Abstract
The molecular mechanism of the cell-cycle machinery in uterine leiomyoma has not yet been fully elucidated. Among the various types of cell-cycle regulators, p27Kip1 (p27) is considered to be a potent tumor suppressor. To provide further molecular basis for understanding the progression of uterine leiomyoma, our objective was to evaluate the expression level of p27 in normal myometrium and uterine leiomyoma tissue and its effect on cytogenic growth. Western blot analysis, real-time polymerase chain reaction (PCR) and immunohistochemical staining revealed that p27 protein and messenger RNA were down-regulated in uterine leiomyoma tissue and cultured cells compared to normal myometerium. Full-length human p27 cDNA was transferred using a replication-deficient recombinant adenoviral vector (Ad.p27) into uterine leiomyoma cells and evaluated the effect on cell proliferation. Transfection of Ad.p27 into uterine leiomyoma cells resulted in the induction of apoptosis, reduction in viability and proliferation of uterine leiomyoma cells. Our results suggest a new paradigm that down-regulated p27 protein expression is the possible underlying mechanism for the growth of uterine leiomyoma and over-expression of p27 induces cell death. This study provides better understanding of the control exerted by p27 in regulating growth and disease progression of uterine leiomyoma.
Keywords: Uterus, Leiomyoma, Cyclin-Dependent Kinase Inhibitor p27, Apoptosis
INTRODUCTION
Uterine fibroids or leiomyoma arise from smooth muscle cells of the uterine myometrium. As other leiomyoma, they are benign, but may lead to excessive menstrual bleeding (menorrhagia), often cause anemia and may lead to infertility (1). Both the economic cost and the effect of uterine leiomyoma on quality of life are substantial. Progression through the mammalian cell cycle is regulated by cyclins, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs). The function of these proteins in the irreversible growth arrest associated with terminally differentiated cells is largely unknown. p27Kip1 (p27) is a CKI whose specific late G1 destruction allows progression of the cell across the G1/S boundary. The protein is ubiquitinated by S-phase kinase-interacting protein-2 (Skp2) following its specific phosphorylation, and is subsequently degraded by the 26s proteosome (2). There is a direct relationship between low level of p27 and rapid proliferation occurring in several benign states and in many malignancies. It has been reported that p27 levels are markedly reduced in several malignancies, such as those of the skin (3), hepatic (4), bladder (5), thyroid (6), breast (7), prostate (8) and endometrium (9). In some of the tumors studied, a strong correlation has been found between the low level of p27, the aggressiveness of the disease and poor prognosis of the patients (7). Interestingly, p27 in all these tumors is of the wild-type species, and its deregulation has been attributed to aberrant accelerated ubiquitin-mediated degradation of the protein. Expression of p27 in certain tissues is regulated by hormones, as in the case of endometrial tissue where the level of p27 is hormone-dependent. Estrogen exposure has been considered as an important risk factor in developing endometrial cancer (10). Up-regulation of p27 by progesterone has been demonstrated in glandular cells (11).
Although many studies have suggested that genetic and epigenetic factors play a role in the progression of uterine leiomyoma via oncogene activation or tumor suppressor gene inactivation, the potent gatekeeper in uterine leiomyoma development and progression remains unclear. Recent progress in molecular biology techniques has revealed that deregulation of the cell cycle machinery is deeply involved in many types of cancer cells (12). Histological (13, 14) and gene expression profiling studies (15) have shown differential expression of p27 in uterine leiomyoma. Nevertheless, though historically thought of as antiproliferative, a number of recent studies have shown that p21 and p27 can assume both pro- and anti-apoptotic, and even oncogenic functions depending on cell type and cellular context (16). In the wake of such literature, our primary aim was to investigate the expression of p27 in uterine leiomyoma in order to identify molecular pathways that could be important in the development of uterine leiomyoma. A secondary aim was to examine if regulating the expression of p27 would influence the growth of uterine leiomyoma, which would have direct clinical implications.
MATERIALS AND METHODS
Tissues and cell culture
Nine sets of uterine leiomyoma and their adjacent normal myometrium tissues were obtained from hysterectomies that were conducted on benign diseases at Dongsan Medical Center, Daegu, Korea. No-objection written consents were obtained from patients, and the study was approved by the Keimyung University ethical committee. We have obtained consent from the patients ranging in age from 40 to 49. The stage of their menstrual cycle was established from each woman's menstrual history and verified by performing a histologic examination of the endometrium. Five of the patients were in the proliferative phase at the time of surgery and four were in the secretory phase. Three sets of fresh uterine leiomyoma and normal myometrial tissue samples were minced and digested by incubation in Hanks solution for 4 hr at 37℃, containing HEPES 0.0065 g/mL, collagenase 0.0015 g/mL and DNase 0.0002 g/mL with periodic agitation. A portion of each tissue was stored at -70℃ for isolation of mRNA and proteins. The dispersed uterine leiomyoma and myometrial cells were plated in Ham F12: DMEM and grown to confluence.
Construction of recombinant ad-p27
Ad.p27 was a kind contribution from Choon-Taek Lee, Department of Internal Medicine and Lung Institute of Medical Research Center, Seoul National University, Seoul, Korea. Construction of Ad.p27 and Ad.null is described elsewhere (17). Briefly, the cDNA of human p27 was subcloned into the KpnI and BamHI sites of the polylinker of adenoviral shuttle vector, pAC CMV pLpA. The resulting pAC CMV-27 and pJM17 were co-transfected into 293 cells (human renal embryonal cells immortalized by stable transfection with E1 of adenovirus) using the standard calcium phosphate precipitation method. 293 cells were maintained in RPMI with 2% FBS until the onset of the cytopathic effect. The generating adenovirus was purified three times by plaque assay and confirmed by DNA sequencing of viral DNA and Western blot of p27. A large-scale stock of adenovirus was prepared by the standard CsCl method. Ad.null was a recombinant adenovirus without any therapeutic gene.
Immunohistochemical staining
The subcellular localization of p27 was studied using polyclonal antibody directed against unique sequence of p27. This antibody was devoid of any cross-reaction with other related proteins. Formalin-fixed and paraffin-embedded specimens were cut into 4 mm-thick sections, deparaffinized and preincubated with normal bovine serum to prevent non-specific binding. The sections were incubated overnight at 4℃ with primary polyclonal antibody to human p27 (Santa Cruz, CA, U.S.A.), followed by alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (Santa Cruz). The reaction products were visualized using a mixture of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; Roche Diagnostic Corp., Indianapolis, IN, U.S.A.). Negative controls replaced the primary antibody with non-immunized rabbit serum.
Western blot analysis
Uterine leimyoma cell extracts were prepared in lysis buffer (10 mM Tris [pH7.4], 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, PMSF [10 µg/mL], leupeptin [10 µg/mL], aprotinin [10 µg/mL], 5 mM phenanthroline, and 28 mM benzamidine-HCl). Protein concentrations were measured using Bio-Rad Protein Assay Reagent (Bio-Rad, Richmond, CA, U.S.A.) following the manufacturer's suggested procedure. Aliquots of protein were separated by 8-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidine difluoride (PVDF) membrane (Millipore, Bedford, MA, U.S.A.). The membrane was blocked with Tris-bufferd saline (TBS) with 5% skim milk and 0.2% Tween 20, reacted with primary antibodies, and washed. The following antibodies were used: anti-p27, anti-Rb, anti-p21, cdk2, cdk4 and β-tubulin (Santa Cruz). After reaction with horseradish peroxidase conjugated secondary antibodies (Amersham Lifescience, Buckinghamshire, England), immune complexes were visualized by using an enhanced chemiluminescence's (ECL) system (Amersham Life-science) following the manufacturer's suggested procedure.
Cell proliferation assay
The ability of p27 to inhibit DNA synthesis was determined by estimating the amount of BrdU incorporation into DNA by a colorimetric immunoassay, using Cell Proliferation ELISA, BrdU-colorimetric kit (Roche Diagnostics GmbH, Penzberg, Germany). Uterine leiomyoma cells were cultured in 48-well plates (1 × 104 cells/well). The 24-hr post-seeding cells were transfected with Ad.p27 and Ad.null vectors, 24-hr post-transfection the assay was carried out according to manufacturer's instructions. The developed assay color was measured at 490 nm. The color intensity and the absorbance values directly correlate to the amount of BrdU incorporated into DNA. The results are expressed as percent inhibition of BrdU incorporation by Ad.p27 and Ad.null vectors over the control. Data were calculated as percentage inhibition using the formula:
Inhibition (%)=(100-(ODt/ODs)×100
Where ODt and ODs are the optical density of the test substance and solvent control, respectively.
Fluorescence-activated cell sorting (FACS) analysis
To determine the cell cycle distribution, FACS analysis was done in uterine leiomyoma cells transfected with Ad.p27 and Ad.null vectors. Twenty-four hours after transfection, cells were harvested, washed with PBS, and fixed in cold 70% ethanol. Fixed cells were suspended in 0.1% RNase A and propidium iodide (50 µg/mL in PBS) to determine cell cycle dynamics. DNA fluorescence was measured by flow cytometer (FACS Calibur™, Becton Dickinson, Franklin Lakes, NJ, U.S.A.). The percentage of cells in each cell cycle phase was determined using the ModFit LT™ software (Becton-Dickinson) based on the DNA histogram.
Statistical evaluation
Data are expressed as means±SD for all experiments. Student-t test was used to assess statistical significance between means. p<0.05 was considered significant.
RESULTS
Down-regulation of p27 in human uterine leiomyoma tissues and cultured cells
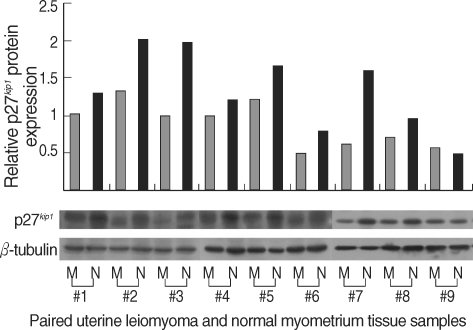
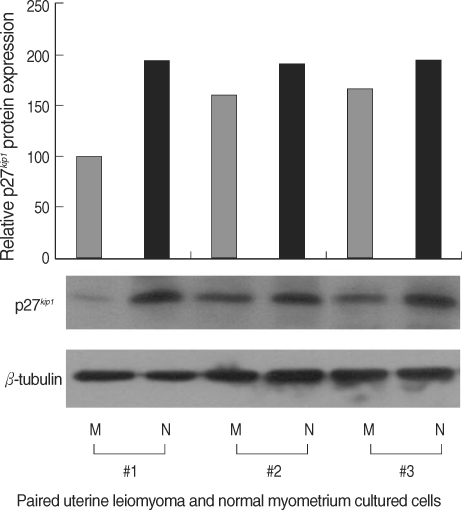
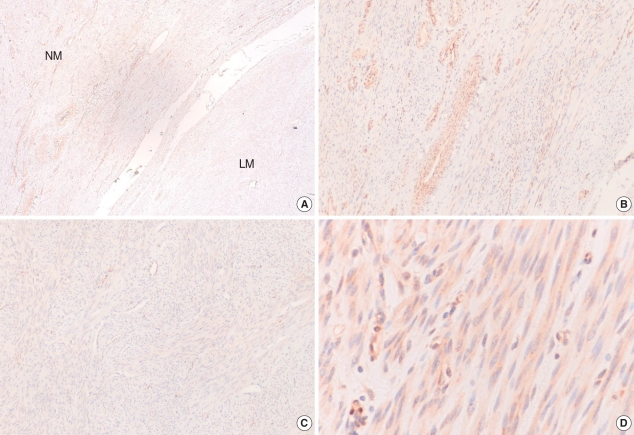
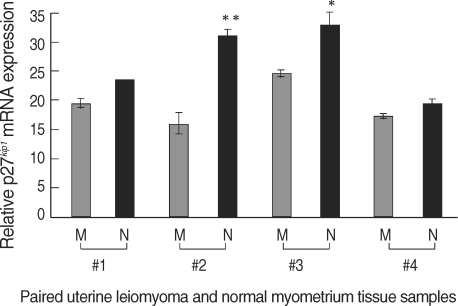
The expression levels of p27 protein and mRNA in human normal myometrium and uterine leiomyoma tissues were examined by Western blot analysis and semi-quantitative real-time RT-PCR analysis, respectively. We found a significant decrease in p27 protein expression levels in uterine leiomyoma tissue samples (Fig. 1), which paralleled data from cultured uterine leiomyoma tissues as well (Fig. 2). Comparison of immunohistochemical staining for p27 protein in formalin-fixed paraffin-embedded sections of leiomyoma (LM) and myometrial tissues (NM) revealed that myometrial smooth muscle cells showed more predominant immunostaining for p27 protein than uterine leiomyoma cells. Cytoplasmic and nuclear brown-staining cells are positive for p27 protein expression (Fig. 3). To determine whether the altered expression in p27 protein level reflects changes in its mRNA level, real-time PCR analysis for p27 expression was performed in pairs of uterine leiomyoma and corresponding myometrium (Fig. 4). The results were in concordance with the observation with p27 protein expression.
Fig. 1.
Decreased p27 protein expression levels in human uterine leiomyoma tissue.Total protein was isolated from uterine leiomyoma and normal myometrial tissues. Nine pairs of tumor and normal tissues; i.e., each pair of samples was obtained from same patient - M; uterine leiomyoma, N; normal myometrium, 5 of the tissue samples belonged to the proliferative phase (#1 to #5) and the rest to the secretory phase (#6 to #9). Fifty micrograms of the total isolated protein were resolved by SDS-polyacrylamide gel electrophoresis and electroblotted onto PVDF membranes. The blot was incubated with antibody against p27. Reactive bands were visualized with an ECL labeling and detection system. β-tubulin was used as the loading control. Densitometric analysis was also done to the western blots to determine the relative protein expression levels.
Fig. 2.
Decreased p27 protein expression levels in cultured human uterine leiomyoma tissue. Total protein was isolated from cultured, well-grown, confluent uterine leiomyoma and normal myometrium tissue samples (3 pairs of tumor and normal tissue, obtained from same patient - M; uterine leiomyoma, N; normal myometrium, 2 of the cultured cells were from proliferative phase tissue samples (#1 and #2) and #3 was cultured cells of the secretory phase tissue sample). Fifty micrograms of the total isolated protein were resolved by SDS-polyacrylamide gel electrophoresis and electroblotted onto PVDF membranes. The blot was incubated with antibody against p27. Reactive bands were visualized with an ECL labeling and detection system. β-tubulin was used as the loading control. Densitometric analysis was also done to the Western blots to determine the relative protein expression levels.
Fig. 3.
Comparison of immunohistochemical staining for p27 protein. Immunohistochemical staining for p27 formalin-fixed paraffin-embedded sections of uterine leiomyoma (LM) and myometrial tissues (NM). (A) Myometrial smooth muscle cells showed more predominant immunostaining for p27 protein than the leiomyoma cells (magnification ×40), (B). Normal myometrial cells showed positive for p27 protein (magnification ×100), (C). Leiomyoma cells showed negative for p27 protein (magnification ×100), (D). Cytoplasmic and nuclear brown-staining cells are positive for p27 protein expression (magnification ×400).
Fig. 4.
Decreased p27 mRNA levels in human uterine leiomyoma tissue. Total RNA was isolated from uterine leiomyoma and normal myometrial tissues (4 pairs of tumor and normal tissues; i.e., each pair of samples was obtained from same patient; tissue samples #1 and #2 were isolated at the proliferative phase and tissue samples #3 and #4 were isolated at the secretory phases of the menstrual cycle). cDNA templates for RT were prepared from the total RNA extracted. The level of p27 mRNA was determined by quantitative real-time PCR. Relative p27 mRNA levels (normalized to GAPDH) in paired samples represented as M; uterine leiomyoma, N; normal myometrium. *, p<0.05; **, p<0.01.
Confirmation of adenoviral expression of p271 in uterine leiomyoma cells
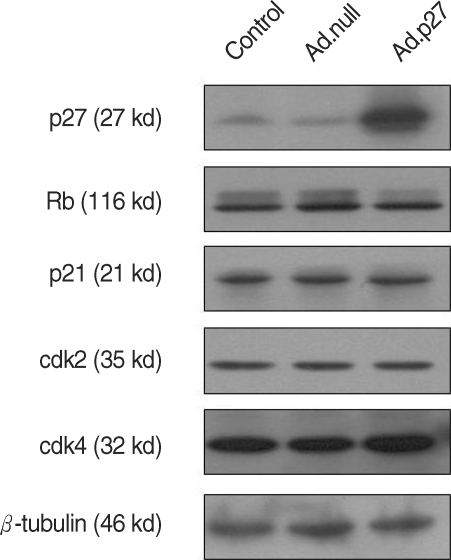
The expression of p27 protein following Ad.p27 infection was confirmed by Western blot (Fig. 5). Fig. 5 shows that ectopic expression of p27 (mediated through infection with recombinant adenovirus Ad.p27) mRNA in uterine leiomyoma cells. To gain insight into the cell-cycle status of p27-transfected cells, we examined the phosphorylation pattern of Rb protein, p21, cdk2 and cdk4. Ad.p27-transfected myoma cells show principally hypophosphorylated Rb attributing to their slow-dividing nature. No such effect was observed with Ad.null infected cells (Fig. 5).
Fig. 5.
Effect of Ad.p27 transfection on uterine leiomyoma cells. Cultured uterine leiomyoma cells were transfected with Ad.null and Ad.p27 recombinant, non-replicating adenoviral vectors. Twenty-four hours after transfection, immunoblotting was performed to detect the expression levels of p27, p21, Rb, cdk2 and cdk4. β-tubulin was used as the loading control. Transfection of Ad.p27 onto uterine leiomyoma cells caused increased expression levels of p27 and dephosphorylation of Rb.
Adenoviral expression of p27 in uterine leiomyoma cells inhibits cell proliferation
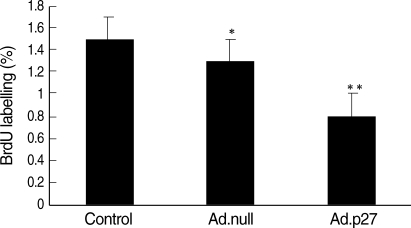
Because p27 is an important control mechanism of the cell cycle, we studied the effects of expression of Ad.p27 on uterine leiomyoma cell proliferation. The proliferation rate of uterine leiomyoma cells transfected with empty adenovirus (which does not carry the recombinant gene-Ad.null) was indistinguishable from that of untransfected cells (Control cells). In contrast, the rate of proliferation of cells in which Ad.p27 was expressed was 40-50% lower than that observed in uterine leiomyoma cells infected with an equal amount of the empty adenovirus (Ad.null) (Fig. 6).
Fig. 6.
Inhibition of the synthesis of DNA in uterine leiomyoma cells by Ad.p27. The antiproliferative effects were determined after transfecting uterine leiomyoma cells for 24 hr with Ad.null and Ad.p27 adenoviral vectors and labeling with BrdU (10 µM) for 24 hr. Results are expressed as percentage inhibition of BrdU incorporation relative to control cells. Values are the means (±SD) of three experiments with triplicate determinations. *, p<0.05; **, p<0.01.
Expression of p27 in uterine leiomyoma cells causes cell death and increased cells in subG1 phase
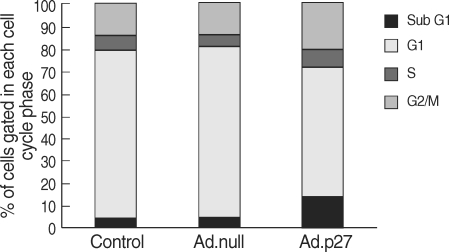
To investigate the mechanism by which expression of p27 inhibits uterine leiomyoma cell proliferation, we performed cell cycle analysis. Uterine leiomyoma cells transfected with empty adenovirus (Ad.null) showed almost similar patterns and timing of progression through the different phases of the cell cycle that were indistinguishable from cells that were not transfected with the adenovirus (Control cells), whereas Ad.p27 transfected uterine leiomyoma cells had a surging increase in sub-G1 population of cells (Fig. 7). The increase in sub-G1 peak can be attributed to cells that became arrested at G1 phase which was confirmed by the appearance of hypophosphorylated Rb specific to G1 phase (Fig. 5) and the lack of BrdU incorporation (Fig. 6).
Fig. 7.
Effect of Ad.p27 on the cell cycle profile. Ad.null and Ad.p27 transfected uterine leiomyoma cells were harvested, fixed, stained with PI, and analyzed by flow cytometry analysis. The values represent the number of cells in each phase of the cell cycle as a percentage (%) of total cells. Following transfection with Ad.p27, the growth of uterine leiomyoma cells was affected with an increase in the percentage of cells in the sub-G1 phase.
DISCUSSION
The transition from G1 to S is controlled by the concerted actions of protein kinases, the activities of which are modulated by families of regulatory proteins in both a positive (cyclins) and a negative (cyclin-dependent kinase inhibitors [CKI]) manner. A family of CKI plays a major role in the cell cycle machinery (18). Among them, p27 is thought to represent the most bona fide inhibitor as its level oscillates throughout the cell cycle with the highest level observed during G1 and the lowest during S and G2/M phases. p27 is frequently inactivated in tumorigenic cells, and is considered to be a potent tumor suppressor.
High-level expression of p27 was detected in some highly proliferative breast cancer and colorectal cells (19). This evidence seems to provide a novel aspect of the role of p27 in cancer cells because an inverse correlation between p27 expression and cell proliferation is generally observed in normal human tissues. It has been reported that a subset (35%) of colorectal carcinomas displayed diffuse cytoplasmic staining for p27 by immunohistochemical staining (20). Cytoplasmic localization of p27 was associated with decreased survival in Barrett's associated adenocarcinoma (21), suggesting that the subcellular localization of p27 may be an adverse prognostic factor. Recent reports claim decreased p27 expression in cervical (22), lung cancer (23), and prostate (24). Histological staining analysis of p27 in uterine leiomyoma and leiomyosarcoma showed that p27 as a significant prognostic indicator (14). The biological behavior of p27 may be somewhat complex in some types of cancer cells, and comprehensive analyses of the p27 status should be performed to precisely assess the clinical value of p27.
To date, the prognosis of uterine leiomyoma patients has remained poor, and identification of useful molecular prognostic marker for this clinical condition is required. Because deregulated cell-cycle progression is one of the most significant alterations in cancer cells, many studies have investigated cell-cycle regulators. To comprehensively investigate the biological significance of p27 in uterine leiomyoma, we thoroughly examined the p27 status in primary tumors. Our immunohistochemical staining results revealed that myometrial smooth muscle cells showed more predominant p27 staining when compared to uterine leiomyoma cells. To compare protein expression levels in uterine leiomyoma and myometrial tissues and to confirm the results of immunohistochemical staining, immunoblotting analysis was performed using lysates obtained from available paired normal/tumor tissue samples. The level of p27 protein expression correlated with results determined by immunohistochemistry. The detectable levels of expression in myometrial tissues seem to be attributable to the expression in various kinds of cells as observed in immunohistochemistry. Western blotting and real-time PCR analysis revealed remarkable correlation of p27 expression with uterine leiomyoma formation.
Controlled proliferation and differentiation of uterine leiomyoma cells plays an important role in leiomyoma development. Therefore, demonstrating the mechanism of uterine leiomyoma cell proliferation not only helps us to understand leiomyoma development, but it could also lead to a strategy to regulate its development. In this work we utilized the adenoviral mediated expression of p27 in uterine leiomyoma cells and demonstrated that the p27 does play an important role in the control of leiomyoma cell cycle.
The CKI p27, once activated, have been shown to phosphorylate various substrates (25), including the retinoblastoma tumor suppressor gene product. pRb negatively regulates the cell cycle when hypophosphorylated during G0 and early G1, and pRb is progressively phosphorylated during mid to late G1 (26). In this hypophosphorylated state, pRb functions as a transcriptional repressor. To gain an insight into the cell cycle status of Ad.p27 transfected uterine leiomyoma cells, we initially examined the phosphorylation pattern of endogenous Rb protein. Western blot analysis of control and Ad.null transfected uterine leiomyoma cells showed both hyperphosphorylated and unphosphorylated forms of Rb protein, indicative of cells progressing through the cell cycle. In contrast, Ad.p27 transfected cells show principally hypophosphorylated Rb that possibly attributes to the slow-dividing nature of these cells. These data unequivocally demonstrate that p27 expression plays an important role in controlling cell cycle regulators and the proliferation of uterine leiomyoma cells.
If hyperploid progression occurs through continued cell cycling without cell division, then one should be able to block it by expressing G1-specific CKI's. p27 is an inhibitor of cyclin-dependent kinases cdk4 and cdk6 controlling passage through early G1, as well as cdk2 controlling progression through late G1 phase (27). Our results show that ectopic expression of p27 in uterine leiomyoma cells resulted in the absence of a hyperploid peak along with an increase in the G2/M peak. The increase in the G2/M peak is due to cells that have traversed through M phase, but most cells were arrested at G1 phase, which was confirmed by the appearance of unphosphorylated Rb specific to G1 phase. This was further validated with our results from the BrdU assay. To observe DNA synthesis at a single-cell level, BrdU incorporation assay was performed. Reduced DNA synthesis in the presence of Ad.p27 in uterine leiomyoma cells emphasizes the repressor activity of p27.
The p27 is a nodal factor controlling normal cell cycle. Its expression in normal myometrium raised the question whether it has a mutual exclusive or redundant role in uterine leiomyoma. The possible reason for the suppressed expression of p27 in uterine leiomyoma is thought to be the role played by hormones. Estrogen plays a pivotal role in the proliferation of uterine leiomyoma cells and the role of estrogen in suppressing p27 expression have been elucidated in endometrial cancer tissues (10) and this role of estrogen could also be attributed to uterine leiomyoma. Further studies are required to elucidate the correlated role between estrogen and p27 in uterine leiomyoma. Results from our comparative comprehensive analysis in a series of uterine leiomyoma tissues and p27 over expression studies reveal that this cell cycle regulatory protein acts as a checkpoint in uterine leiomyoma growth and development. This provides a unique opportunity to develop a therapy that is specific against tumors lacking the G1 checkpoint. By exploiting the hyperploid-progression-mediated death, a therapy that is tumor specific at the genetic level may be developed and it may have a wide therapeutic applicability in clinical management of uterine leiomyoma.
Footnotes
This work was supported by Korea Research Foundation Grant (KRF-2004-002-E00092).
References
- 1.Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Tchernev G, Orfanos CE. Downregulation of cell cycle modulators p21, p27, p53, Rb and proapoptotic Bcl-2-related proteins Bax and Bak in cutaneous melanoma is associated with worse patient prognosis: preliminary findings. J Cutan Pathol. 2007;34:247–256. doi: 10.1111/j.1600-0560.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda Y, Ichida T. p16 and p27 are functionally correlated during the progress of hepatocarcinogenesis. Med Mol Morphol. 2006;39:169–175. doi: 10.1007/s00795-006-0339-2. [DOI] [PubMed] [Google Scholar]
- 5.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481–487. doi: 10.1016/j.juro.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Saltman B, Singh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006;140:899–905. doi: 10.1016/j.surg.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 1997;57:1259–1263. [PubMed] [Google Scholar]
- 8.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–548. [PubMed] [Google Scholar]
- 9.Lahav-Baratz S, Ben-Izhak O, Sabo E, Ben-Eliezer S, Lavie O, Ishai D, Ciechanover A, Dirnfeld M. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol Hum Reprod. 2004;10:567–572. doi: 10.1093/molehr/gah084. [DOI] [PubMed] [Google Scholar]
- 10.Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiozawa T, Horiuchi A, Kato K, Obinata M, Konishi I, Fujii S, Nikaido T. Up-regulation of p27Kip1 by progestins is involved in the growth suppression of the normal and malignant human endometrial glandular cells. Endocrinology. 2001;142:4182–4188. doi: 10.1210/endo.142.10.8455. [DOI] [PubMed] [Google Scholar]
- 12.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Dobashi Y, Noguchi T, Nasuno S, Katayama K, Kameya T. CDK-inhibitors-associated kinase activity: a possible determinant of malignant potential in smooth muscle tumors of the external soft tissue. Int J Cancer. 2001;94:353–362. doi: 10.1002/ijc.1479. [DOI] [PubMed] [Google Scholar]
- 14.Leiser AL, Anderson SE, Nonaka D, Chuai S, Olshen AB, Chi DS, Soslow RA. Apoptotic and cell cycle regulatory markers in uterine leiomyosarcoma. Gynecol Oncol. 2006;101:86–91. doi: 10.1016/j.ygyno.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 15.Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146:1097–1118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21 (WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat. 2003;6:183–195. doi: 10.1016/s1368-7646(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 17.Park KH, Seol JY, Yoo CG, Kim YW, Han SK, Lee EH, Kim CM, Shim YS, Lee CT. Adenovirus expressing p27Kip1 induces growth arrest of lung cancer cell lines and suppresses the growth of established lung cancer xenografts. Lung Cancer. 2001;31:149–155. doi: 10.1016/s0169-5002(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 18.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 19.Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O'Hare MJ, Lu X. High level expression of p27Kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27Kip1 and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA. 1997;94:6380–6385. doi: 10.1073/pnas.94.12.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB. Localization and expression of p27Kip1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58:114–122. [PubMed] [Google Scholar]
- 21.Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 22.Chen TP, Chen CM, Chang HW, Wang JS, Chang WC, Hsu SI, Cho CL. Increased expression of SKP2 and phospho-MAPK/ERK1/2 and decreased expression of p27 during tumor progression of cervical neoplasms. Gynecol Oncol. 2007;104:516–523. doi: 10.1016/j.ygyno.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Pateras IS, Apostolopoulou K, Koutsami M, Evangelou K, Tsantoulis P, Liloglou T, Nikolaidis G, Sigala F, Kittas C, Field JK, Kotsinas A, Gorgoulis VG. Downregulation of the KIP family members p27 (KIP1) and p57 (KIP2) by SKP2 and the role of methylation in p57 (KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119:2546–2556. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- 24.Dvorackova J, Uvirova M. A molecularly genetic determination of prognostic factors of the prostate cancer and their relationships to expression of protein p27kip1. Neoplasma. 2007;54:149–154. [PubMed] [Google Scholar]
- 25.Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci USA. 1992;89:7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg RA. Tumor suppressor genes. Science. 1991;254:1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- 27.Hong FD, Chen J, Donovan S, Schneider N, Nisen PD. Taxol, vincristine or nocodazole induces lethality in G1-checkpoint-defective human astrocytoma U373MG cells by triggering hyperploid progression. Carcinogenesis. 1999;20:1161–1168. doi: 10.1093/carcin/20.7.1161. [DOI] [PubMed] [Google Scholar]