Abstract
Mediastinal (N2) lymph node involvement is heterogenous with huge variation in the extent and grouped together under stage IIIA. However, they showed a different survival even in the same stage. We tried to determine the prognostic implication of the multiple station N2 lymph node metastasis in stage IIIA N2 non-small cell lung cancer (NSCLC). The survival of stage IIIA N2 was analyzed according to the number of N2 station and their survival was compared with that of stage IIIB. In stage IIIA N2 NSCLC, multivariate analysis indicated that multiple station N2 was one of the independent prognostic factors for poor survival. The 5-yr survival of multiple station N2 IIIA (20.4%) was lower than that of single station N2 IIIA (33.8%) significantly (p=0.016). but when it was compared with that of stage IIIB (15.5%), there was no difference. Therefore, we suggest that multiple station N2 should be considered similar to stage IIIB disease with regard to predicting survival and accordingly should receive a new position in the TNM staging system.
Keywords: Lung Neoplasms; Lymph Node, Neoplasm Metastasis; Prognosis
INTRODUCTION
Nodal involvement is the most important prognostic factor in determining survival for patients with non-small cell lung cancer (NSCLC) (1, 2). However, conventional lymph node (LN) staging does not effectively reflect the wide differences in LN involvement and patient survival, even in patients classified within the same LN stage (3, 4). Furthermore, the characteristics of the mediastinal node (N2) are rather heterogeneous. Consequently, several subclassifications with regard to LN involvement have been proposed. Some investigators have also postulated the possible classification of a subgroup of multiple station N2 patients who have more unfavorable results compared to single station N2 patients with stage IIIA N2 NSCLC (3, 5-7).
In this retrospective study, we attempted to clarify the prognostic significance of multiple station N2 patients with stage IIIA N2 lung cancer and to propose an adequate TNM staging position for multiple station N2 disease by comparing the survival of multiple station N2 patients to the survival patients with stage IIIB disease.
MATERIALS AND METHODS
Between 1990 and 2005, 1,326 patients underwent operations for non-small cell lung cancer at our institution. Of these patients, 430 patients were diagnosed with either stage IIIA N2 or stage IIIB disease by pathological examination. Patients who received preoperative induction therapy (n=55) and those who died within 1-month postoperatively (n=17) were excluded from our study. Ultimately, a total of 358 patients were analyzed retrospectively. All patients underwent a thoracic computed tomography (CT) scan. Clinical staging was determined by bronchoscopy, chest CT, abdominal ultrasonogram, and bone scans. Lymph nodes with a shortest diameter of 10 mm or more were considered enlarged. The mediastinal node status was assessed according to the system defined by Mountain and Dresler (8). Systematic node dissection of the both hilar and mediastinal lymph nodes was performed in all patients except for in 14 open and closure cases and 2 wedge resection cases. Six cycles of cisplatin-based adjuvant chemotherapy were considered for all patients with stage II through stage III from the early 1990s to 2004. After 2005, four cycles of adjuvant chemotherapy were considered. Postoperative radiotherapy was considered for patients who had N2-positive and those who had positive resection margin. A Multiple station N2 was defined as lymph node metastsis involving more than one N2 station. Skip N2 was defined as metastasis-free hilar and intrapulmonary lymph nodes. The pathological staging was based on the 1997-TNM classification system (9).
The group was comprised of 75 female and 283 male patients, with a median age of 61 yr (range, 31 to 78 yr). 262 patients were classified with stage IIIA N2 disease and 96 patients were classified with stage IIIB disease. Among the stage IIIB patients, 14 patients were open and closure cases. Of these 14 patients, 10 cases did not have a proven pathological node stage. The median follow-up duration was 18.5 months (range, 1.2 to 184.2 months). All patients were followed up until either death or the last follow-up date (December 31, 2006). The patient characteristics are summarized in Table 1.
Table 1.
Clinical and pathological patient characteristics
*, Wedge resection in 2 patients and open and closure cases in 14 patients; †, large cell in 12, adenosquamous cell in 11, sarcomatoid carcinoma in 3 and neuroendocrine tumor in 5; ‡, 10 open and closure cases did not prove a pathological node factor.
The clinocopathological records of each patient with stage IIIA N2 disease were examined for prognostic factors such as age, sex, right or left side, type of surgical procedure (pneumonectomy or other procedure), histology, adjuvant chemotherapy, tumor location (upper or lower), T stage, metastatic node station (single or multiple station), and distribution of metastatic nodes (skip N2 or non skip N2). The survival rates of patients with stage IIIA N2 disease according to significant prognostic factors were compared with those of patients with stage IIIB disease. The Institutional Review Board granted us permission to retrospectively review and publish the patient records.
Statistical analysis
The association between variables was analyzed by either chisquare or analysis of variances (ANOVA) tests. The duration of survival was defined as the interval between the date of surgery and either the date of death or the last follow-up date. Survival rates were calculated using the Kaplan-Meier method, and univariate analyses were performed using the log-rank test. Multivariate analyses were performed by means of the Cox proportional hazard model in variables that had p values less than 0.05 as determined by univariate analyses. A p value less than 0.05 was considered significant.
RESULTS
Overall survival rate
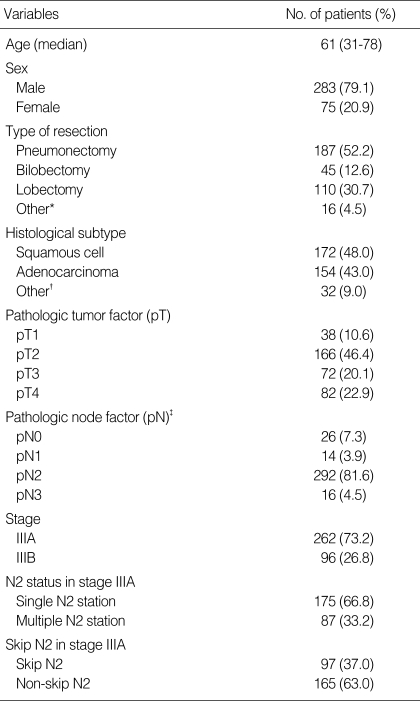
The overall 5-yr survival rates were 29.1% (95% confidence interval [CI], 23.6 to 34.6%) for patients with stage IIIA N2 disease and 15.5% (95% CI, 8.3 to 22.7%) for patients with stage IIIB disease, with median survival durations of 21.6 months (95% CI, 17.5 to 25.6 months) and 15.8 months (95% CI, 11.0 to 20.6 months), respectively. This difference in survival rates between the two patient groups was statistically significant (p=0.011) (Fig. 1).
Fig. 1.
Overall survival curves of patients with stage IIIA N2 and stage IIIB NSCLC.
Prognostic significance of multiple station N2 in patients with Stage IIIA N2 NSCLC
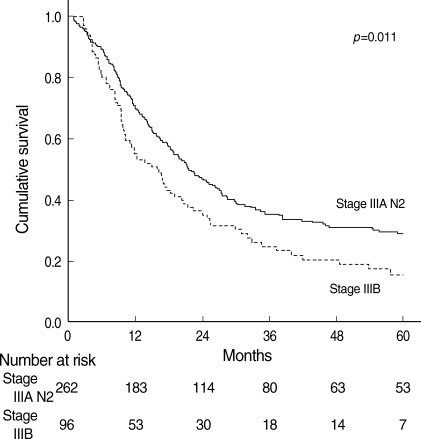
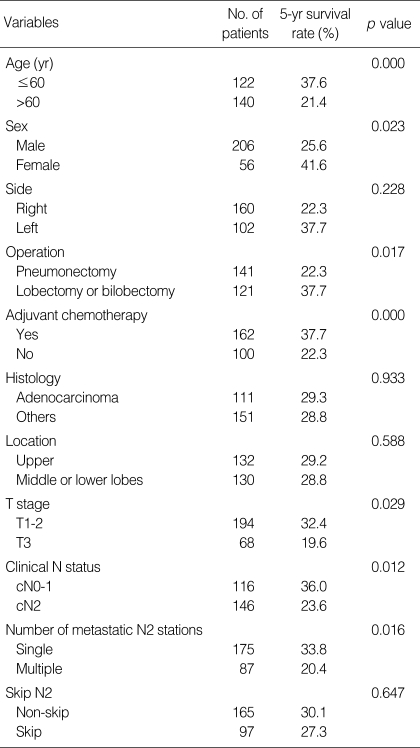
In stage IIIA N2 NSCLC, a univariate analysis using the variables listed in Table 2 showed that the following factors were significantly associated with poor outcome: age>60, male, T3 factor, pneumonectomy, clinical N2, no adjuvant chemotherapy, and multiple station N2. Using multivariate analysis, we found that the independent unfavorable prognostic factors were age>60, no adjuvant chemotherapy, and multiple station N2 (Table 3).
Table 2.
Prognostic factors in stage IIIA N2 patients by univariate analysis
Table 3.
Prognostic factors in N2 IIIA patients as determined by Multivariate analysis
Survival rate according to the number of metastatic N2 stations
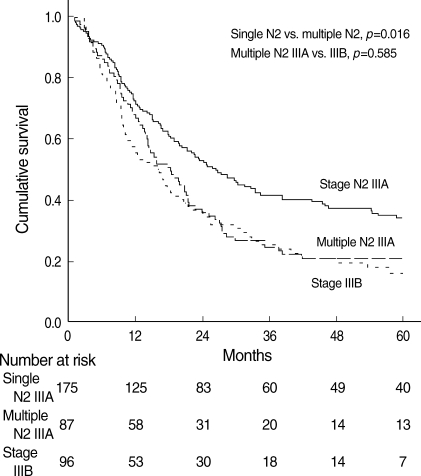
We evaluated the effect of the number of metastatic N2 stations upon stage IIIA patient survival. The overall 5-yr survival rates were 33.8% (95% CI, 26.8 to 40.8%) in single station N2 patients and 20.4% (95% CI, 13.3 to 27.5%) in multiple station N2 patients. The median survival times in single station N2 and multiple station N2 patients were 26.4 months (95% CI, 20.3 to 32.5 months) and 18.2 months (95% CI, 12.8 to 23.6months), respectively. The difference was statistically significant (p=0.016). Furthermore, the survival rate of multiple station N2 patients in stage IIIA was compared with the survival rate of stage IIIB patients, and we found no significant difference (p=0.585) (Fig. 2).
Fig. 2.
Overall survival curves of patients with single station N2, multiple station N2, and stage IIIB NSCLC.
Among the three groups, we found no difference in clinocopathological variables such as age, sex, histopathology, tumor size, number of resected LNs, or type of procedure performed (except for the 14 open and closure cases).
DISCUSSION
Our results indicate that the multiple station N2 criteria was one of the most important prognostic factors for poor outcome after surgery in patients with stage IIIA N2 NSCLC. Furthermore, we found that the multiple station N2 patients with stage IIIA had a survival rate that was similar to the stage IIIB patient survival rate.
Several factors, such as the clinical N2 factor, the number of metastatic N2 stations, T factor, tumor location, and skip N2, have been reported as important prognostic factors in patients with stage IIIA N2 cancer (7, 8, 10-13). Our uninvariate analysis study showed that the significant unfavorable prognostic factors were age>60, male, T3 factor, pneumonectomy, clinical N2, no history of adjuvant chemotherapy, and multiple station N2. Tumor location, histology, and skip N2 status showed no prognostic significance in this study. Furthermore, multivariate analysis confirmed that multiple station N2, age >60, and no history of adjuvant chemotherapy were significant, unfavorable prognostic factors. Several studies have shown that the number of metastatic mediastinal LNs was an important prognostic factor (14-16). Vansteenkiste and associates (17) reported that the 5-yr survival for patients with single station N2 was 29.6% versus 20.8% for those with multiple station N2 (p=0.008). In our study, the overall 5-yr survival rates were 33.8% in patients with single station N2 and 20.4% in patients with multiple station N2 (p=0.016). Besides the number of the metastatic mediastinal LNs, the clinical N factor and skip metastatis have also been considered prognostic factors in nodal factors (13, 17). In our study, patients with skip metastasis did not gain a survival benefit. In addition, clinical N2 patients showed significantly unfavorable clinical outcomes on univariate analysis but did not show a survival difference on to multivariate analysis. With these results, the number of the involved mediastinal LN stations can be recognized as a single and reliable finding that indicates highly advanced N2 lung cancer. The role of adjuvant chemotherapy is considered controversial, but recent clinical trials have begun to demonstrate the survival benefit of adjuvant chemotherapy in select cases of NSCLC patients. Recent guidelines generally recommend adjuvant chemotherapy for patients with completely resected stage IB through stage III NSCLC (18, 19). Our data support these results.
The current staging system for NSCLC has served us well for a number of years. This system has helped us design a treatment plan and discuss patient prognoses. However, the most complex and unsatisfactory aspect of the current TNM staging system is the method of assessing nodal disease. At present, mediastinal node diseases are grouped together under stage IIIA. However, there is a huge variation in the extent of N2 disease, ranging from incidental nodal metastasis to bulky multiple station, unresectable lymphadenopathy. Clearly, these variations in stage IIIA N2 disease have far-reaching implications with respect to both therapy and prognosis. Grouping all stage IIIA N2 disease into one stage is clearly inappropriate. Furthermore, we need formal recognition for a subclassification of stage IIIA N2 that considers variation. Previous proposals have called for such a change in N2 disease classification (3, 5, 6). The previous subclassifications of LN metastsis largely depended on the clinical node factor, which were then decided by the size of the LNs, but these classifications were not always correct because LN size is influenced by several inflammatory processes, such as tuberculosis and anthracosis (20, 21). In our study, clinical N2 did not have a statistical significance.
The number of metastatic mediastinal LNs is another prognostic factor that has been considered a subclassification indicator. Multiple station N2s have been accepted as one of the most important prognostic factors (14-17). In our study, multiple station N2 patients showed a significantly poor outcome. These results confirm the relevance of subclassification based on the number of lymph node stations involved. Therefore, stage IIIA N2 NSCLC patients should be classified into two basically distinct patient subgroups: single station N2 IIIA; multiple station N2 IIIA.
Furthermore, the survival outcome in the present study showed no difference between multiple station N2 IIIA and stage IIIB patients. Andre and associates (5) reported that the prognosis of clinical N2 and multiple station N2 patients with stage IIIA disease was close to the prognosis of stage IIIB disease patients. According to these results, we suggest that multiple station N2 disease should be considered similar to stage IIIB disease for predicting patient survival and therefore that multiple station N2 disease should receive a new position in the TNM staging system.
In our study, we compared multiple station N2 with stage IIIB for survival, but a better comparison might be between multiple station N2 and N3 disease as a subgroup of stage IIIB NSCLC. Pathologically proven N3 was not available in surgically resected patients because these patients are not usually indicated for surgery. In our institution, we treated only 16 patients with N3 who underwent surgery and then were proven to be N3 after the operation. Since this number of patients was not enough to compare, we included all surgically proven stage IIIB patients.
In conclusion, multiple station N2 was one of the most important single prognostic factors indicating a poor survival rate in pathological stage IIIA N2 NSCLC patients. We found no difference in survival between multiple station N2 stage IIIA and stage IIIB patients. Therefore, we suggest that multiple station N2 disease should be considered similar to stage IIIB disease for predicting patient survival and that these criteria should receive a new position in a new TNM staging system.
References
- 1.Naruke T, Tsuchiya R, Kondo H, Asamura H. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg. 2001;71:1759–1764. doi: 10.1016/s0003-4975(00)02609-6. [DOI] [PubMed] [Google Scholar]
- 2.Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stage II and IIIA non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70:358–366. doi: 10.1016/s0003-4975(00)01673-8. [DOI] [PubMed] [Google Scholar]
- 3.Grunenwald D, Le Chevalier T. Re: Stage IIIA category of non-small cell lung cancer: a new proposal. J Natl Cancer Inst. 1997;89:88–89. doi: 10.1093/jnci/89.1.88-a. [DOI] [PubMed] [Google Scholar]
- 4.Pearson FG, Delarue NC, Ilves R, Todd TR, Cooper JD. Significance of positive superior mediastinal nodes identified at mediastinoscopy in patients with resectable cancer of the lung. J Thorac Cardiovasc Surg. 1982;83:1–11. [PubMed] [Google Scholar]
- 5.Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 6.Ruckdeschel JC. Combined modality therapy of non-small cell lung cancer. Semin Oncol. 1997;24:429–439. [PubMed] [Google Scholar]
- 7.Osaki T, Nagashima A, Yoshimatsu T, Tashima Y, Yasumoto K. Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung cancer. 2004;43:151–157. doi: 10.1016/j.lungcan.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 9.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe Y, Hayashi Y, Shimizu J, Oda M, Iwa T. Mediastinal nodal involvement and prognosis of non-small cell lung cancer. Chest. 1991;100:422–428. doi: 10.1378/chest.100.2.422. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, Watanabe Y, Mitsudomi T, Yoshimura M, Tsuboi M, Japanese Clinical Oncology Group Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg. 2001;122:803–808. doi: 10.1067/mtc.2001.116473. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Sawabata N, Takeda S, Ohta M, Ohno Y, Maeda H. Results of surgical intervention for p-stage IIIA (N2) non small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J Thorac Cardiovasc Surg. 2004;127:1100–1106. doi: 10.1016/j.jtcvs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Riquet M, Assouad J, Bagan P, Foucault C, Barthes F, Dujon A, Danel C. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg. 2005;79:225–233. doi: 10.1016/j.athoracsur.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 14.Martini N, Flehinger B. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037–1049. doi: 10.1016/s0039-6109(16)44341-0. [DOI] [PubMed] [Google Scholar]
- 15.Naruke T, Goya T, Tsuchiya R, Suemasu K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg. 1988;46:603–610. doi: 10.1016/s0003-4975(10)64717-0. [DOI] [PubMed] [Google Scholar]
- 16.Mountain CF. Surgery for stage IIIa-N2 non-small cell lung cancer. Cancer. 1994;73:2589–2598. doi: 10.1002/1097-0142(19940515)73:10<2589::aid-cncr2820731021>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Vansteenkiste JF, Leyn PR, Deneffe GR, Lerut TE, Demedts MG. Clinical prognostic factors in surgically treated stage IIIA-N2 nonsmall cell lung cancer: analysis of the literature. Lung Cancer. 1998;19:3–13. doi: 10.1016/s0169-5002(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 18.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, Bethune D, Ayoub J, Ding K, Seymour L, Graham B, Tsao MS, Gandara D, Kesler K, Demmy T, Shepherd F, National Cancer Institute of Canada Clinical Trials Group. National Cancer Institute of the United States Intergroup JBR.10 Trial Investigators Vinorelbine plus cisplatin vs observation in resected non-smallcell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 19.Douillard J, Rosell R, Delena M, Legroumellec A, Torres A, Carpagnano F. ANITA: Phase III adjuvant vinorelbine and cisplatin versus observation in completely resected (stage I-III) non small cell lung cancer (NSCLC) patients: Final results after 70-month median follow-up, On behalf of the Adjuvant Navelbine International Trialists Association. J Clin Oncol. 2005;23:624. (Suppl; abstr 7013) [Google Scholar]
- 20.Murray JG, O'Driscoll M, Curtin JJ. Mediastinal lymph node size in an Asian population. Br J Radiol. 1995;68:348–350. doi: 10.1259/0007-1285-68-808-348. [DOI] [PubMed] [Google Scholar]
- 21.Arita T, Matsumoto T, Kuramitsu T, Kawamura M, Matsunaga N, Sugi K, Esato K. Is it possible to differentiate malignant mediastinal nodes from benign nodes by size? Reevaluation by CT, transesophageal echocardiography and nodal specimen. Chest. 1996;110:1004–1008. doi: 10.1378/chest.110.4.1004. [DOI] [PubMed] [Google Scholar]