier method was used in all survival analysis. The Statistics Package for the Social Sciences (SPSS) program for Windows (Version 13.0) was applied for analysis.
RESULTS
Patient characteristics
Forty-five patients were initially enrolled in this study. Of these, four patients were dropped out; one had another primary tumor (esophageal cancer), two were lost to follow-up after one cycle of chemotherapy, and one did not want further chemotherapy after one cycle. The median age of patients was 56 yr, with a range of 21 to 75 yr (Table 1). Thirty patients were male (68%) and 14 were female (32%); most patients were in good general condition with a performance status of 0 or 1 (82%). All patients had histologically confirmed adenocarcinoma. Twenty-seven patients (61%) had recurrent disease after operation with or without adjuvant chemotherapy and seventeen patients (39%) had no prior treatment in metastatic gastric cancer. The median follow-up duration was 12.8 months (95% CI, 6.2-24.8)
Table 1.
Patient characteristics
ECOG, Eastern Cooperative Oncology Group; FP, 5-FU and cisplatin; FAM, 5-FU, adriamycin and mitomycin-C.
Chemotherapy delivery
The median number of treatment cycles was six, with a range from three to nine cycles. The number of patients receiving more than six cycles was 33 (80%). The median duration of treatment for all patients was 147 days, with a range of 46 to 245 days. All patients received more than three cycles of chemotherapy, and forty one patients were able to evaluate their response. The total number of cycles delivered was 269. The number of patients who were received at least 1 dose reduction and at least 1 cycle delay were 13 (29.5%) and 7 (15.9%), respectively. The target doses for paclitaxel, cisplatin, and 5-FU were 58.3, 25, and 1,250 mg/m2/week, respectively, and the achieved doses for paclitaxel, cisplatin, and 5-FU were 52.3 (89.7%), 22.3 (89.2%), and 1,131.2 mg/m2/week (90.5%), respectively.
Efficacy
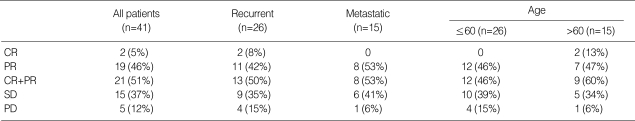
Initially, 34 patients were treated as scheduled. Of these patients, one patient showed CR, 18 patients showed PR, 12 patients showed SD and 3 patients showed PD. Therefore, we advanced this study up to 45 patients. The best responses are shown in Table 2. Two patients achieved CR (4.9%; 95% CI, 1-16) and 19 patients PR (46.3%; 95% CI, 31-63). The ORR was 51.2% (95% CI, 35-67) with a median response duration of 23 weeks. The responses of the 26 patients who had recurrent disease were 2 CR, 11 PR, 9 SD, and 4 PD, and the responses who had no prior treatment in metastatic disease were 8 PR, 6 SD, and 1 PD. No statistically significant difference was observed between the two groups. Among 12 patients who had carcinomatosis peritonei with ascites, four showed marked reduction of ascites after chemotherapy, and three did not need further paracentesis (SD). Two patients were treated with palliative surgery after the sixth and ninth cycles, and they had no evidence of disease until data analysis.
Table 2.
Tumor responses
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Survival outcome
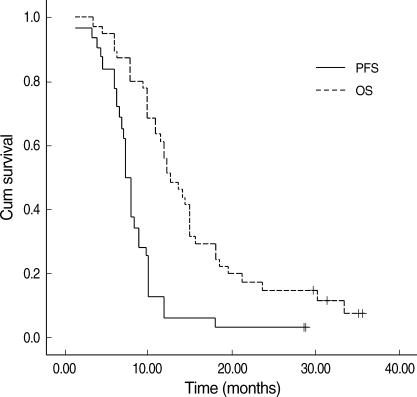
Of the 44 patients, four (9.1%) remained alive at a median follow-up of 12.8 months. The median progression-free survival was 6.9 months (95% CI, 5.86-7.94). The median OS was 12.7 months (95% CI, 9.9-15.5) and the 1-yr OS rate was 62.5% (Fig. 1).
Fig. 1.
Survival curves of progression-free survival (PFS) and overall survival (OS).
Toxicity
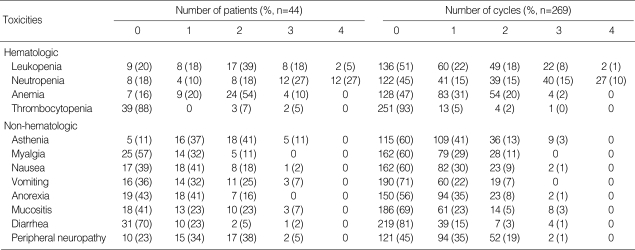
Chemotherapy-related toxicity is summarized in Table 3. The main hematological toxicity was neutropenia. Grade 3 or 4 neutropenia was observed in 67 cycles (25%) and 54% of patients. Greater than grade 3 thrombocytopenia developed in only two patients (5%). Eighteen patients (41%) received growth factor before starting the next cycle of chemotherapy to prevent delayed treatment. Febrile neutropenia developed in three patients (7.3%) and all of them recovered after treatment; two patients recovered after a short course of antibiotic treatment and one patient needed intensive care treatment. Grade 1 or 2 peripheral sensory neuropathy was observed in 32 patients (72%), and greater than grade 3 peripheral sensory neuropathy occurred in two patients (5%). The major non-hematological toxicity was asthenia, which spontaneously resolved after 1 or 2 weeks of rest. No treatment-related mortality occurred.
Table 3.
Toxicities
DISCUSSION
Taxanes have been used widely as single agents in the treatment of gastric cancer. Paclitaxel is well tolerated and demonstrates response rates in the 17-23% range (19). Docetaxel has also been found to be active in advanced gastric cancer, with ORRs between 17 and 29% (20, 21). Both taxanes are known for their synergistic effect with cisplatin in vivo and in vitro, especially when paclitaxel precedes platinum administration (22). Based on these results, taxanes have been widely used in combination with cisplatin, with or without 5-FU.
Docetaxel has been widely used in advanced gastric cancer and a randomized phase III trial of DCF versus CF in metastatic gastric adenocarcinoma was reported (15). The doses and schedule of the DCF arm were docetaxel 75 mg/m2 on day 1, cisplatin 75 mg/m2 on day 1, and 5-FU 750 mg/m2/day as continuous infusion on days 1-5, repeated every 3 weeks. The overall response of DCF group showed 37% (CF group: 25%) and the duration of TTP and OS were significantly longer in DCF group than in CF group. Although OS was slightly increased in DCF group (9.2 months) compared with in CF group (8.6 months), a considerable benefit was observed with the 2-yr survival rate of 18% for DCF and 9% for CF. Despite these results, the DCF regimen showed a high rate of toxicities. The rate of grade 3 or 4 neutropenia and febrile neutropenia were 82.3 and 29%, respectively. In the view of palliative aim for chemotherapy in advanced gastric cancer, although docetaxel-based chemotherapy is effective in disease control, it is thought to be too toxic. Therefore, triple combination chemotherapy would be better in disease control and prolongation of survival, but further study is needed to reduce the toxicity. As mentioned above, paclitaxel had less myelosuppressive effect than docetaxel (16, 17).
Previously, the combination chemotherapy with paclitaxel, cisplatin, and 5-FU was reported by Kim et al. (11) with at the following doses: paclitaxel 175 mg/m2 on day 1, 5-FU 750 mg/m2 by 24-hr continuous infusion on days 1-5, and cisplatin 20 mg/m2 on days 1-5, every 28 days and it showed promising report as triple chemotherapy with a high response rate of 51%, similar with our results. However, the response duration (17 weeks) and the median survival time about 6 months (26 weeks) seems disappointing. To make up for this point, we enhanced the dose intensity of chemotherapy. Compare with the previously used dosage, the doses of paclitaxel and 5-FU in our study were increased by 25% because the schedule was repeated every 3 weeks. Following the treatment, the response rate was similar, but the response duration and PFS were prolonged. Especially the PFS in this study was comparable to the OS previously reported. The PFS would be a more reasonable parameter in the evaluation of the chemotherapy because the OS could be affected by 2nd-line chemotherapy. Actually, 24 (58.6%) patients received the 2nd-line chemotherapy such as oral fluorouridine, irinotecan or oxaliplatin and it may be related with the gap between PFS and OS. Nonetheless, the prolongation of the response duration and the PFS were meaningful. It also demonstrated effectiveness in controlling carcinomatosis peritonei, frequently seen in advanced gastric cancer. In terms of toxicity, this regimen was fairly well tolerated. Greater than grade 3 neutropenia developed in 25% of total cycles and 54% of patients. Taken into consideration about 86% of patients who developed greater than grade 3 neutropenia in DCF chemotherapy (14), the frequency of neutropenia was decreased. The most common non-hematological toxicity was asthenia. Greater than grade 3 asthenia was developed in five patients, but these patients were able to receive further chemotherapy after resting about one week. Sensory neuropathy was seen in 55% of cycles, but most of these were grade 1 or 2. Neuropathy seemed to be associated with dose reduction, and no severe sensory neurotoxicity, including motor weakness, was observed.
In conclusion, combination chemotherapy with paclitaxel, cisplatin, and 5-FU at the dose used in this study is an effective regimen, and its safety may be useful for palliative treatment in patients with advanced gastric cancer. For more convenient regimen with reduced toxicity, further clinical trials on combination with oral fluorouracil are needed.
References
- 1.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with nonresectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 5.Comis RL, Carter SK. A review of chemotherapy in gastric cancer. Cancer. 1974;34:1576–1586. doi: 10.1002/1097-0142(197411)34:5<1576::aid-cncr2820340503>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813–3818. doi: 10.1002/1097-0142(19930615)71:12<3813::aid-cncr2820711205>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 9.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxnes-induced apoptosis. Curr Med Chem Anticancer Agents. 2003;3:291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 10.Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. A Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999;22:580–586. doi: 10.1097/00000421-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. [PubMed] [Google Scholar]
- 12.Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Herrmann R, Honegger H, Cavalli F, Alberto P, Castiglione M, Goldhirsch A. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO) Ann Oncol. 2000;11:301–306. doi: 10.1023/a:1008342013224. [DOI] [PubMed] [Google Scholar]
- 13.Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P. Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric cancer. 2002;5:142–147. doi: 10.1007/s101200200025. [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastoesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–5667. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 16.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, Parkin D, Paul J, Hay A, Kaye SB, Scottish Gynaecological Cancer Trials Group Phase III randomized trial of docetaxel-carboplain versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 17.Hsu Y, Sood AK, Sorosky JI. Docetaxel versus paclitaxel for adjuvant treatment of ovarian cancer: case-control analysis of toxicity. Am J Clin Oncol. 2004;27:14–18. doi: 10.1097/01.coc.0000045849.95834.6b. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, Cho EK, Shin DB, Lee JH. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006;17:225–229. doi: 10.1097/00001813-200602000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, Catalano G. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs. 1998;9:307–310. doi: 10.1097/00001813-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, Wanders J, Franklin H, LeBail N, Verweij J. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994;70:380–383. doi: 10.1038/bjc.1994.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, Benson AB., 3rd Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996;13:87–93. doi: 10.1007/BF02993858. [DOI] [PubMed] [Google Scholar]
- 22.Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA, Ettinger DS, Lubejko BG, Clark B, Sartorius SE. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991;9:1692–1703. doi: 10.1200/JCO.1991.9.9.1692. [DOI] [PubMed] [Google Scholar]