Abstract
This study was undertaken to investigate age-dependent and postmenopausal changes in the serum levels of anti-Müllerian hormone (AMH), inhibin B, insulin-like growth factor (IGF)-I, IGF-binding protein-3 (IGFBP-3), and follicle-stimulating hormone (FSH), and to determine which of these markers best reflects the aging process in women. A total of 144 women aged 20-59 yr were enrolled in this cross-sectional study. Blood samples were obtained on cycle day 3 of regularly menstruating women (n=111), or at random in postmenopausal women (n=33). Data were analyzed with respect to premenopausal women age groups and compared in pre- and postmenopausal women. Area under the receiver operating characteristic curve (ROCAUC) analyses were performed to assess the ability of each marker to discriminate between the pre- and postmenopausal status. Serum levels of AMH, IGF-I, and IGFBP-3 decreased and serum levels of FSH increased significantly with age in premenopausal women. Serum luteinizing hormone (LH) was higher and inhibin B was lower in women in their 20-30's than in 40's. Serum levels of AMH and IGF-I showed a consistent decrease with all age groups. ROCAUC analysis showed that the diagnostic accuracy of AMH for menopausal status was similar to those of FSH, LH, and inhibin B, and was better than that of IGF-I. In conclusion, the serum AMH level appears to be the best marker of the aging process in premenopausal women.
Keywords: Reproductive Aging, Anti-Müllerian Hormone, Inhibin B, Insulin-Like Growth Factor I, Insulin-Like Growth Factor Binding Protein 3, FSH
INTRODUCTION
Reproductive aging is associated with a decline in fertility, which begins in a moderate and steady fashion starting in the third to fourth decade but then accelerates rapidly after age 35 (1). A gradual diminution in the pool of ovarian follicles seems to underlie this decline (2). This age-related decrease in the follicle number and fertility is marked by an increase in follicular-phase serum follicle-stimulating hormone (FSH) levels. This usually starts in women approaching 40 yr of age (3) and is followed several years later by an increase in luteinizing hormone (LH) (4, 5). In addition, changes in estradiol (E2) levels have been described in women with advanced reproductive age (5).
Various endocrinological markers have been used to assess the ovarian reserve, and the accurate and reliable determinations of serum ovarian aging marker levels are essential for safe and successful treatment (6). Previous studies have suggested that anti-Müllerian hormone (AMH) and inhibin B play important roles as ovarian aging markers (7). AMH is secreted by granulosa cells of ovarian follicles and appears to regulate the early follicular development (8). According to a previous study, serum AMH levels decrease with increasing age (9). On the other hand, inhibin B is produced by granulosa cells which characteristically suppress the synthesis and secretion of FSH (10). In addition to its role as a FSH modulator, inhibin B appears to regulate the follicular development and promote follicular growth (11).
Other putative intraovarian regulators of follicle growth include the insulin-like growth factor (IGF) family of proteins. The IGF system is one of several growth factor systems that probably serve adjunctive roles in ovarian follicle development (12). Moreover, IGFs may play an important role in the human preovulatory process, and IGF-binding proteins (IGFBPs) may be valuable biochemical markers in the evaluation of oocytes maturity (13). IGF-I in blood either circulates freely or is bound to binding proteins, mainly to IGFBP-3. Furthermore, serum IGFBP-3 levels have been reported to be positively regulated by IGF-I (14). A small number of studies have explored the positive relationships between follicular fluid IGF-I and ovarian reserve and between follicular fluid IGF-I and serum IGF-I (5). Although serum IGF-I levels have been reported to be downregulated in elderly women and to influence ovarian function (16), the serum IGF-I level is not widely used as an ovarian aging marker and its usefulness has not been clearly documented.
The present study was focused on the usefulness of several ovarian age markers in one population sample with respect to age and menopausal status. Even though the decreases of these hormones with age have been reported (7, 9, 16), the data published to date are not comprehensive enough to reach a consensus regarding reference ranges. Moreover, no study has yet been conducted in an Asian population on these hormones. The purposes of this study were to investigate age-dependent and postmenopausal changes in the serum levels of AMH, inhibin B, IGF-I, IGFBP-3, and FSH and to identify which of these markers better reflects the aging process in Korean women. Furthermore, this study provides diagnostic reference ranges of novel age markers, such as AMH, inhibin B, and IGF-I.
MATERIALS AND METHODS
Subjects
A total of 144 women participated in this study. We recruited healthy, ovulatory women aged 20-29 yr (n=48), 30-39 yr (n=33), and 40-49 yr (n=30) and postmenopausal women aged 50-59 yr (n=33). Premenopausal women were required to have regular menstrual cycles (24-35 days), and postmenopausal women were required to have been in physiologic menopause for at least 1 yr. No participant had any evidence of endocrine disorders (normal prolactin and thyroid-stimulating hormone levels, and no evidence of polycystic ovarian syndrome), and all participants were required to have a body mass index (BMI) of 19-26 kg/m2, both ovaries present, and an absence of medical or reproductive disorders (including any history of infertility), and no participant was taking hormone medications.
Hormone assays
Blood samples were obtained by venipuncture on cycle day 3 from regularly menstruating women or at random from postmenopausal women to measure the serum levels of AMH, inhibin B, IGF-I, IGFBP-3, FSH, LH, and E2. Serum was separated from blood samples and stored at -20℃ until assayed. Samples from a given subject were analyzed for each hormone in the same assay to avoid inter-assay variation.
Serum levels of LH and FSH were measured by immunoradiometric assay (IRMA) using commercial kits (Biosource, Nivelles, Belgium). The detection limits of the assay were 0.2 mIU/mL for LH and 0.1 mIU/mL for FSH. Intra- and inter-assay coefficients of variation (CV) were 3.2% and 6.7% for LH, and 3.3% and 7.1% for FSH, respectively. Samples were analyzed for E2 using radioimmunoassay (RIA) using commercial kits (Biosource, Nivelles, Belgium). The detection limit of this assay was 10 pg/mL, and intra- and inter-assay CVs were 4.9% and 5.2%, respectively. Serum levels of IGF-I and IGFBP-3 were determined by IRMA using reagents supplied by Diagnostic Systems Laboratories (DSL Inc., Webster, U.S.A.). Its detection limits were 2.06 ng/mL for IGF-I and 0.5 ng/mL for IGFBP-3, and its intra- and inter-assay CVs were 5.1% and 9.1% for IGF-I, and 5.2% and 7.1% for IGFBP-3, respectively.
Serum levels of AMH and Inhibin B were determined using enzyme-linked immunosorbent assays (ELISA) using commercial kits from Diagnostic Systems Laboratories (DSL Inc., Webster, U.S.A.). The detection limits of this assay were 0.017 ng/mL for AMH and 7 pg/mL for Inhibin B, and its intra- and inter-assay CVs were 4.6% and 8.0% for AMH and 5.6% and 7.6% for inhibin B, respectively.
Statistical analysis
Data were analyzed by age group in premenopausal women. Differences between age groups were tested by one-way analysis of variance (ANOVA), followed by Turkey's multiple comparison test. Comparisons between pre- and postmenopausal women were made using Mann-Whitney U test. The abilities of hormone levels to discriminate between pre- and postmenopause women were investigated using receiver operating characteristic (ROC) analysis. The diagnostic values of the various hormone markers were evaluated by comparing areas under the curves (ROCAUC) (23). Statistical analyses were performed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, U.S.A.), and p values of <0.05 were considered statistically significant. We drew fitting plots of hormone levels versus age. Mean data values with error bar are presented in the fitting plots. The x-axis was divided into intervals of 5 yr and each curve was fitted using linear, Gaussian, Lorentzian, and sigmoid functions using Mathematica 5.0 (Wolfram Research Inc., Champaign, IL, U.S.A.)
RESULTS
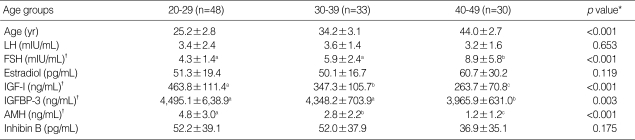
The characteristics of the premenopausal subjects and their serum hormone levels on cycle day 3 are shown in Table 1. Serum levels of AMH, IGF-I, and IGFBP-3 decreased and those of FSH increased significantly with age. Serum levels of E2, LH, and inhibin B showed no significant differences. However, serum LH was higher and inhibin B was lower in women in their 20-30's than in 40's. AMH and IGF-I levels consistently decreased with age, and showed significant changes across all ages, whereas IGFBP-3 and FSH levels showed a significant difference only between women in their 30's and 40's.
Table 1.
Characteristics of study subjects and serum marker levels in premenopausal women by age
LH, luteinizing hormone; FSH, follicle-stimulating hormone; IGF-I, insulin-like growth factor-I; IGFBP-3, IGF-binding protein-3; AMH, anti-Müllerian hormone. Values are means±S.D..
*By ANOVA; †Same letters indicate non-significant differences between groups as determined by Turkey's multiple comparison test.
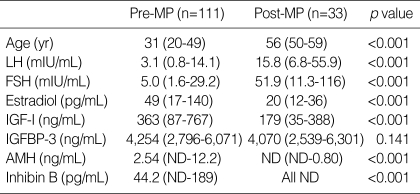
Median ages and serum hormone levels of premenopausal and postmenopausal women are presented in Table 2. Data for AMH and inhibin B were not normally distributed in the postmenopausal women, mainly because the majority of values were below the detection limit. Hence the data were presented as medians and ranges, and a nonparametric method was used for comparison between two groups. Premenopausal and postmenopausal women had median ages of 31 (range 20-49) yr and 56 (range 50-59) yr, respectively. Serum levels of LH, FSH, E2, IGF-I, AMH, and inhibin B showed significant differences between pre- and postmenopausal women (p<0.001). However, serum IGFBP-3 levels did not change significantly after menopause. As expected, LH and FSH levels in postmenopausal women were significantly higher and those of E2, IGF-I, AMH and inhibin B levels were significantly lower than in premenopausal women.
Table 2.
Age and marker levels according to the menopausal status
LH, luteinizing hormone; FSH, follicle-stimulating hormone; IGF-I, insulin-like growth factor-I; IGFBP-3, IGF-binding protein-3; AMH, anti-Müllerian hormone; MP, menopause; ND, non-detectable.
Values are medians (range).
AMH was undetectable in 20 of 33 postmenopausal women, whereas it was only undetectable in 2 of 111 premenopausal women. Inhibin B was undetectable in all postmenopausal women and in 10 of 111 premenopausal women; moreover, these 10 women were older than 40 yr old.
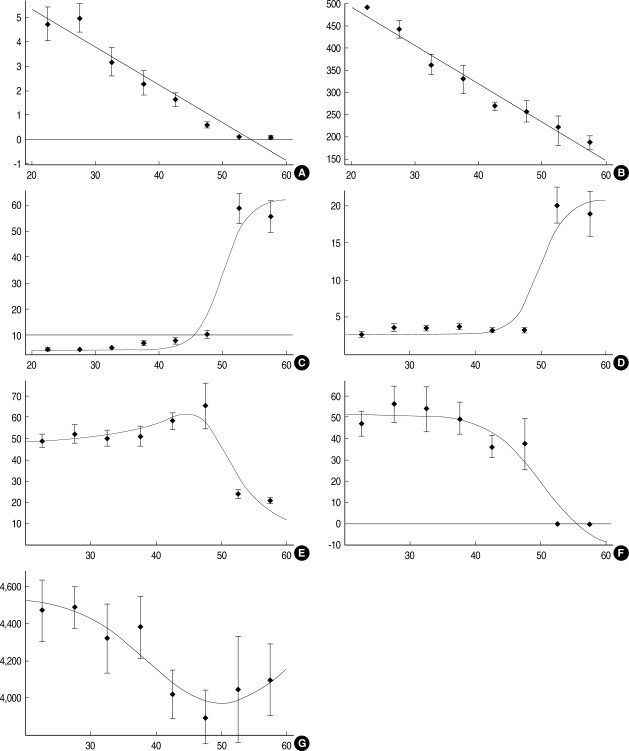
Serum levels of AMH and IGF-I showed linear decreases from the earlier age (Fig. 1A, B), whereas FSH and LH levels showed sigmoid increases and showed greatest increase after menopause (Fig. 1C, D). Serum E2 levels were fitted using sigmoid and Lorentzian functions (Fig. 1E) and inhibin-B levels showed a sigmoid decrease (Fig. 1F), and both of these hormones decreased after menopause. Serum IGFBP-3 levels were fitted using Gaussian function with a minimum value at the boundary between pre- and postmenopause (Fig. 1G).
Fig. 1.
Fitting plots of hormone serum levels according to age. (A) AMH, y=8.438-0.156x; (B) IGF-I, y=667-8.69x; (C) FSH, y=62.8-58.2 Exp[-(x-50)/2]/(1+Exp[-(x-50)/2]); (D) LH, y=21.0-18.2 Exp[-(x-50)/2]/(1+Exp[-(x-50)/2]); (E) E2, y=0.27+45.6 Exp[-(x-50)/2]/(1+Exp[-(x-50)/2])+23.2/(1+(x-50)2/100); (F) Inhibin B, y=-13.4+65.3 Exp[-(x-50)/4]/(1+Exp[-(x-50)/4]); (G) IGFBP-3, y=4540.4-569.66 Exp[-(x-50)2/250].
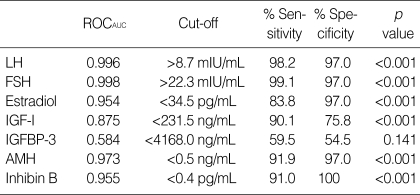
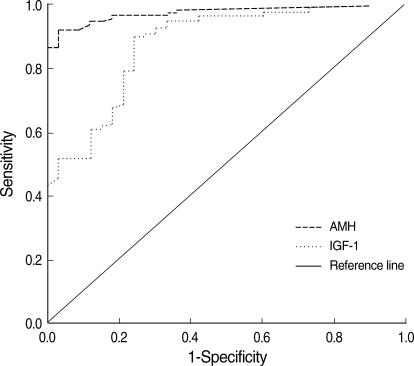
Table 3 shows the results of ROC curve analysis for serum markers. This analysis showed that the diagnostic accuracy of AMH (ROCAUC=0.943) for menopausal status was similar to those of FSH (ROCAUC=0.998), LH (ROCAUC=0.996), and inhibin B (ROCAUC=0.945), and was better than IGF-I (ROCAUC=0.875). Cutoff levels providing desired sensitivities and specificities can be deduced from ROC curves. When the point on a curve closest to the upper left corner of the box corresponding to 100% sensitivity and 100% specificity (0% false positivity) was selected, it resulted in cutoff levels for AMH of <0.46 ng/mL and for inhibin B of <0.4 pg/mL in terms of identifying postmenopausal subjects. The sensitivity and specificity corresponding to these cutoffs were 92% and 97% for AMH, and 91% and 100% for inhibin B, respectively, and the cutoff level of IGF-I was <231.5 ng/mL with a sensitivity and specificity of 90% and 76%, respectively. The sensitivity and specificity of AMH were similar to those of FSH, LH, and inhibin B, and were better than those of IGF-I.
Table 3.
Receiver operating characteristic curve analysis results for serum markers in terms of discriminating menopausal status
LH, luteinizing hormone; FSH, follicle-stimulating hormone; IGF-I, insulin-like growth factor-I; IGFBP-3, IGF-binding protein-3; AMH, anti-Müllerian hormone.
ROCAUC, area under the receiver operating characteristic curve.
DISCUSSION
Several studies have investigated changes in markers of ovarian follicular reserve. Serum FSH levels increase in old reproductive age women, a fact that has been well documented and recognized for many years by many investigators (5). In women approaching 40 yr of age, serum FSH levels usually begin to rise, which reflects a reduction in the number of early antral follicles present that can be recruited to ovulate (3). Serum FSH levels increase over time because inhibin B and E2 production are reduced by a diminished cohort of growing follicles (18, 19). Nevertheless, prior to age 40, FSH levels are not correlated with age, which confirms the lack of correlation between FSH and age in women aged 20-35 (20, 21). However, the proper assessment of ovarian aging at an early stage is crucial in terms of counseling patients about their possibility of pregnancy, either spontaneously or during fertility therapy. Therefore, a new marker of ovarian aging in younger women is needed.
Previous studies have reported that serum AMH levels are closely related to the early antral follicle count, and moreover, this relationship was found to be remarkably more strong than those of inhibin B, E2, FSH, or LH (8, 9, 22). The serum AMH level decreased with ages in premenopausal women and postmenopausal women, which is in line with the findings of de Vet et al. (7). In the present study, it was found that serum AMH levels in normal ovulatory women reduced with advancing age before changes in other markers (e.g., FSH and inhibin B) were apparent, and that AMH was undetectable in most of postmenopausal women. These results are in line with those of previous studies and suggest that AMH could be used as a novel marker of ovarian aging.
Age-dependent decreases of IGF-I may occur secondary to age-dependent reductions in growth hormone secretion (23, 24). IGF-I has been shown to serve as an intraovarian regulator of follicle function in rodents and to exerts a direct effects on human and rodent granulos a cell function (25, 26). Moreover, IGF-I, in conjunction with gonadotropins, appears to promote follicle growth (27) and steroid secretion (28) and to act as an antiatretic hormone (29). Although serum IGF-I levels have been reported to be attenuated in elderly women (16) and follicular fluid IGF-I levels have been demonstrated to be related to the ovarian reserve (15), serum IGF-I levels are not widely used as an ovarian aging marker and the usefulness of serum IGF-I has not been clearly documented in this context. Based on a high correlation between serum and follicular fluid levels of IGF-I and on the positive correlation between follicular fluid level and an ovarian reserve (15), it can be speculated that serum IGF-I and ovarian reserve are positively correlated. The present study demonstrates age-dependent and postmenopausal changes in the serum levels of IGF-I, which suggests that serum IGF-I is an another candidate marker of ovarian reserve.
The results of the present study indicate that serum levels of AMH, IGF-I, and IGFBP-3 decrease and that those of FSH increase significantly with age in premenopausal women, but serum levels of inhibin B do not decrease significantly with age. In addition, FSH levels in normal ovulatory women in their 20's and 30's were similar, which is in line with previous reports on inhibin B and FSH (7, 20, 21). In contrast, serum levels of AMH and IGF-I were significantly different in women in these age groups. It is noteworthy that serum levels of AMH and IGF-I significantly changed at earlier ages than did those of the other hormones in premenopausal women; moreover, the main limitation of conventional ovarian reserve markers such as FSH, LH, and E2 is that their serum levels change relatively late.
All hormones examined showed significant differences pre- to postmenopause except IGFBP-3. Inhibin B was practically undetectable after menopause, and AMH was undetectable in 20 of the 33 postmenopausal women. This is supposed to be because inhibin B and AMH are produced by the granulosa cells of ovarian follicles (7, 10). Several studies have investigated changes in AMH and inhibin B, and also found that they ultimately become undetectable after menopause (7, 30).
To follow the age-dependent changes of each marker more precisely, we fitted their hormone level versus age plots. After menopause, serum FSH and LH levels increase markedly and E2 and inhibin B levels decrease markedly, but no changes in these markers were observed during earlier premenopausal ages. The fitting curves of the AMH and IGF-I serum levels versus ages showed a linear decrease from the earlier age. The present study more precisely characterized the usefulness of AMH and IGF-I during early reproductive ages. These results suggest that AMH and IGF-I are better markers of ovarian reserve in premenopausal women than other hormone markers, such as FSH, LH, E2, and inhibin B.
To determine whether AMH or IGF-I better reflects the aging process in women, we compared the diagnostic accuracies of hormone levels in terms of differentiating between pre- and postmenopausal status (Fig. 2). ROCAUC analysis showed that the diagnostic accuracy of AMH for menopausal status was similar to those of FSH, LH, E2, and inhibin B, and that it was better than IGF-I. The reason for the inferiority of IGF-I to AMH and other markers appears to be that IGF-I levels decrease continuously after menopause, whereas FSH, LH, and E2 levels are fairly stable and AMH and inhibin B levels vanish after menopause.
Fig. 2.
Receiver operating characteristic curves of serum AMH and IGF-I levels on cycle day 3 for differentiating between premenopausal and postmenopausal women.
In conclusion, the present study provides strong evidences that the serum AMH level is an important marker of reproductive aging in women. It was also found that serum IGF-I is a good candidate ovarian aging marker, especially in premenopausal women. In addition, the results of this study provide useful reference data of serum AMH ranges in Korean populations. Further research of large scale and longitudinal design is necessary to confirm our results.
ACKNOWLEDGEMENT
The authors thank Dr. Joon Shik Kim for academic support, and the Shin Jin Medics Company for providing EIA kits and helping EIA tests. This work was supported by a grant from the Korean Science & Engineering Foundation at the Tumor Immunity Medical Research Center at Seoul National University College of Medicine.
References
- 1.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–1394. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 2.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing, and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 3.Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505–510. doi: 10.1016/s0015-0282(97)00557-8. [DOI] [PubMed] [Google Scholar]
- 4.Lenton EA, Sexton L, Lee S, Cooke ID. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas. 1988;10:35–43. doi: 10.1016/0378-5122(88)90129-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod. 1988;3:851–855. doi: 10.1093/oxfordjournals.humrep.a136796. [DOI] [PubMed] [Google Scholar]
- 6.Rose MP, Gaines Das RE, Balen AH. Definition and measurement of follicle stimulating hormone. Endocr Rev. 2000;21:5–22. doi: 10.1210/edrv.21.1.0388. [DOI] [PubMed] [Google Scholar]
- 7.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 8.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 9.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 10.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff TK, Lyon RJ, Hansen SE, Rice GC, Mather JP. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990;127:3196–3205. doi: 10.1210/endo-127-6-3196. [DOI] [PubMed] [Google Scholar]
- 12.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 13.Clemmons DR, Dehoff ML, Busby WH, Bayne ML, Cascieri MA. Competition for binding to insulin-like growth factor (IGF) binding protein-2, 3, 4, and 5 by the IGFs and IGF analogs. Endocrinology. 1992;131:890–895. doi: 10.1210/endo.131.2.1379166. [DOI] [PubMed] [Google Scholar]
- 14.Blum WF, Ranke MB, Kietzmann K, Gauggel E, Zeisel HJ, Bierich JR. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab. 1990;70:1292–1298. doi: 10.1210/jcem-70-5-1292. [DOI] [PubMed] [Google Scholar]
- 15.Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998;15:587–593. doi: 10.1023/A:1020377209952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein NA, Battaglia DE, Miller PB, Soules MR. Circulating levels of growth hormone, insulin-like growth factor-I, and growth hormone binding protein in normal women of advanced reproductive age. Clin Endocrinol (Oxf) 1996;44:285–292. doi: 10.1046/j.1365-2265.1996.670491.x. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742–2745. doi: 10.1210/jcem.81.7.8675606. [DOI] [PubMed] [Google Scholar]
- 19.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 20.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen APN, te Velde ER. Serum antimüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Schipper I, de Jong FH, Fauser BC. Lack of correlation between maximum early follicular phase serum follicle stimulating hormone concentrations and menstrual cycle characteristics in women under the age of 35 years. Hum Reprod. 1998;13:1442–1448. doi: 10.1093/humrep/13.6.1442. [DOI] [PubMed] [Google Scholar]
- 22.Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 23.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchimont P, Urbain-Choffray D, Lambelin P, Fontaine MA, Frangin G, Reginster JY. Effects of repetitive administration of growth hormone-releasing hormone on growth hormone secretion, insulin-like growth factor I, and bone metabolism in postmenopausal women. Acta Endocrinol (Copenh) 1989;120:121–128. doi: 10.1530/acta.0.1200121. [DOI] [PubMed] [Google Scholar]
- 25.Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol. 1990;126:R1–R4. doi: 10.1677/joe.0.126r001. [DOI] [PubMed] [Google Scholar]
- 26.Mason HD, Margara R, Winston RM, Seppala M, Koistinen R, Franks S. Insulin-like growth factor-I (IGF-I) inhibits production of IGF-binding protein-1 while stimulating estradiol secretion in granulosa cells from normal and polycystic human ovaries. J Clin Endocrinol Metab. 1993;76:1275–1279. doi: 10.1210/jcem.76.5.7684393. [DOI] [PubMed] [Google Scholar]
- 27.Di Blasio AM, Vigano P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994;61:483–487. doi: 10.1016/s0015-0282(16)56580-7. [DOI] [PubMed] [Google Scholar]
- 28.Yong EL, Baird DT, Yates R, Reichert LE, Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–849. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 29.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 30.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]