Abstract
Mechanically stressed cells display increased levels of fos message and protein. Although the intracellular signaling pathways responsible for FOS induction have been extensively characterized, we still do not understand the nature of the primary cell mechanotransduction event responsible for converting an externally acting mechanical stressor into an intracellular signal cascade. We now report that plasma membrane disruption (PMD) is quantitatively correlated on a cell-by-cell basis with fos protein levels expressed in mechanically injured monolayers. When the population of PMD-affected cells in injured monolayers was selectively prevented from responding to the injury, the fos response was completely ablated, demonstrating that PMD is a requisite event. This PMD-dependent expression of fos protein did not require cell exposure to cues inherent in release from cell–cell contact inhibition or presented by denuded substratum, because it also occurred in subconfluent monolayers. Fos expression also could not be explained by factors released through PMD, because cell injury conditioned medium failed to elicit fos expression. Translocation of the transcription factor NF-κB into the nucleus may also be regulated by PMD, based on a quantitative correlation similar to that found with fos. We propose that PMD, by allowing a flux of normally impermeant molecules across the plasma membrane, mediates a previously unrecognized form of cell mechanotransduction. PMD may thereby lead to cell growth or hypertrophy responses such as those that are present normally in mechanically stressed skeletal muscle and pathologically in the cardiovascular system.
INTRODUCTION
Mechanical stress can positively regulate the expression of a number of genes (Komuro and Yazaki, 1993; Nerem, 1993, Skalak and Price, 1996). FOS is perhaps the most commonly reported and well studied of these. For example, fos mRNA and/or protein is known to increase when cardiac myocytes are stretched in vitro or in the intact heart (Sadoshima et al., 1992), when skeletal muscle is exercised (Dawes et al., 1995), when fibroblasts contract a collagen gel in vitro (Rosenfledt et al., 1998), and when cultured endothelial or other cell monolayers are injured by scrape removal of narrow zones of cells (Verrier et al., 1986). FOS and other mechanical stress-inducible genes, such as those coding for basic fibroblast growth factor (Ku and D’Amore, 1993), cyclo-oxygenase, platelet-derived growth factor, ICAM-1, and nitric oxide synthase (Topper et al., 1996), appear to promote tissue hypertrophy, repair, and other adaptive responses, a speculation in keeping with the known biological functions of these polypeptides. Importantly, activation of genes by mechanical stress may sometimes lead to maladaptive hypertrophy, such as occurs in restenosis after arterial ballooning (Bauters et al., 1992), or in pathological enlargement of the heart in hypertensive individuals (Mayer and Rubin, 1995).
A fundamental unanswered question concerns how injured cells sense and convert the physical stimulus inherent in a mechanical stressor into a chemical signal that, in turn, activates intracellular signal cascades. Given the variable magnitude of the forces that cells may encounter in vivo, approximately newtons to piconewtons (Gooch and Tennant, 1997), and the complex spatial nature of these forces in relation to diverse cell architecture, it seems unlikely that any single mechanism can explain all mechanotransduction events.
Two “mechanotransduction” hypotheses attempt to explain how externally imposed mechanical stress leads to gene activation. First, stretch-activated plasma membrane ion channels could be responsible (Hamill and McBride, 1993, 1996); however, in many nonsensory cell types that display mechanotransduction potential, such channels have been difficult to identify. For example, the fos response of the cardiac myocyte to stretch was examined extensively in light of this possibility, but no evidence in support of stretch-activated channel mediation was found (Sadoshima et al., 1992; Sadoshima and Izumo, 1993). A second hypothesis, presented in this article, posits that mechanical stress leads to tearing of the cell plasma membrane and consequent signal generation. A plasma membrane disruption (PMD)1 allows influx and efflux of normally impermeant molecules, such as Ca2+ and growth factors, down steep concentration gradients. Therefore it is predicted that multiple signal cascades will be initiated by PMD.
Plasma membrane continuity is not necessarily a constant feature of the life of the normal, healthy cell, and a disruption in continuity does not invariably lead to cell death. In fact, the plasma membrane is vulnerable to mechanically induced “wear and tear,” as has now been documented in numerous studies of mechanically active tissues (McNeil and Steinhardt, 1997). The cells of such tissues, which include skeletal muscle, cardiac myocyte, and endothelial cells, normally and frequently experience disruptions in plasma membrane integrity (McNeil and Khakee, 1992; Yu and McNeil, 1992; Clarke et al., 1995). Cells moving in culture periodically tear off small pieces of cytoplasm and hence tear their plasma membrane, because their trailing end is drawn out into long retraction fibers that eventually break (Chen, 1981; Galbraith and Sheetz, 1997). Pathological levels of mechanical stress can exacerbate, of course, these constitutive levels of cell “wounding.” An active, complex resealing mechanism rapidly (1–5 s) repairs disruptions, preventing influx of potential toxins such as Ca2+ and loss of vital cytosolic constituents (Steinhardt et al., 1994; Bi et al., 1995; Terasaki et al., 1997). Indeed, many cells survive surprisingly large membrane disruptions. Skeletal muscle cells and certain free-living amoebae, for example, survive after being cut in half, and sea urchin eggs can be fertilized and undergo cleavage after a 20 × 40 μm2 patch of plasma membrane and underlying cortex is ripped from their surface (Terasaki et al., 1997).
In the present study, we have rigorously tested the hypothesis that a form of mechanically induced cell damage, PMD, can regulate expression of fos protein. Our analysis, which pertains to several widely different cell types, suggests that a flux of molecules entering or leaving cytosol through a survivable PMD constitutes the initiating step of a previously unrecognized mode of mechanotransduction. This “damage sensor” hypothesis may explain how cells initiate appropriate adaptational responses to injurious but not necessarily cell-lethal or tissue-disruptive levels of mechanical stress.
MATERIALS AND METHODS
Cell Culture
Bovine aortic endothelial (BAE) cells were purchased (Clonetics, Pittsburgh, PA), as were NIH 3T3 cells (American Type Culture Collection, Bethesda, MD). Human umbilical vein endothelial cells and human umbilical vein smooth muscle cells (HUSMCs) were the kind gift of Dr. Carlos Isales (Medical College of Georgia, Augusta, GA). Cells were cultured in DME containing 10% fetal bovine serum and penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere, except for BAE cells, which were grown in endothelial growth medium (Clonetics). Media and supplements used in the maintenance of these cells were obtained from Life Technologies (Grand Island, NY). Cultures were passaged at confluence with trypsin–EDTA in HBSS (Life Technologies). Cells were plated onto 22-mm-square glass coverslips (Fisher, Pittsburgh, PA) for monolayer injury experiments and used 24–48 h later.
Monolayer Injury Protocol
Fluorescein-labeled, lysine-fixable dextran (FDx) of 10,000 Mr (Molecular Probes, Eugene, OR) was dissolved at a concentration of 3–10 mg/ml in PBS. All monolayers were rinsed with three volumes of PBS at 37°C. The above FDx solution at 37°C was added to the monolayers, and the cells were injured by slowly scratching the coverslips multiple times with a sterile 30-gauge hypodermic needle from Becton-Dickinson (Rutherford, NJ), which removed a two to four cell-wide swath. Cultures were allowed to stand for approximately 3 min and were washed with three volumes of PBS at 37°C. The coverslips were returned to normal culturing conditions for an interval ranging from 1 to 4 h. In additional experiments, a protein synthesis inhibitor, gelonin (Pierce Chemical, Rockford, IL), was added to the above FDx solution at a final concentration of 25 μg/ml, or cells were washed into iso-osmolar (to PBS) NaCl-based, HEPES (10 mM)-buffered saline containing 50 mM Ca2+ before initiation of the scratch injury.
Immunostaining
Coverslips containing wounded and control monolayers were fixed in 4% formaldehyde for 10 min at room temperature. Cells were then rinsed with three volumes of PBS and permeablized in 0.1% Triton X-100 (Sigma Chemical, St. Louis, MO) for 1 min or by 15 s immersion in cold acetone. After an additional washing with PBS (containing 1% BSA), monolayers were incubated with a polyclonal antibody to c-fos protein (Santa Cruz Biotechnology, Santa Cruz, CA) or NF-κB (Santa Cruz Biotechnology) diluted 1:100 in PBS containing 1% bovine serum albumin at 37°C or room temperature for 1 h. Cells were then incubated with a biotinylated goat anti-rabbit IgG (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA) for 0.5–1 h. Finally, the coverslips were exposed to Texas Red/Avidin (Vector Laboratories) at a concentration of 10 μg/ml for 15 min and mounted onto glass slides using ProLong (Molecular Probes). Fluorescein and rhodamine fluorescence images were taken on Kodak slide film using a Zeiss Photomicroscope II (Zeiss, Oberkochen, West Germany) equipped with Zeiss Neofluor 16, 20, and 40× phase-contrast objectives.
Image Analysis
Fluorescent images were acquired from a Zeiss Photomicroscope II through a SIT-camera (Hamamatsu, Japan) and stored on an optical disk drive for further analysis. Individual cells along the wound edge were chosen at random for collection of data. This included both undisturbed cells and those that suffered a plasma membrane disruption. Image analysis software (Universal Imaging or NIH image) was used to evaluate the intensity in digitized images of fluorescein and rhodamine fluorescence in individual cells along the denuded zone. Microscope illumination intensity and video camera black and gain levels remained constant for analysis of all monolayers under comparison. Using the mouse, a binary template (∼10 × 10 pixel box) was positioned over the digitized fluorescent image of each cell. The light intensity level was measured on a scale ranging from 1 (black) to 255 (white). The digitized fluorescent images of control and experimental groups were quantitatively analyzed for fos expression and PMD events using the above method, and the results were recorded for statistical analysis.
PMD-Conditioned Medium
BAE cells (9 × 106) were trypsinized and washed with PBS. A 1 ml suspension of the cells was then taken up into and expelled from a tuberculin syringe fitted with a 30-gauge needle using an automated apparatus (Clarke et al., 1994) that controls ejection/intake pressure (40 psi). After the fourth uptake into the syringe, these shear-wounded cells were either (0.5 ml) expelled by hand directly onto monolayer coverslips for fos analysis or (remaining 0.5 ml) centrifuged to remove cells and debris. This latter conditioned medium was then applied to a second set of coverslips ∼2 min after the last syringe movement. The monolayer coverslips, containing BAE cell cultures, injured as described above ∼5 min before its receipt, were exposed to the conditioned medium, or PBS only, for 5 min before being washed thoroughly in PBS and returned as above to normal culturing conditions. The fos response was then analyzed as above for duplicate coverslips exposed to each condition.
RESULTS
Fos mRNA and protein content in injured cell monolayers have been analyzed by Northern, Western, and immunolocalization approaches. Only the last of these allows a cell-by-cell correlation of fos expression directly with an individually variable cell-signaling event, such as PMD. Therefore we developed a microscopic method that uses quantitative image analysis for measuring the magnitude of both fos protein expression and PMD events on a cell-by-cell basis.
Colocalization of Fos Expression and PMD in the Injured Monolayer
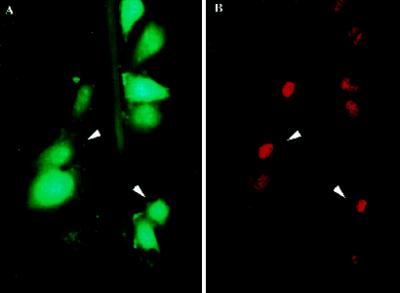
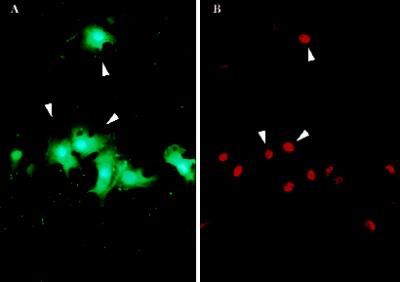
Narrow zones of fibroblasts and endothelial and smooth muscle cells were denuded from confluent monolayers by scratching substrata with a sharp implement in the presence of a marker for survivable PMD (FDx). FDx (Mr, ∼10 kDa) enters only those cells that incur a PMD. It is retained only in “wounded” cells that successfully reseal. Next, cultures were fixed 3 h later and immunostained to reveal fos protein localization. We observed, as in previous reports (Verrier et al., 1986; Sosnowski et al., 1993), that fos protein in these cultures was localized to cells lining the denudation tracts (Figure 1B). PMD events too were localized to this same general subpopulation (Figure 1A). This suggested that PMD events might be the trigger for fos expression.
Figure 1.
Fos protein expression and PMD events apparently occur within the same discrete subpopulation of cells in the injured monolayer, namely those immediately lining the denudation zone. (A) Many (arrowheads indicate two examples), but not all, BAE cells lining the scratch site (green steak) are labeled in their cytosol with the PMD marker, FDx. (B) In a paired image, this same general subpopulation (arrowheads) is seen to have up-regulated fos protein expression, as indicated by the increased immunostaining of nuclei with a fos antibody. This monolayer was fixed 3 h after injury by scratching of the plastic substratum with a sharp implement in the presence of FDx and then immunostained and photographed.
Quantitative Relationship between Fos Expression and PMD
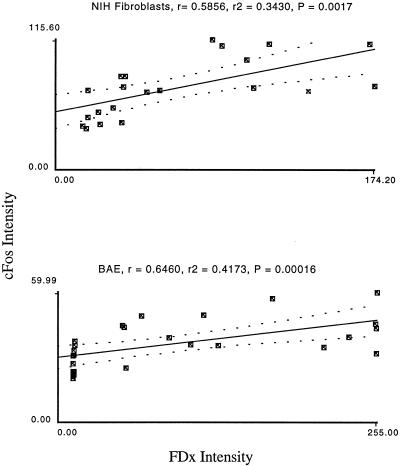
To determine whether, in addition to the obvious subpopulation correlation of PMD and fos expression events, these two parameters were quantitatively correlated at the individual cell level, we used image analysis to measure fos protein and PMD levels on a cell-by-cell basis. For fibroblasts and endothelial and smooth muscle cells, we found a quantitative correlation (p values ranging from 0.002 to <0.0001) between these two variables, suggesting a functional connection between them (Figure 2).
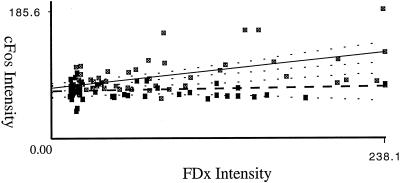
Figure 2.
Fos immunostaining intensity is quantitatively related to membrane disruption severity in the cell population lining denuded substratum. Depicted are typical plots of fos immunostaining intensities as a function of PMD extent (FDx Intensity) in an injured monolayer of NIH fibroblast cells and of BAE cells. For each plot, the linear regression coefficient R, R2, and an ANOVA p value are given. This last value tests the null hypothesis that the two parameters are not significantly related.
Lack of a Requirement for Exposure to Bare or Denuded Substratum
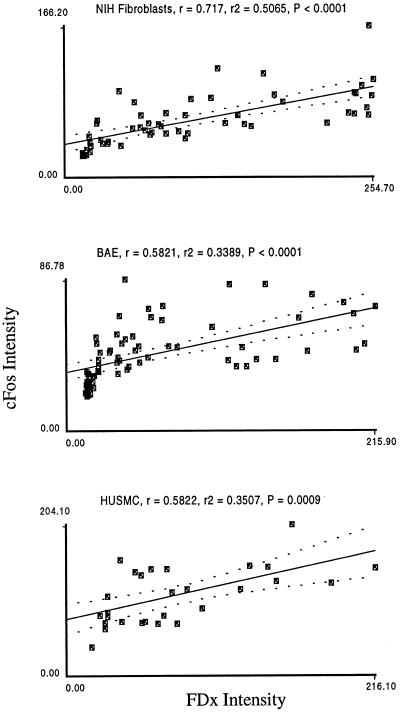
Another possible stimulus for fos expression in the monolayer injury model is exposure of the cells to the denuded substratum. In this interpretation (Heimark and Schwartz, 1985), the cells are responding to release from cell–cell contact inhibition and/or some aspect of the denuded substratum. To determine whether this type of stimulus was required in addition to PMD to induce fos expression, we injured subconfluent (∼80% substratum coverage) monolayers in which all the cells were exposed to bare substratum. In the absence of injury, fos expression was present at low levels (our unpublished results), but as in the case of confluent monolayers, injury potently stimulated fos expression that was highly correlated with PMD (Figure 3). This shows that there is no requirement for cues from the substratum or contact with other cells for the fos response to occur.
Figure 3.
In subconfluent monolayers, fos immunostaining intensity is also quantitatively related to membrane disruption severity. Depicted are typical analyses of fos expression as a function of PMD extent in subconfluent monolayers of NIH fibroblasts, BAE cells, and HUSMC.
Direct Evidence that PMDs Are Required for Expression
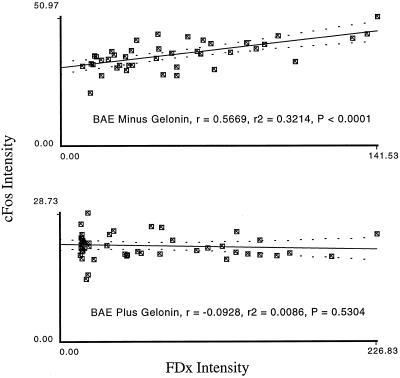
Because the above data are correlative only, and because the measured correlation was not absolute, this does not, by itself, establish that a PMD-event is required for fos expression in the mechanically injured monolayer. Fos expression in the injured monolayer might be activated by other, PMD-independent mechanisms, for example, stretch-activated Ca2+ channel activity. One strategy for distinguishing between these two possibilities is to prevent PMD-affected cells, but not other mechanically stressed (stretched, compressed, etc.) cells, from responding, and then to determine how the fos response is affected. We devised two independent ways of accomplishing this. In the first, confluent monolayers of bovine aortic endothelial cells were injured as above except that in addition to the FDx marker of PMD, the protein synthesis inhibitor gelonin was added to the saline present during injury. Gelonin, a protein of ∼40,000 Mr, can only exert its effect, via proteolytic inactivation of ribosomes, in cytosol and can only gain access to this domain when a membrane barrier has been breached (Lambert et al., 1988). Injury in the presence of gelonin completely inhibited the fos response (Figure 4).
Figure 4.
Gelonin that enters the cell through plasma membrane disruptions abolishes fos protein expression in the injured monolayer. Confluent monolayers of BAE cells were injured as above, except that in addition to the FDx, the protein synthesis inhibitor gelonin was added as well (lower panel). Gelonin is a protein (Mr, ∼40,000 ) that can only exert its effect in cytosol and can only gain access to this domain when a membrane barrier has been breached. Thus, cells in culture that do not experience a PMD are not affected by gelonin, even when it is present at high (mg/ml) concentrations and despite the fact that one gelonin molecule is enough, when present in cytosol, to completely abolish protein synthesis. Controls (upper panel) received dextran only (no gelonin).
To rule out the possibility that gelonin was inhibiting cells that had not incurred PMD, we next injured monolayers first in the presence of gelonin. Then, making additional scratches on the same coverslip but in a direction perpendicular to the first set, we injured the monolayer a second time after washing away external gelonin. Fos expression was entirely absent along the scratch sites created in the presence of the inhibitor but displayed the expected quantitative correlation with PMD along the scratch site created in its absence (Figure 5).
Figure 5.
Gelonin entry through a plasma membrane disruption, and not merely cell exposure to gelonin, is required for inhibition of fos expression. Coverslips containing human umbilical vein endothelial cells were scratched multiple times in two sets of perpendicular directions. During one set, gelonin was present (filled squares); during the second set, it had been washed away and was replaced with an FDx-only saline (open squares). Thus, some cells suffered disruptions in the presence of gelonin (filled squares), and this inhibitor could enter cytosol of individual cells in this population. Another subpopulation was exposed to gelonin (open squares), but direct access to cytosol was not induced until after gelonin had been removed. Such cells could take up gelonin only by other mechanisms that, theoretically, might lead to its toxic effect on protein synthesis, such as endocytosis. Then the fos and dextran intensities were determined as above for both sets of scratches.
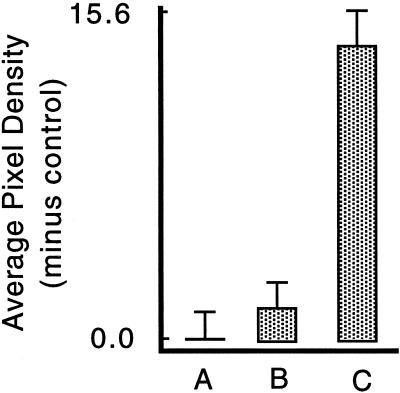
As a second test of the requirement for PMD, monolayers were injured in medium containing elevated Ca2+ (50 mM in iso-osmolar saline). Cells labeled with the wound marker FDxLys were absent along the injury site created in high Ca2+ (our unpublished results), showing that this treatment eliminated PMD-affected cells, presumably because excessive Ca2+ entry through a PMD is toxic. The fos response was absent in the presence of supranormal Ca2+: it was not significantly different from the undisturbed control culture value (p = 0.3933), whereas it was significantly (p < 0.0001) reduced relative to the cultures scratched as usual in PBS (1.5 mM Ca2) (Figure 6). Thus two independent lines of experimentation—involving gelonin and elevated Ca2+—demonstrate that in the absence of a PMD-affected cell population (high Ca2+) or the functional absence (gelonin), fos expression does not occur in mechanically injured monolayers. Because other cells were mechanically stressed in these monolayers, but not to the point of PMD, these results rule out other mechanisms, such as stretch activation of Ca2+ channels, as an important factor. Indeed, the fact that there was no fos response in the presence of elevated Ca2+ is incompatible with fos activation by a stretch-activated Ca2+ channel mechanism. Such a mechanism predicts that activation will increase rather than decrease under conditions of elevated Ca2+.
Figure 6.
Fos expression is inhibited by elevated extracellular Ca2+. BAE cell monolayers were washed into PBS saline (A, C), or iso-osmolar NaCl, HEPES-buffered saline containing 50 mM Ca2+ (B), both of which contained FDx. Five minutes later, all except two control PBS-washed cultures (A) were scratched in these salines, and the fos expression level was quantitated in every cell encountered along scratch zones or at random in the undisturbed cultures (A). The high calcium treatment eliminated FDx-positive cells along the scratch zone (our unpublished results) and hence selectively eliminated PMD-affected cells from the responding monolayer. Therefore, unlike in other experiments, we could not correlate FDx and c-fos intensities in this analysis because the cultures injured in high Ca2+ entirely lack FDx-positive cells. The values shown represent the mean and SEs of the fos intensity measured by image analysis from ∼150 cells on replicate coverslips after subtraction of the mean value measured from an equivalent number of control, undisturbed cells (A), which represents “autofluorescence” background. The fos response of the elevated Ca2+ cultures (B) was not significantly different from the control, undisturbed culture (A) (p = 0.3993), but was significantly reduced relative to the PBS (C) cultures (p < 0.0001).
Direct Evidence That a Diffusible Enhancer Is Not Released through PMD
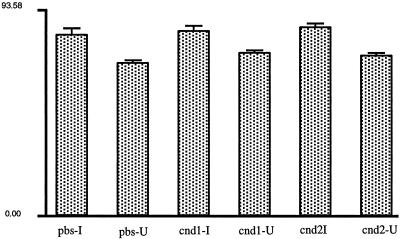
To examine the question of whether the signal generated by PMD was one that was released or that entered through a PMD, we collected medium conditioned by the mechanically injured monolayer and determined whether it was capable, by itself, of stimulating fos expression. We produced such a PMD-conditioned medium by syringing (Clarke et al., 1994) a large number of endothelial cells (9 × 106 in 1 ml of PBS), a technique that efficiently generates PMDs and, because it is performed on the trypsinized cells, eliminates the confounding variable produced by a scrape injury of conditioning the medium with disrupted extracellular matrix as well. We immediately placed this conditioned medium onto test monolayers, injured by scratching in the usual way. The fos expression response along the injury site, and also in undisturbed regions of the monolayer, were then measured. Compared with controls that received the saline vehicle only, no significant increase in fos protein levels was detected in undisturbed portions of experimental monolayers that received conditioned medium. This is direct evidence that release of a soluble factor is not involved in PMD-induced fos expression, suggesting that signal entry is responsible. The fos response along the scratch sites was also unchanged by the conditioned medium (Figure 7). This rules out the possibility that an inhibitor, potentially released as a result of syringing a large number of cells, was released and inhibited the normal fos response to injury.
Figure 7.
PMD-conditioned medium fails to stimulate fos expression. Approximately 9 × 106 BAE cells suspended in 1 ml of PBS were taken up into and expelled from a syringe fitted with a 30-gauge needle for a total of four complete passes. At the end of the fourth pass, these shear-injured cells, most of which have incurred PMD, were either (0.5 ml) expelled directly onto replicate coverslips containing a subconfluent monolayer of BAE cells (cnd1) or (the remaining 0.5 ml) were centrifuged to remove cells and debris, and the resulting supernatant was applied to separate monolayers (cnd2). Controls (pbs) received the saline vehicle only. The fos immunostaining was then measured along the scratch injury site of replicate treatment group monolayers or in undisturbed regions of treatment group monolayers. Plotted are the mean fos staining intensities, and the SEs of measurements of 100–150 cells in each region (injured, I; undisturbed, U) of each treatment group. Analysis of each treatment group revealed the expected highly significant difference (all values, p < 0.0002) in the fos response of cells along the injury zone compared with that in cells in undisturbed zones; however, between the different treatment groups, we failed to detect a significant difference in either zone of cells. Error bars represent SEM.
Evidence That NF-κB Activity also May Be Induced by PMD
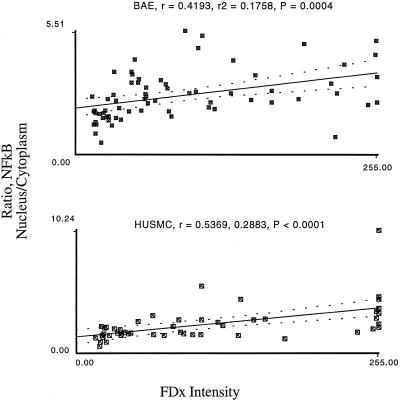
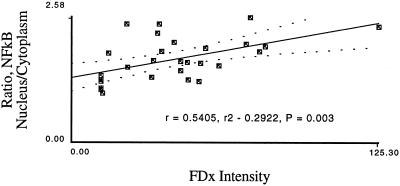
NF-κB, a transcriptional activator (Wulczyn et al., 1996), is translocated into the nuclei of mechanically injured endothelial and smooth muscle cells (Figure 8). To determine whether, like the fos expression event, the NF-κB translocatory event also is regulated by PMD, we applied image analysis techniques. We found that in endothelial and smooth muscle cells, but not fibroblasts, NF-κB translocation events show a strong correlation with PMD events (Figure 9). This result suggests that gene expression regulation will be differentially affected based on responsive cell type. Subconfluent HUSMC also showed a strong correlation between NF-κB translocation and PMD (Figure 10), suggesting that, as with fos, NF-κB activation does not required a release from contact inhibition event.
Figure 8.
NF-κB translocation into the nucleus is also induced along monolayer injury sites where PMD is common. (A) A confluent monolayer of BAE cells was scratched in FDx. As in Figure 1, cells incurring a survivable PMD are labeled with this marker. (B) A paired image showing immunostaining for NF-κB. Note the correspondence of PMD and NF-κB events, as was the case for the fos event.
Figure 9.
The ratio of NF-κB nuclear/cytoplasmic staining intensity is quantitatively related to PMD severity. Cell monolayers (BAE and HUSMC) were injured and analyzed for PMD and immunostaining intensity as in the fos experiments above, except that two measurements are made of NF-κB intensity, one over the nucleus and the other over an adjacent area of cytoplasm. Then an intensity ratio (nucleus/cytoplasm) is calculated and plotted as a function of FDx intensity.
Figure 10.
In subconfluent monolayers the ratio of NF-κB nuclear/cytoplasmic staining intensity is also quantitatively related to membrane disruption severity. Subconfluent monolayers of smooth muscle cells were analyzed for NF-κB staining as a function of FDx (wound) intensity.
DISCUSSION
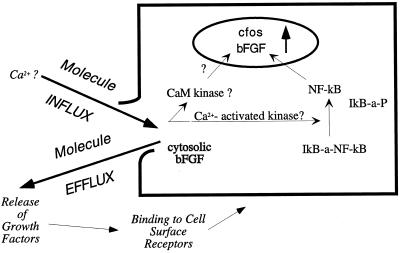
We demonstrate that PMD is quantitatively related to, and required for, fos protein expression in mechanically injured monolayers. Fos expression scaled directly on a cell-by-cell basis with the extent of PMD injury, and when PMD-affected cells were selectively removed from the injured monolayer, fos expression was absent. We propose a damage sensor model for cell mechanotransduction. Unlike other models, it makes no requirement of a cell that it possess a specialized molecular apparatus for converting a physical force into a chemical signal. Instead, the crucial transduction event is a form of cell damage, a resealable disruption in the cell’s external-most permeability barrier that generates a potent flux of normally impermeant molecules that might initiate any of a number of well characterized intracellular signaling pathways (Figure 11).
Figure 11.
The damage sensor hypothesis explains how injurious mechanical stress is transduced into intracellular signaling events. Flux of molecules through a PMD signal that a mechanically injurious event has occurred locally and elicit changes in gene expression in the injured cell, acting ultimately to promote tissue adaptation to the mechanical stress that caused the cell injury. One candidate signal for changes in gene expression is Ca2+ influx, although this remains to be demonstrated experimentally. Efflux of an unknown, cytosolic inhibitor of fos expression is also consistent with the data of this paper. Early changes in gene expression are driven by an increase in fos expression and translocation of NF-κB into the nucleus. The suggestion that bFGF expression increases as a function of PMD is based on published data (Ku and D’Amore, 1993). Release of such growth factors as bFGF through PMD may also promote local adaptive responses, in both injured and neighboring cells.
Given the simplicity of the mechanism proposed, mechanotransduction by damage sensing could operate in a wide variety of cell types and over a wide range of stress levels, an important feature in light of the wide range of cell types and stresses known to elicit mechanotransduction events in vivo. Is then PMD a common event under normal conditions in vivo? Using special staining techniques that reveal the occurrence of survivable PMDs, it has been shown that in several tissues under physiological conditions, including endothelium, skeletal, cardiac muscle, and skin (McNeil, 1993), wounded cells are present at high levels in the general population and that the level of cell wounding appears to scale with imposed mechanical load. Moreover, imposition of mechanical load beyond normal levels, e.g., into the “pathological” range, is predicted and demonstrated to increase “constitutive” levels of PMD cell injury in vivo.
Indeed, many studies have documented fos expression in intact tissues under conditions similar to those known also to generate PMD events. Examples are the exercise of skeletal muscle (Osbaldeston et al., 1995), ballooning and other forms of mechanical injury to the arterial wall (Bauters et al., 1992), contraction by fibroblasts of collagen gels (Rosenfeldt et al., 1998), and stretch or pressure overload of cardiac muscle (Schunkert et al., 1991) and constitutively in skin (Basset-Seguin et al., 1990). Fos expression is induced, moreover, under various conditions in which PMD has not been investigated but might occur. For example, in smooth muscle cells of the distended bladder wall (Chen, 1981), in chondrocytes exposed to compressive forces (Closs et al., 1990), and in endothelial cells exposed to shear stress (Hsieh et al., 1993).
The fact that gross cell injury is not detectable in biological and pathological situations of mechanical stress does not mean that PMD has not occurred. PMD is a survivable injury that is not efficiently detected by the methods commonly used to define cell death, such as trypan blue or similar dye exclusion tests or lactate dehydrogenase release. After resealing, vital dyes will not stain cells that survived a PMD. Because a survivable PMD generally remains open for < ∼5 sec, it may not lead to release of significant quantities of large proteins, such as lactate dehydrogenase. Recent studies have shown that even under well controlled in vitro conditions of mechanical stress in which cell death is not detected, for example in cell stretching protocols, survivable PMD is detectable if appropriate methods are used for its detection (Cheng et al., 1996; Clarke and Feeback, 1996). The results of this study suggest that whenever mechanical stress is applied to a cell, it is important in interpreting the mechanism of signal generation to test whether a sublethal plasma membrane disruption has been induced. Lack of an indication of overt death is not sufficient in ruling out cell injury as a causative agent in such situations.
Two general signal modalities are generated by PMD. In the first place, while the disruption is open, localized entry down a concentration gradient is permitted of molecules that are normally present outside. Ca2+ is the most prominent such molecule. It enters down an ∼10,000-fold concentration gradient and, most importantly, has a well characterized intracellular messenger function. A number of known properties of fos favor Ca2+ as a PMD-induced messenger. The fos gene contains a Ca2+-responsive element (Ghosh and Greenberg, 1995). Fos expression can be induced in some cells by treatment with Ca2+ ionophore and similar manipulations that raise cytosolic Ca2+ concentration (Bajpai et al., 1989). The fos expression response of fibroblasts contracting a collagen gel, where PMDs are known to occur (Lin et al., 1996), was dependent on the extracellular presence of Ca2+ ions (Rosenfeldt et al., 1998). Unfortunately, we were unable to perform the simple but informative test of the role of Ca2+ that consists of removing this cation from the culture medium during PMD generation and then asking whether fos expression is inhibited. This is because Ca2+ is required for two events proximal to PMD-generated fos expression in our model system: 1) continuous cell adherence to the substratum, which has to occur for PMDs to be generated during scratching of the monolayer as well as for subsequent immunostaining analysis; and 2) resealing of PMDs (Steinhardt et al., 1994). Thus, the role of Ca2+ in PMD-generated fos expression remains unproven.
A second general signaling modality that must be considered is localized exit of normally impermeant cytosolic factors. For example, PMD might allow soluble enhancers of fos expression to escape, or it could allow release of endogenous, constitutively active inhibitors of c-fos expression. The first of these possibilities is supported by earlier studies of PMD. Growth factors, such as basic fibroblast growth factor, are known to be released through PMD (McNeil et al., 1989; Muthukrishnan et al., 1991). Moreover, a soluble signal, probably ATP, released from endothelial cells along scrape monolayer injuries like those studied here was shown to mediate propagation of a rise in cytosolic Ca2+ from one cell for up to five cells distant from the denudation zone (Sammak et al., 1997); however, our data argue against the importance of release of a stimulatory factor. Selective elimination of PMD-affected cells as responders in the injured monolayer population, by loading gelonin into such cells or killing them by allowing excessive Ca2+ entry, abolished the fos response. Because PMD and hence release are expected to occur in these experiments at normal (gelonin) or increased (high Ca2+) levels, this result—complete ablation of fos expression—does not support release as a mechanism. Additionally, more direct evidence that exit of a positive regulator is unimportant was our finding that PMD-conditioned medium fails to elicit a fos response in the absence of local PMD events.
NF-κB is present in an inactive form in the cytoplasm of unstimulated cells by virtue of its binding to an inhibitory factor, IκB. On appropriate stimulation, IκB is degraded proteolytically and releases NF-κB, which can then enter the nucleus where it acts as a potent transcriptional activator (Read et al., 1994). The strong correlation between the NF-κB translocatory response and PMD in mechanically injured monolayers suggests that activation of this transcription factor, too, is evoked by PMD, although it was not possible to investigate the signaling modality as in the case of fos. The NF-κB response was absent from fibroblasts, as was also the case in fibroblasts contracting collagen gels (Rosenfeldt et al., 1998), suggesting that gene expression responses to PMD will vary depending on cell type.
In conclusion, PMD is a key event in initiating expression of fos protein by cells placed in mechanically stressful situations. It may also regulate NF-κB translocation into the nucleus. Because both of these events are predicted to increase the expression of numerous additional genes, it seems likely that PMD will stimulate a complex cellular response. The likely signaling event initiated by PMD, based on our experiments, can be narrowed down to either signal entry into cytosol or loss of a cytosolic inhibitor of fos expression. Mechanotransduction resulting from PMD, here termed the damage sensor hypothesis, can explain the well known fact that a diverse array of cell types are able to sense injurious levels of mechanical stress under normal and pathological conditions in vivo and that these cells respond with appropriate repair/adaptational behaviors such as growth/hypertrophy.
ACKNOWLEDGMENTS
This work was supported by grants from National Institutes of Health (48091) and the Muscular Dystrophy Foundation to P.L.M.
Abbreviations used:
- BAE
bovine aortic endothelial
- FDx
fluorescein-labeled dextran
- HUSMC
human umbilical vein smooth muscle cells
- PMD
plasma membrane disruption
REFERENCES
- Bajpai A, Andrews GK, Ebner KE. Induction of c-fos mRNA in rat lymphoma Nb-2 cells. Biochem Biophys Res Commun. 1989;165:1359–1363. doi: 10.1016/0006-291x(89)92753-8. [DOI] [PubMed] [Google Scholar]
- Basset-Seguin N, Escot C, Blanchard JM, Kerai C, Verrier B, Mion H, Guilhou JJ. High levels of c-fos proto-oncogene expression in normal human adult skin. J Invest Dermatol. 1990;94:418–422. doi: 10.1111/1523-1747.ep12874493. [DOI] [PubMed] [Google Scholar]
- Bauters C, de Groote P, Adamantidis M, Delcayre C, Hamon M, Lablanche JM, Bertrand ME, Dupuis B, Swynghedauw B. Eur. Heart J. 13:556–559. 1992. Proto-oncogene expression in rabbit aorta after wall injury. First marker of the cellular process leading to restenosis after angioplasty? [DOI] [PubMed] [Google Scholar]
- Bi G-Q, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT. J. Cell Biol. 90:187–200. 1981. Mechanism of retraction of the trailing edge during fibroblast movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GC, Libby P, Grodzinsky AJ, Lee AT. Role of fibroblast growth factor. Circulation 93:99–105. 1996. Induction of DNA synthesis by a single transient mechanical stimulus of human vascular endothelial cells. [DOI] [PubMed] [Google Scholar]
- Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- Clarke MS, Feeback DL. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated skeletal muscle cultures. FASEB J. 1996;10:502–509. doi: 10.1096/fasebj.10.4.8647349. [DOI] [PubMed] [Google Scholar]
- Clarke MS, Vanderburg CR, Hay ED, McNeil PL. Cytoplasmic loading of dyes, protein and plasmid DNA using an impact-mediated procedure. Biotechniques. 1994;17:1118–1125. [PubMed] [Google Scholar]
- Closs EI, Murray AB, Schmidt J, Schon A, Erfle V, Strauss PG. c-fos expression precedes osteogenic differentiation of cartilage cells in vitro. J Cell Biol. 1990;111:1313–1323. doi: 10.1083/jcb.111.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes NJ, Lee DM, Cox VM, Nga H, Goldspink DF. The effect of various stretch and electrical stimulation regimes on proto-oncogene induction in skeletal muscle. Biochem Soc Trans. 1995;23:327S. doi: 10.1042/bst023327s. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Sheetz MP. A micromachined device provides a new bend on fibroblast traction forces. Proc Natl Acad Sci USA. 1997;94:9114–9118. doi: 10.1073/pnas.94.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gooch KJ, Tennant CJ. Mechanical Forces: Their Effects on Cells and Tissues. Berlin: Springer; 1997. , 182. [Google Scholar]
- Hamill O, McBride D. Molecular clues to mechanosensitivity. Biophys J. 1993;65:17–18. doi: 10.1016/S0006-3495(93)81028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- Heimark RL, Schwartz SM. The role of membrane-membrane interactions in the regulation of endothelial cell growth. J Cell Biol. 1985;100:1934–1940. doi: 10.1083/jcb.100.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol. 1993;154:143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- Komuro I, Yazaki Y. Control of cardiac gene expression by mechanical stress. Annu Rev Physiol. 1993;55:55–75. doi: 10.1146/annurev.ph.55.030193.000415. [DOI] [PubMed] [Google Scholar]
- Ku PT, D’Amore PA. Regulation of basic fibroblast growth factor (bFGF) gene and protein expression following its release from sublethally injured endothelial cells. J Cell Biochem. 1993;58:328–343. doi: 10.1002/jcb.240580307. [DOI] [PubMed] [Google Scholar]
- Lambert JM, Blattler WA, McIntyre GD, Goldmacher VS, Scott CF., Jr Immunotoxins containing single-chain ribosome-inactivating proteins. Cancer Treat Res. 1988;37:175–209. doi: 10.1007/978-1-4613-1083-9_12. [DOI] [PubMed] [Google Scholar]
- Lin Y-C, Ho C-H, Grinnell F. Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up FITC-dextran and Ca2+ Mol Cell Biol. 1997;8:59–67. doi: 10.1091/mbc.8.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer NJ, Rubin SA. The molecular and cellular biology of heart failure. Curr Opin Cardiol. 1995;10:238–245. doi: 10.1097/00001573-199505000-00002. [DOI] [PubMed] [Google Scholar]
- McNeil PL. Cellular and molecular adaptations to injurious mechanical force. Trends Cell Biol. 1993;3:302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Muthukrishnan L, Warder E, D’Amore PA. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989;109:811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan L, Warder E, McNeil PL. Basic fibroblast growth factor is efficiently released from a cytosolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991;148:1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Hemodynamics and the vascular endothelium. J Biomech Eng. 1993;115:510–514. doi: 10.1115/1.2895532. [DOI] [PubMed] [Google Scholar]
- Osbaldeston NJ, Lee DM, Cox VM, Hesketh JE, Morrison JF, Blair GE, Goldspink DF. The temporal and cellular expression of c-fos and c-jun in mechanically stimulated rabbit latissimus dorsi muscle. Biochem J. 1995;308:465–471. doi: 10.1042/bj3080465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read MA, Whitley MZ, Williams AJ, Collins T. NF-κ B and I κ B α: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt H, Lee DJ, Grinnell F. Increased c-fos mRNA expression by human fibroblasts contracting stressed collagen matrices. Mol Cell Biol. 1998;18:2659–2667. doi: 10.1128/mcb.18.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Mechanotransduction in stretch-induced hypertrophy of cardiac myocytes. J Recept Res. 1993;13:777–794. doi: 10.3109/10799899309073692. [DOI] [PubMed] [Google Scholar]
- Sadoshima J-I, Takahashi T, Jahn L, Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA. 1992;89:9905–9909. doi: 10.1073/pnas.89.20.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak PJ, Hinman LE, Tran PO, Sjaastad MD, Machen TE. How do injured cells communicate with the surviving cell monolayer? J Cell Sci. 1997;110:465–475. doi: 10.1242/jcs.110.4.465. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Jahn L, Izumo S, Apstein CS, Lorell BH. Localization and regulation of c-fos and c-jun protooncogene induction by systolic wall stress in normal and hypertrophied rat hearts. Proc Natl Acad Sci USA. 1991;88:11480–11484. doi: 10.1073/pnas.88.24.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak TC, Price RJ. The role of mechanical stresses in microvascular remodeling. Microcirculation. 1996;3:143–165. doi: 10.3109/10739689609148284. [DOI] [PubMed] [Google Scholar]
- Sosnowski RG, Feldman S, Feramisco JR. Interference with endogenous ras function inhibits cellular responses to wounding. J Cell Biol. 1993;121:113–119. doi: 10.1083/jcb.121.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier B, Muller D, Bravo R, Muller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986;5:913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulczyn FG, Krappmann D, Scheidereit C. The NF-κ B/Rel and I κ B gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–69. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- Yu QC, McNeil PL. Transient disruptions of aortic endothelial cell plasma membranes. Am J Pathol. 1992;141:1349–1360. [PMC free article] [PubMed] [Google Scholar]