Abstract
Wiskott-Aldrich syndrome (WAS) is an X-linked congenital immune-deficiency syndrome, and bone marrow transplantation (BMT) has become a curative modality. However, the transplant with the alternative donor needed more intensive conditioning with increased treatment-related toxicities. Recently, fludarabine-based reduced toxicity myeloablative conditioning regimens have been developed for adult myeloid malignancies with promising results of good engraftment and low treatment-related toxicities. To increase the engraftment potential without serious complications, a boy with WAS received successful unrelated BMT with a reduced toxicity myeloablative conditioning regimen composed of fludarabine (40 mg/m2 on days -8, -7, -6, -5, -4, -3), busulfan (0.8 mg/kg i. v. q 6 hr on days -6, -5, -4, -3), and thymoglobulin (2.5 mg/kg on days -4, -3, -2). This novel conditioning regimen could improve the outcome of allogeneic transplantation for other non-malignant diseases such as congenital immune-deficiency syndromes or metabolic storage diseases.
Keywords: Wiskott-Aldrich Syndrome, Bone Marrow Transplantation, Busulfan, Fludarabine
INTRODUCTION
Wiskott-Aldrich syndrome (WAS) is an X-linked congenital immune-deficiency syndrome characterized by the triad of recurrent infection, eczema, and thrombocytopenia, and bone marrow transplantation (BMT) from an HLA-matched related donor is an effective treatment (1, 2). Patients without an appropriate related donor could receive an alternative stem cell source such as matched unrelated donor or cord blood. However, the transplant with the alternative donor needed more intensive conditioning to overcome the hematologic and immunologic barrier with increased treatment-related toxicity. Recently, fludarabine-based reduced toxicity myeloablative conditioning regimens have been developed for adult myeloid malignancies with promising results of good engraftment and low treatment related toxicities (3-5). To increase the engraftment potential without serious complications, a boy with WAS received BMT from a matched unrelated donor with a reduced toxicity myeloablative conditioning regimen.
CASE REPORT
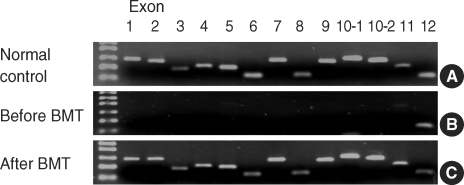
A 3-yr-old boy was admitted because of eczema, recurrent infection, and thrombocytopenia with a platelet count less than 10×109/L and was diagnosed as having WAS with a large deletion in the exon 1 to 11 of the WAS gene (Fig. 1). For the detection of mutation, the following primers were used; exon 1 forward 5'-GGT TTT TTG CAT TTC CTG TTC-3' and exon 1 reverse 5'-AGG AAG AGG AAG AAA CGG TG-3', exon 2 forward 5'-CCT GAC CAG ACT CCA CTG AC-3' and exon 2 reverse 5'-CTT GAA GCT ATG GAC ACA TAT G-3', exon 3 forward 5'CCT CAG TGC CAC TGT GCC TC3' and exon 3 reverse 5'-TTC CCA TCT CCT CTC CAC AC-3', exon 4 forward 5'-GTG TGG AGA GGA GAT GGG AA-3' and exon 4 reverse 5'-CAC TCA CCT CTG CCC AAC TT-3', exon 5 forward 5'-AAG TTG GGC AGA GGT GAG TG-3' and exon 5 reverse 5'-AGA GAG TTA TCA CAG CCC TG-3', exon 6 forward 5'-GGC TGT GAT AAC TCT CTA CA-3' and exon 6 reverse 5'-CCA TCC ATC CAG AGA CAC AG-3', exon 7 forward 5'-TGG TAA GTG GGT CAA TGA GC-3' and exon 7 reverse 5'-CAG CTG TCC ACT TGT TCA TG-3', exon 8 forward 5'-AAG GAA GGG CAG TGA GGA TT-3'and exon 8 reverse 5'-GGT GGA AGT TTA GTG GAG TC-3', exon 9 forward 5'-CGC CTT ATT CCT CTA CTC CT-3'and exon 9 reverse 5'-GAC TGA GTG ACT TAG TGC GT-3', exon 10-1 forward 5'-TCA GTC AGG AGT TGG TCA GT-3'and exon 10-1 reverse 5'-GTC CAG AAC GTC CAG TAG CT-3', exon 10-2 forward 5'-CAG CTA CTG GAC GTT CTG GA-3'and exon 10-2 reverse 5'-CAG TAT CCT GAC TTA GAC GG-3', exon 11 forward 5'-GAG AAA TGC TCC TTT CCC AG-3'and exon 11 reverse 5'-TAG CCC TGG GAG CCA GGT TT-3', exon 12 forward 5'-CTC CCA GGG CAT CTT ATC TT-3'and exon 12 reverse 5'-AGC ACA GGG CAG CAA GTA AC-3'. He received transplantation from an HLA-A, B, C, DR, and DQ-matched unrelated male donor confirmed by a high-resolution molecular method, after the conditioning regimen composed of fludarabine (40 mg/m2 on days -8, -7, -6, -5, -4, -3), busulfan (0.8 mg/kg i.v. q 6 hr on days -6, -5, -4, -3), and thymoglobulin (2.5 mg/kg on days -4, -3, -2). Written informed consent was obtained from parents of the patient before transplantation. Graft versus host disease (GVHD) prophylaxis was composed of cyclosporin, methotrexate (15 mg/kg on day 1, 10 mg/kg on days 3 and 6), and thymoglobulin (1.25 mg/kg on days 7, 9, 11). Other supportive care was performed according to the guideline for stem cell transplantation of our center (6). The number of infused nucleated cells and CD34-positive cells were 7.6×108/kg and 6.2×106/kg, respectively. He received G-CSF from 7 days after stem cell infusion and the nadir of white blood cells (0.09×109/L) occurred 12 days after transplantation. The number of days required for absolute neutrophil count of more than 0.5×109/L and 1.0× 109/L were 14 days and 15 days, respectively, and spontaneous platelet recovery to more than 20×109/L and 100× 109/L required 16 days and 43 days, respectively. Grade I hematuria, grade II proteinuria, and grade II hepatic toxicity occurred during the transplantation but recovered spontaneously. No other serious complications occurred during transplantation. Grade II acute GVHD occurred 17 days after transplantation but resolved after the treatment with steroid. Bone marrow examination performed at one month post-transplantation revealed the donor type chimerism at 98.4% analyzed by short tandem repeat analysis and the normal polymerase chain reaction pattern in exons of the WAS gene (Fig. 1). After 3 months of transplantation, complete donor type chimerism was achieved. Post-transplatation lymphoproliferative disease occurred 4 months after BMT but completely resolved with rituximab therapy. Chronic GVHD did not occur, and he is alive at 16 months after transplantation without any disease characteristics of WAS.
Fig. 1.
A patient diagnosed as Wiskott-Aldrich syndrome with a large deletion in the exon 1 to 11 of the WAS gene (B), compared with a normal control (A) by polymerase chain reaction (PCR) method, received bone marrow transplantation with a reduced toxicity myeloablative conditioning regimen. Bone marrow examination at one month post-transplantation revealed normal PCR pattern for all exons of the WAS gene (C).
DISCUSSION
Purine-analog, in particular, fludarabine, has an advantage over cyclophosphamide. It has an immunosuppressive property that allows the engraftment of hematopoietic stem cells with minimal extramudullary toxicities (3-5). However, fludarabine-based non-myeloablative conditioning with a reduced myeloablative activity by reduction in busulfan dosage than the conventional conditioning regimen was not so successful owing to the poor engraftment in congenital immune-deficiency syndromes (7, 8). Transplantation with fludarabine-based reduced toxicity but fully myeloablative conditioning regimen using the conventional dose of busulfan resulted in good engraftment without serious complications in WAS. This kind of conditioning regimen could improve the outcome of allogeneic transplantation for non-malignant diseases such as congenital immune-deficiency syndromes or metabolic storage diseases.
Footnotes
This study was supported by grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A050089).
References
- 1.Filipovich AH, Stone JV, Tomany SC, Ireland M, Kollman C, Pelz CJ, Casper JT, Cowan MJ, Edwards JR, Fasth A, Gale RP, Junker A, Kamani NR, Loechelt BJ, Pietryga DW, Ringden O, Vowels M, Hegland J, Williams AV, Klein JP, Sobocinski KA, Rowlings PA, Horowitz MM. Impact of donor type on outcome of bone marrow transplantation for Wiskott-Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood. 2001;97:1598–1603. doi: 10.1182/blood.v97.6.1598. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS. Management of primary immune deficiency. J Asthma Allergy Clin Immunol. 2000;20:447–458. [Google Scholar]
- 3.Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, Gyonyor E, Andersson BS. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 4.Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, McDonald GB, Nichols WG, Radich J, Woolfrey A, Jenke A, Schleyer E, Thiede C, Ehninger G, Anasetti C. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 5.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, Shahjahan M, Pierre B, Giralt S, Korbling M, Russell JA, Champlin RE, Andersson BS. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 6.Kang HJ, Shin HY, Choi HS, Ahn HS. Fludarabine, cyclophosphamide plus thymoglobulin conditioning regimen for unrelated bone marrow transplantation in severe aplastic anemia. Bone Marrow Transplant. 2004;34:939–943. doi: 10.1038/sj.bmt.1704720. [DOI] [PubMed] [Google Scholar]
- 7.Longhurst HJ, Taussig D, Haque T, Syndercombe-Court D, Cavenagh J, Edgar JD, Helbert MR. Non-myeloablative bone marrow transplantation in an adult with Wiskott-Aldrich syndrome. Br J Haematol. 2002;116:497–499. doi: 10.1046/j.1365-2141.2002.03269.x. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz ME, Barrett AJ, Brown MR, Carter CS, Childs R, Gallin JI, Holland SM, Linton GF, Miller JA, Leitman SF, Read EJ, Malech HL. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med. 2001;344:881–888. doi: 10.1056/NEJM200103223441203. [DOI] [PubMed] [Google Scholar]