Abstract
Birt-Hogg-Dubé syndrome (BHDS) is an autosomal dominant genodermatosis characterized by cutaneous hair follicle tumors (fibrofolliculoma or trichodiscoma), pulmonary cysts, and increased risk of renal neoplasia. The genetic alteration for BHDS has been mapped to chromosome 17p12q11, and the gene in this region has been cloned and believed to be responsible for the BHDS. Mutations in the BHD gene (also known as FLCN) have been described in the patients with BHDS. We present a case of a 30-yr-old Korean woman with multiple mildly pruritic papules on her face and neck area. The patient had several firm, flesh-colored, dome-shaped, papular lesions measuring between 2 to 5 mm. Except for a history of pneumothorax her medical records were not remarkable. Mutation analysis of the BHD gene was performed, and a novel deletion mutation (p.F519LfsX17 [c.1557delT]) causing truncation of the gene product, folliculin, was found in the exon 14. The actual incidence of BHDS is unknown, but it is most likely underdiagnosed. So it is imperative that doctors recognize the skin lesions of BHDS and institute proper screening to detect other manifestations of the disease. Here, we report a case of BHDS with a novel mutation, which is the first report in Korea.
Keywords: Birt-Hogg-Dubé Syndrome; Fibrofolliculoma, Trichodiscoma
INTRODUCTION
Birt-Hogg-Dubé syndrome (BHDS) is an inherited autosominal dominant genodermatosis that predisposes individuals to the development of fibrofolliculomas (FF), trichodiscomas (TD), and acrochordons of the face, neck, and upper trunk (1). There is also an increased risk for lung cysts, spontaneous pneumothorax, and renal carcinomas in affected people, and some patients may also develop colorectal polyps and cancers (2-4). The disease is caused by germline mutations in the BHD (also known as FLCN) gene located in 17p11.2 encoding folliculin, a new protein with unknown function (5). We report a patient with BHDS in which mutation analysis subsequently revealed a novel mutation.
CASE REPORT
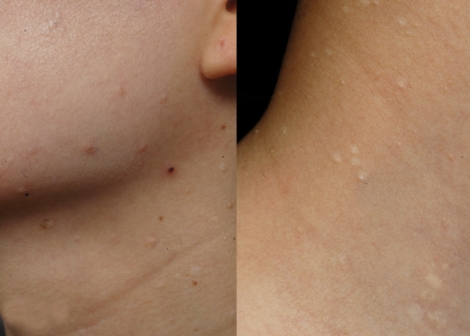
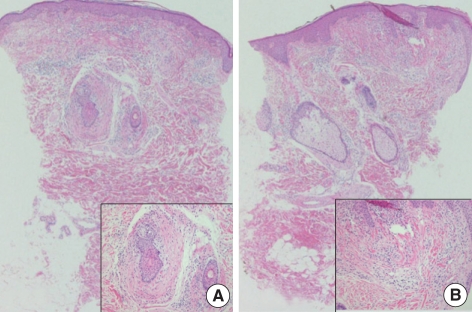
A 31-yr-old woman visited the Department of Dermatology for evaluation of long-standing mildly pruritic facial papules that she had had for 10 yr (Fig. 1). Previously she had been treated with CO2 laser and Aldara ointment with the impression of verruca or syringoma but with little effect. Physical examination revealed several firm, flesh-colored, dome-shaped, papular lesions ranging from 2 to 5 mm in diameter, distributed on the face and neck area. The patient had a history of pneumothorax; otherwise, her medical history was not notable. Skin biopsies of the facial papules were performed. One sample showed concentric layers of cellular fibrous tissue around hair follicles with an aritifactual cleft separating the fibroma from adjacent connective tissue (Fig. 2A). Also in another sample, a raised papule composed of connective tissue surrounded by a lateral collarette was seen (Fig. 2B). Staining with alcian blue and elastin stain revealed an unencapsulated loosely woven admixture of collagen and thin elastic fibers with mucin. Periodic acid-schiff (PAS) staining showed multiple thin and thick walled blood vessels with PAS-positive basement membrane within the substane of the tumor. The biopsy findings were consistent with perifollicular fibroma and trichodiscoma, respectively.
Fig. 1.
Multiple small dome-shaped, flesh-colored papules on the face and neck.
Fig. 2.
(A) Concentric layers of cellular fibrous tissue around hair follicles representing perifollicular fibroma (hematoxylin-eosin stain; original magnification ×40, inset ×200). (B) Raised papule composed of connective tissue surrounded by a lateral collarette representing trichodiscoma (hematoxylin-eosin stain; original magnification ×40, inset ×200).
Computed tomographic scan of the chest, abdomen, and pelvis showed multiple cysts on both lower lung zone, with sizes ranging from 1.4-2.4 cm and a small amount of pneumothorax noted on the left anterior basal hemithorax. The scan did not show any abnormalities in the kidney or colon. There was no family history of renal tumors, colon carcinoma, or other benign or malignant neoplasm. However, the patient reported that her two aunts died of 'lung disease' (diagnosis unknown) and one of her older sisters was remembered to have had multiple tumorous lesions. Both of her older sisters passed away at an early age but she did not know the cause of death.
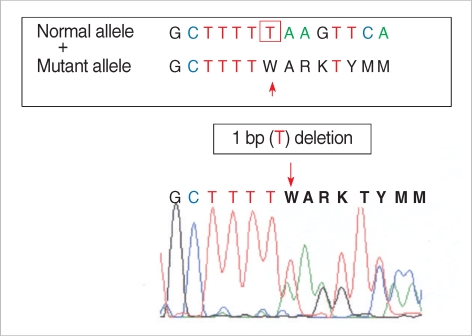
Molecular analysis of the BHD gene was performed after informed consent from the patient. Genomic DNA was extracted from peripheral blood leukocytes, and subjected to mutation analysis. All the coding regions of the BHD gene (exons 4-14) were amplified using polymerase chain reaction (PCR) with oligonucleotide primers we designed (Table 1). The amplified PCR products were sequenced using the ABI 3100 Genetic Analyzer (Applied Biosystems/Hitachi, Foster City, CA, U.S.A.). Sequencing analysis revealed a novel deletion mutation (p.F519LfsX17 [c.1557delT]) in the BHD gene exon 14 of the patient (Fig. 3). This mutation results in producing the truncated protein folliculin, which is caused by frameshift of amino acid sequence in the BHD gene product. Also, this deletion mutation has not yet been found in the normal populations, indicating that this mutation is the real cause of the syndrome in this case.
Table 1.
The primer sequences used in the amplification of the BHD gene
Fig. 3.
Sequence analysis of the Birt-Hogg-Dubé gene, showing deletion of a thymidine in the exon 14 (c.2012delT).
DISCUSSION
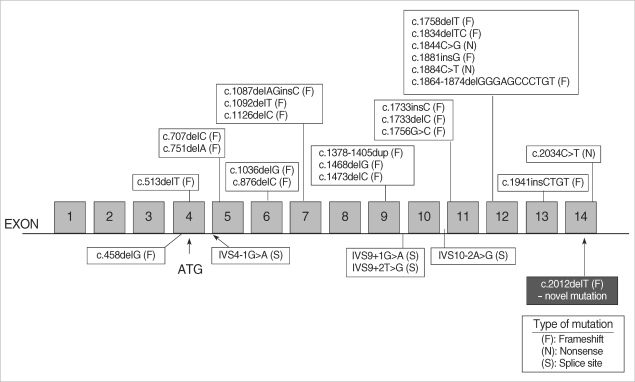
BHDS was recently found to be caused by heterozygous loss-of-function mutations in the BHD tumor suppressor gene on chromosome 17p11.2, which encodes the novel protein folliculin. Mutations are predicted to result in truncation of folliculin. Fluorescent in situ hybridization has shown the expression of BHD mRNA in many normal tissues including the skin and its appendages, the distal nephron of the kidney, stromal cells, and type 1 pneumocytes of the lung (6). The function of the BHD gene in tumorigenesis, however, has not yet been elucidated. The defective protein in patients with BHDS may affect the cell's cytoskeleton, disrupting the extracellular matrix and affecting the regulation of cellular proliferation (7). Because patients with BHDS have inactivating mutations in the BHD gene, and many BHDS tumors exhibit loss of heterozygosity, the BHD gene is considered to be a tumor suppressor gene (7). Fluorescent in situ hybridization showed that BHD mRNA was strongly expressed in the proliferating epithelial strands of FFs but not expressed in the renal tumors of patients with BHDS. This further supports a tumor suppressor role for BHD in renal cancer (8). Genotype-phenotype correlation remains to be evaluated for BHDS, but it was recently suggested that significantly fewer renal tumors were observed in patients with the C-deletion than those with the C-insertion mutation in the exon 11 hotspot (9). To date, several different BHD germline mutations have been identified with mutations affecting all translated exons (4-11) with the exception of exons 8 and 10. Our patient showed novel mutation in the exon 14 (Fig. 4).
Fig. 4.
Distribution of mutations reported in Birt-Hogg-Dubé syndrome.
Clinical diagnosis of BHDS requires a minimum of 10 skin lesions clinically compatible with FFs and at least one histologically proven FF (5, 9). FF and TD present as asymptomatic single or multiple dome-shaped papules commonly located on the head, neck, back, and arms. Biopsy from our patient showed features of perifollicular fibroma and trichodiscoma. However, many researchers now consider perifollicular fibroma in patients with BHDS to actually represent FF (10). Thus, based on clinical and histological findings, our patient could be diagnosed as BHDS.
Since its description in 1977, BHDS has been linked to tumors of nearly every organ system. BHDS patients are 9.3 times more likely to develop a renal tumor than unaffected persons, and are 32.3 times more likely to develop a spontaneous pneumothorax (12). Older age and male gender increase the risk for renal tumors, whereas the risk of spontaneous pneumothorax is inversely associated with age (12). Between 15% and 30% of BHDS-affected patients develop renal cancers (13). Because of the inherited predisposition, patients develop renal tumors much earlier than unaffected persons, typically developing tumors in their 20s or 30s instead of in their 70s and often presenting with multiple, bilateral renal cancers. In the absence of strictly defined guidelines, it is recommended that patients with clinical manifestations of BHDS undergo abdominal computed tomography and/or renal ultrasound at the time of diagnosis. Interval screening may be performed every 3 to 5 yr. Siblings should have a complete physical examination with biopsy of lesions suspicious for FF/TDs beginning in their 20s. Routine screening for renal pathology may begin if they have FF or at age 40 yr. Tumors less than 3 cm are observed, whereas tumors larger than 3 cm are treated with nephron-sparing procedures including partial nephrectomy (14). Spontaneous pneumothorax was more likely to occur in younger patients with BHDS (12). Pneumothorax typically presents with dyspnea and chest pain. Clinical findings include tachypnea, diminished or absent breath sounds, and hyperresonace. Radiographic examination confirms the diagnosis. Patients with BHDS should be informed of the increased risk and educated on the signs and symptoms of pneumothorax. Our patient was diagnosed with pneumothorax in her 20s but other concominant abnormalities were not found. However, as the risk of renal tumors increases with age we advised the patient to have regular screening. The risk for colon polyps and colon cancer was not increased in BHDS (12), so screening for colon pathology may not be warranted. However, routine screening by colonoscopy is included in the currently recommended guideline for all patients aged >50 yr. The decision to undergo examination at an earlier age should be discussed with the patient.
The actual incidence of BHDS is unknown, and the disease is most likely underdiagnosed. It is imperative that physicians recognize the skin lesions of BHDS and institute proper screening to detect other manifestations of the disease, such as renal tumors and lung cysts. Patients with numerous facial and truncal papules resembling FFs are candidates for biopsy. In summary, we report a case of BHDS with a novel mutation, which is the first report in Korea.
References
- 1.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 2.Adley BP, Smith ND, Nayar R, Yang XJ. Birt-Hogg-Dubé syndrome, Clinicopathologic findings and genetic alterations. Arch Pathol Lab Med. 2006;130:1865–1870. doi: 10.5858/2006-130-1865-BSCFAG. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt LS. Birt-Hogg-Dubé syndrome, a genodermatosis that increases risk for renal carcinoma. Curr Mol Med. 2004;4:877–885. doi: 10.2174/1566524043359773. [DOI] [PubMed] [Google Scholar]
- 4.Khoo SK, Giraud S, Kahnoski K, Chen J, Motorna O, Nickolov R, Binet O, Lambert D, Friedel J, Lévy R, Ferlicot S, Wolkenstein P, Hammel P, Bergerheim U, Hedblad MA, Bradley M, Teh BT, Nordenskjöld M, Richard S. Clinical and genetic studies of Birt-Hogg-Dube syndrome. J Med Genet. 2002;39:906–912. doi: 10.1136/jmg.39.12.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, Duray P, Merino M, Choyke P, Pavlovich CP, Sharma N, Walther M, Munroe D, Hill R, Maher E, Greenberg C, Lerman MI, Linehan WM, Zbar B, Schmidt LS. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 6.Warren MB, Torres-Cabala CA, Turner ML, Merino MJ, Matrosova VY, Nickerson ML, Ma W, Linehan WM, Zbar B, Schmidt LS. Expression of Birt-Hogg-Dubé gene mRNA in normal and neoplastic human tissues. Mod Pathol. 2004;17:998–1011. doi: 10.1038/modpathol.3800152. [DOI] [PubMed] [Google Scholar]
- 7.Kahnoski K, Khoo SK, Nassif NT, Chen J, Lobo GP, Segelov E, Teh BT. Alterations of the Birt-Hogg-Dubé gene in sporadic colorectal tumours. J Med Genet. 2003;40:511–515. doi: 10.1136/jmg.40.7.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JH, Shin YK, Ku JL, Jeong SY, Hong SH, Park SY, Kim WH, Park JG. Mutations of the Birt-Hogg-Dubé gene in sporadic colorectal carcinomas and colorectal carcinoma cell lines with microsatellite instability. J Med Genet. 2003;40:364–367. doi: 10.1136/jmg.40.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, Turner ML, Choyke PL, Sharma N, Peterson J, Morrison P, Maher ER, Walther MM, Zbar B, Linehan WM. Germline BHD-mutation spectrum and phenotypic analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent A, Farley M, Chan E, James WD. Birt-Hogg-Dubé syndrome: a review of the literature and the differential diagnosis of firm facial papules. J Am Acad Dermatol. 2003;49:698–705. doi: 10.1067/s0190-9622(03)01582-2. [DOI] [PubMed] [Google Scholar]
- 11.Narvaez D, Kanitakis J, Faure M, Claudy A. Immunohistochemial study of CD34 positive dendritic cells of the human dermis. Am J Dermatopathol. 1996;18:283–288. doi: 10.1097/00000372-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, Walther M, Choyke P, Weirich G, Hewitt SM, Duray P, Gabril F, Greenberg C, Merino MJ, Toro J, Linehan WM. Risk of renal and colonic neoplasm and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 13.Choyke PL, Glenn GM, Walther MM, Zbar B, Linehan WM. Hereditary renal cancers. Radiology. 2003;226:33–46. doi: 10.1148/radiol.2261011296. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, Merino MJ. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]