Abstract
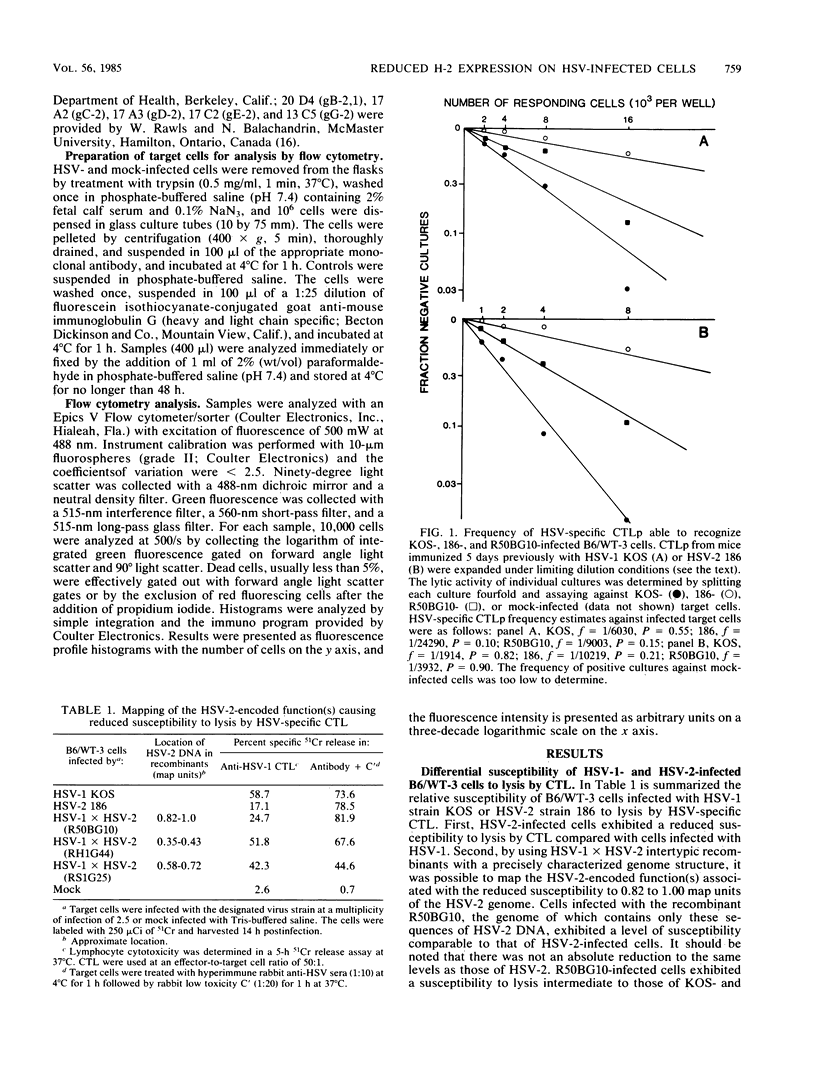
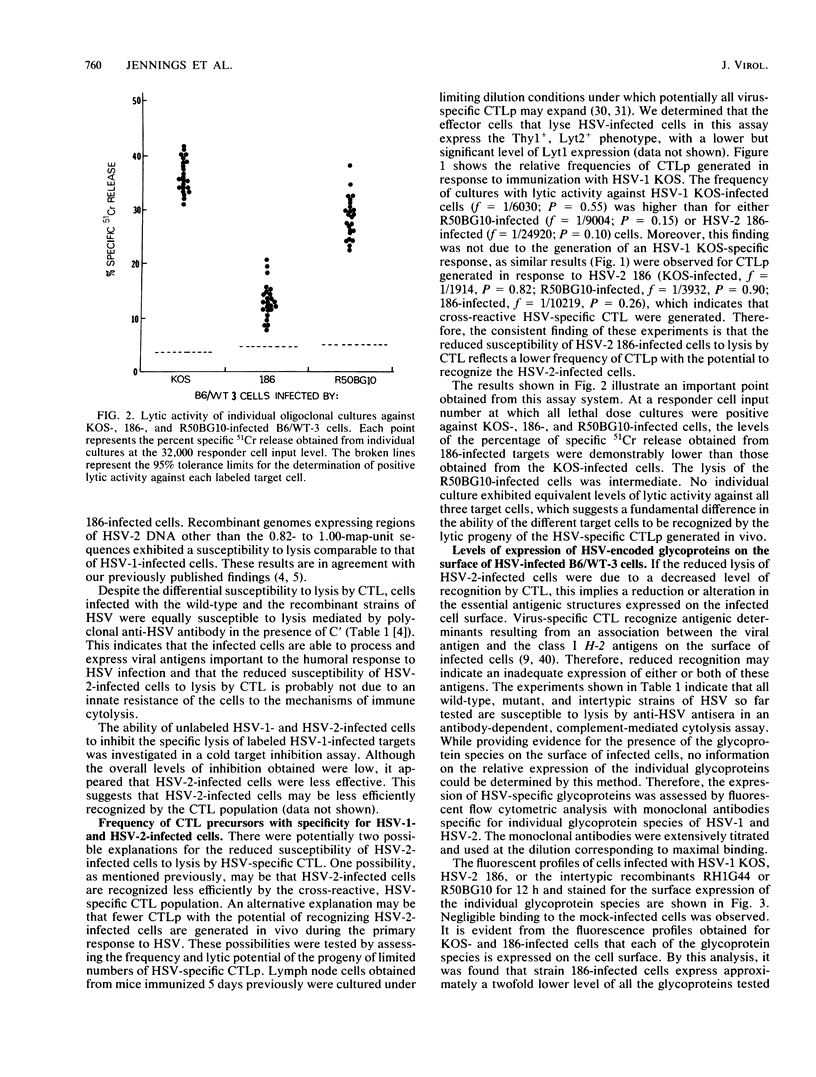
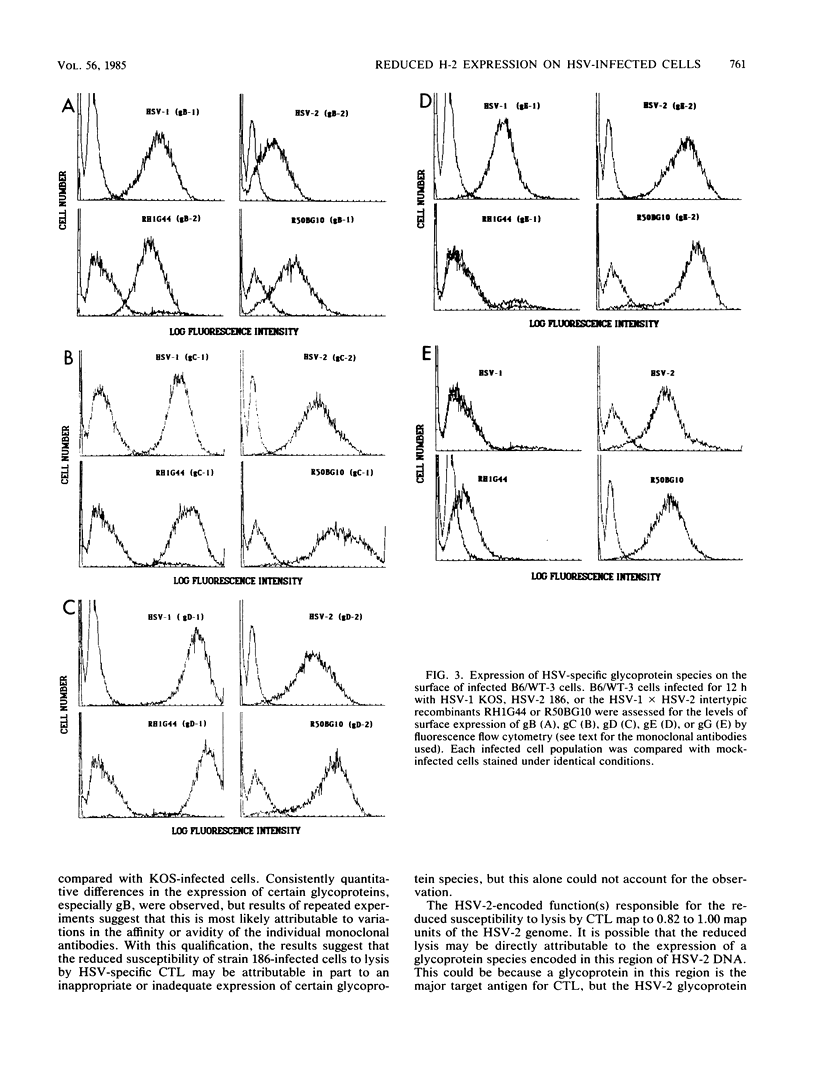
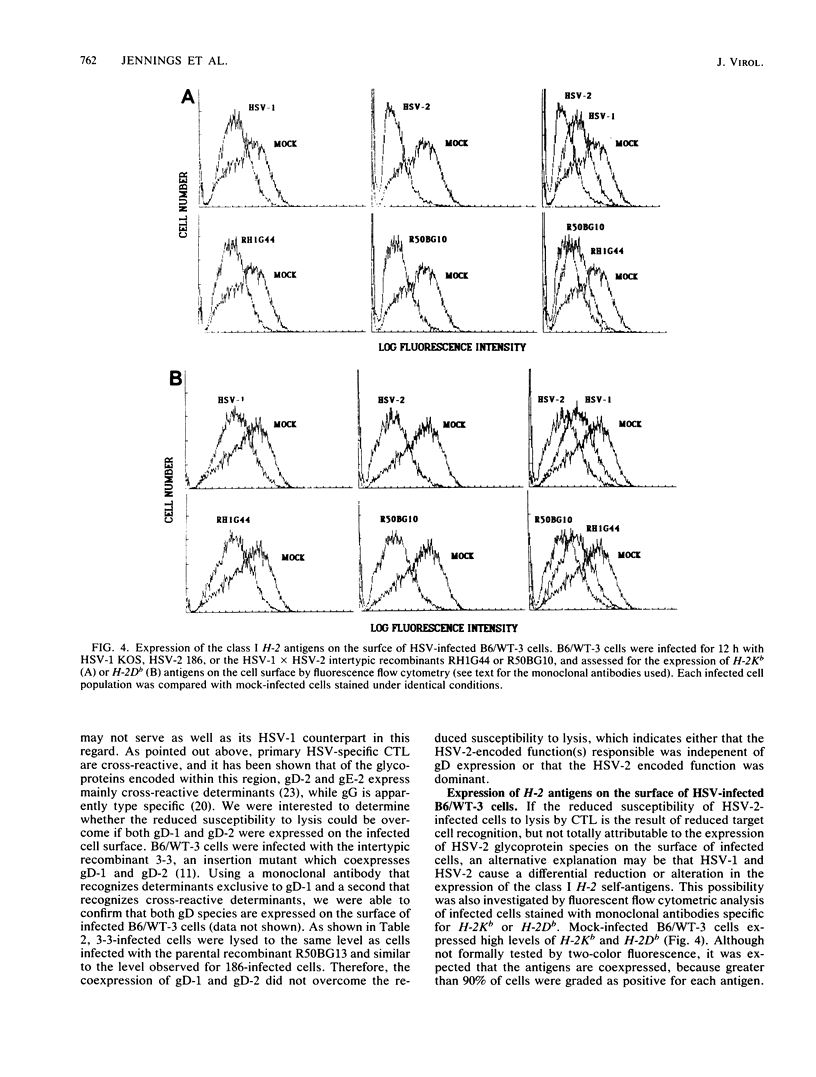
Cytotoxic T lymphocytes (CTL) generated in C57BL/6 (H-2b) mice in response to infection with the serologically distinct herpes simplex virus type 1 (HSV-1) or type 2 (HSV-2) were cross-reactive against target cells infected with either serotype. However, HSV-2-infected cells were shown to be much less susceptible to CTL-mediated lysis, and analysis through the use of HSV-1 X HSV-2 intertypic recombinants mapped the reduced susceptibility to a region contained within 0.82 to 1.00 map units of the HSV-2 genome. The study reported here was undertaken to determine the possible reasons for the reduced susceptibility of HSV-2-infected cells to lysis by CTL. Competition for the specific lysis of labeled HSV-1-infected cells by either HSV-1- or HSV-2-infected, unlabeled inhibitor cells and frequency analysis of the CTL precursor able to recognize HSV-1- and HSV-2-infected cells suggested that the reduced susceptibility of HSV-2-infected cells to lysis could be explained, at least in part, by reduced levels of target cell recognition. A determination of the surface expression of the critical elements involved in target cell recognition by CTL following infection with HSV-1 or HSV-2 revealed that all the major HSV-specific glycoprotein species were expressed. Infection with both HSV-1 and HSV-2 caused a reduction in the expression of the class I H-2 antigens. However, this reduction was much greater following infection with HSV-2. This suggested that one important factor contributing to reduced lysis of HSV-2-infected cells may be the altered or reduced expression of the class I H-2 self-antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berke G. Interaction of cytotoxic T lymphocytes and target cells. Prog Allergy. 1980;27:69–133. [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. E., Foley F. L., Tevethia S. S. Demonstration of multiple antigenic sites of the SV40 transplantation rejection antigen by using cytotoxic T lymphocyte clones. J Immunol. 1983 Jan;130(1):490–492. [PubMed] [Google Scholar]
- Carter V. C., Jennings S. R., Rice P. L., Tevethia S. S. Mapping of a herpes simplex virus type 2-encoded function that affects the susceptibility of herpes simplex virus-infected target cells to lysis by herpes simplex virus-specific cytotoxic T lymphocytes. J Virol. 1984 Mar;49(3):766–771. doi: 10.1128/jvi.49.3.766-771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Rice P. L., Tevethia S. S. Intratypic and intertypic specificity of lymphocytes involved in the recognition of herpes simplex virus glycoproteins. Infect Immun. 1982 Jul;37(1):116–126. doi: 10.1128/iai.37.1.116-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XI. Apparent clustering of functions effecting rapid inhibition of host DNA and protein synthesis. J Virol. 1979 Feb;29(2):825–827. doi: 10.1128/jvi.29.2.825-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg R., Benacerraf B. Induction, control and consequences of virus specific cytotoxic T cells. Immunol Rev. 1981;58:157–180. doi: 10.1111/j.1600-065x.1981.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Bowern N. A., Blanden R. V. Cell-medicated cytotoxicity against ectromelia virus-infected target cells. III. Role of the H-2 gene complex. Eur J Immunol. 1975 Feb;5(2):122–127. doi: 10.1002/eji.1830050210. [DOI] [PubMed] [Google Scholar]
- Gibson M. G., Spear P. G. Insertion mutants of herpes simplex virus have a duplication of the glycoprotein D gene and express two different forms of glycoprotein D. J Virol. 1983 Nov;48(2):396–404. doi: 10.1128/jvi.48.2.396-404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Sinden R. R., Sadler J. R. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis by different mechanisms in Friend erythroleukemia cells. J Virol. 1983 Jan;45(1):241–250. doi: 10.1128/jvi.45.1.241-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Pan S., Knowles B. B., Tevethia S. S. Recognition of herpes simplex virus antigens on the surface of mouse cells of the H-2b haplotype by virus-specific cytotoxic T lymphocytes. J Immunol. 1984 Jan;132(1):475–481. [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killington R. A., Newhook L., Balachandran N., Rawls W. E., Bacchetti S. Production of hybrid cell lines secreting antibodies to herpes simplex virus type 2. J Virol Methods. 1981 Mar;2(4):223–236. doi: 10.1016/0166-0934(81)90012-4. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Buckmaster A., Palfreyman J. W., Hope R. G., Minson A. C. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J Virol. 1984 May;50(2):547–554. doi: 10.1128/jvi.50.2.547-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Quartey-Papafio R., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980 Aug;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. Use of monoclonal antibodies to HSV-1 and HSV-2 for serological analysis of the viral glycoproteins. Dev Biol Stand. 1982;52:115–131. [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Potter T. A., Boyer C., Verhulst A. M., Golstein P., Rajan T. V. Expression of H-2Db on the cell surface in the absence of detectable beta 2 microglobulin. J Exp Med. 1984 Jul 1;160(1):317–322. doi: 10.1084/jem.160.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretell J., Greenfield R. S., Tevethia S. S. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). V In vitro demonstration of SV40 TrAg in SV40 infected nonpermissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979 Aug;97(1):32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Ju G., Rajan T. V., Bloom B. R. Decreased expression of H-2 antigens following acute measles virus infection. Cell Immunol. 1981 Apr;59(2):319–329. doi: 10.1016/0008-8749(81)90412-3. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Wagner H. Frequency of herpes simplex virus-specific cytotoxic T lymphocyte precursors in lymph node cells of infected mice. Immunology. 1984 Jan;51(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Specifically immune mouse T-cells can destroy H-2 compatible murine target cells infected with herpes simplex virus types 1 or 2. Z Immunitatsforsch Immunobiol. 1977 Jul;153(2):162–173. [PubMed] [Google Scholar]
- Sonoda S., Hitsumoto Y., Utsumi S., Takami S., Oseto M., Minamishima Y. Preparation of stable target cells for anti-herpes simplex virus cytotoxic T lymphocytes. Microbiol Immunol. 1981;25(10):999–1010. doi: 10.1111/j.1348-0421.1981.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Tognon M., Furlong D., Conley A. J., Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral fraction which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981 Dec;40(3):870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


