Abstract
Dioscorea batatas is widely used in Asia as a herbal medicine or food product with potential health benefits. There have been several reports of occupational asthma caused by inhalation of D. batatas dust. However, there has been no report of systemic allergic reactions after oral administration of D. batatas. Two patients with D. batatas allergy were enrolled. One had experienced severe urticaria and angioedema after indigestion, and the other had been exposed to D. batatas dust and was diagnosed as having occupational asthma. Both patients had high serum-specific IgE and IgG4 antibodies to D. batatas. And IgE immunoblot demonstrated that both sera bound to a 27-kDa protein with an IgE-binding motif, which was revealed by 2-D-electrophoresis to have the sequence Asn-Val-Glu-Asp-Glu-Phe-Ser-X-Ile-Glu-Gly-Asn-Pro-X-X-Pro-Glu-Asn-X-Gly (pI 6.40, 6.04). In conclusion, discorin from D. batatas (DB3S) was identified as the major allergen of D. batatas in patients sensitized via an oral or inhalant route.
Keywords: Allergen, Dioscorea batatas, Sanyak, Food Hypersensitivity, Inhalant Allergy
INTRODUCTION
Dioscorea batatas, Sanyak, is widely used in Asia as a herbal medicine or food product with potential health benefits. There have been several reports of IgE-mediated rhinitis and asthma caused by inhalation of D. batatas dust (1-4). Recently, investigators have revealed several IgE-binding components in D. batatas extracts in patients with occupational asthma and rhinitis (1-3), although there has been no report identifying the major allergen of D. batatas. Furthermore, there has been no report of systemic allergic reaction after oral administration of D. batatas.
In this study, we compared the IgE-binding components of D. batatas in patients sensitized through the oral and inhalation routes with identification of the major allergen.
MATERIALS AND METHODS
Case reports
Two individuals with histories of allergy to D. batatas were enrolled. Regarding clinical history, one patient had severe urticaria and angioedema from eating fresh D. batatas powder, and the other patient, who had been working in a herbal shop as a herbal merchant, complained of cough, wheezing, and dyspnea upon exposure to D. batatas dust. Skin prick testing with common inhalant allergens, food allergens, and D. batatas extracts was performed. The results of the skin prick tests are expressed as the ratios of mean wheal diameter of allergen to histamine (A/:H ratio).
Patient 1 was a 26-yr-old female, who complained of severe urticaria and angioedema following indigestion of D. batatas with water as a health food. She had been suffering from multiple food allergies including shellfish and peaches, as well as allergic rhinitis.
Patient 2 was a 29-yr-old female who had been working as a merchant dealing in several herbal materials. She presented at the emergency department with sudden onset of dyspnea following exposure to D. batatas dust. Patient 2 had allergies to foodstuffs, including chestnuts and potatoes, as well as allergic rhinitis.
Preparation of D. batatas extracts
D. batatas powder was purchased at a local market and was extracted with phosphate-buffered saline (PBS [pH7.5], 1:5 w/v) at 4℃ overnight. Then it was centrifuged at 10,000 RPM at 4℃ for 30 min, and supernatant was dialyzed against 2L of PBS at 4℃ for 48 hr and then used for the enzyme-linked immunosorbent assays (ELISAs), immunoblot analysis, and 2-dimensional electrophoresis. For the skin prick tests, the supernatants were mixed with an equal amount of sterile glycerin.
Bronchoprovocation testing with D. batatas extracts
Airway responsiveness to methacholine was tested using the 5-breath dosimeter protocol described previously (5). Bronchoprovocation tests were performed according to the procedure used in previous occupational asthma studies (3). The concentrations of inhaled antigen extracts ranged from 1:1,000 w/v to 1:10 w/v.
ELISAs for specific IgE, IgG1, and IgG4 antibodies to D. batatas extract
The presence of specific antibodies to D. batatas extracts was determined by ELISA using a modified method as described previously (2). A 96-well ELISA plate (Corning, Action, MA, U.S.A.) was coated with 1 µg of antigen. The sera of two patients and eighteen non-atopic healthy controls were 1:2 diluted for specific IgE antibody, and 1:10 diluted for specific IgG1 and IgG4 antibodies. The presence of serum specific IgE, IgG1, and IgG4 antibodies was determined by positive cut-off values, which were derived from the mean plus three standard deviations of readings for the sera of the healthy controls.
SDS-PAGE, IgE immunoblot, and 2D gel electrophoresis
D. batatas extract (0.6 µg/well) were applied to a Cambrex precast Tris-glycine homogenous gel (4-20% acrylamide). Electrophoresis was performed with a Novex Mini-cell (Novex, San Diego, CA, U.S.A.) for 90 min at 130 constant voltages. The gel was fixed and stained with Coommassie Brilliant Blue. For immunoblotting the proteins of the gel was transferred to polyvinylidine difluoride membrane (Millipore, Billerica, MA, U.S.A.), which was then treated with a 0.5% fetal bovine serum-Tris-buffered saline solution for 1 hr to block nonspecific protein binding. The membrane was then incubated with the 1:1 vol:vol diluted sera (with TBS) for 2 hr at room temperature, and then washed with TBS with 0.1% Tween-20 (TBS-Tween). Bound specific IgE was detected by biotin-conjugated anti-human IgE antibody (1:1,000 vol/vol, Vector Laboratories Inc.) conjugated with streptavidin alkaline phosphatase (1:1,000 vol/vol, Sigma-Aldrich) followed by the substrate solution (NBT/BCIP kit, Sigma-Aldrich).
2D gel electrophoresis was performed using a modified method as described previously with D. batatas extracts (15 µg per well) (6).
N-terminal amino acid sequencing analysis
To confirm the major allergenic components via N-terminal sequencing, the 2D gel electrophoresed proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane. The protein spots were excised and micro-sequencing was performed using the Procise 492 c1c protein sequencer (Applied Biosystems, Foster City, CA, U.S.A.).
RESULTS
Subject characteristics and clinical findings
Clinical features of two subjects are demonstrated in Table 1.
Table 1.
Clinical features and allergy test results for the two patients
In patient 1, skin prick test showed positive responses to Dermatophagoides pteronyssinus (6.0), Dermatophagoides farinae (3.25), cockroach (2.0), and D. batatas (4.5). Airway hyperresponsiveness to methacholine was confirmed at PC20, 3.563 mg/mL. Oral provocation test with D. batatas could not be done because she had certain histories of severe food allergy reactions twice and refused the provocation test. In patient 2, skin prick test showed positive responses to D. pteronyssinus (1.65), alder (4.4), birch (0.85), and D. batatas (1.0). Airway hyperresponsiveness to methacholine was confirmed at PC20, 0.75 mg/mL, and the bronchoprovocation test to D. batatas extracts (1:1,000 w/v) showed early asthmatic response.
ELISAs for specific IgE, IgG1, and IgG4 antibodies to D. batatas extract
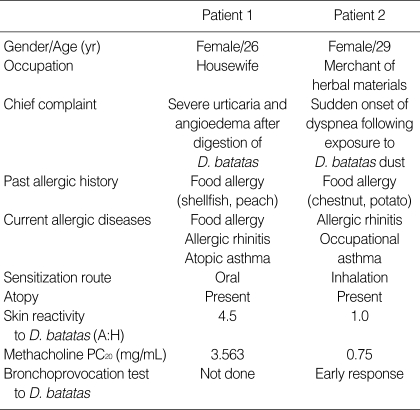
The levels of serum-specific IgE antibodies to D. batatas extracts were significantly higher in the two patients than in non-atopic healthy controls. Serum-specific IgG1 and IgG4 antibodies were also detected in the sera from the two patients (Fig. 1).
Fig. 1.
The levels of specific IgE (A), IgG1, and IgG4 antibodies (B) to D. batatas extracts in the sera of patients (1 and 2) and unexposed healthy controls, as assessed by ELISA. The cut-off value was greater than the mean plus three standard deviations.
IgE immunoblot analysis and 2D gel electrophoresis
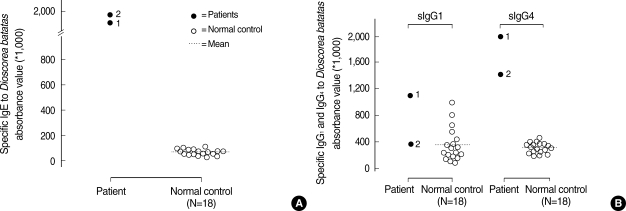
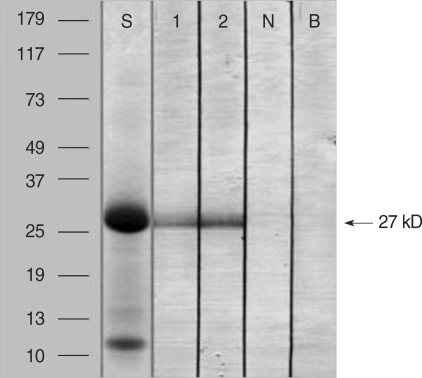
Fig. 2 shows the result of the SDS-PAGE and IgE immunoblot analyses of the D. batatas extracts. A 27-kDa IgE-binding component was detected in the sera of the two patients but not in the serum of the non-atopic healthy control. Fig. 3 shows the results of the 2D electrophoresis and IgE immunoblot. Several spots were noted for the 27-kDa IgE-binding component, and the two largest spots were selected for amino acid sequencing.
Fig. 2.
Analyses by 4-20% SDS-PAGE (S) and IgE immunoblot (1, 73, N, B) of D. batatas extracts.
S, SDS-PAGE; 1 and 2, individual sera from the two patients; N, non-atopic unexposed healthy control; B, buffer control.
Fig. 3.
2D electrophoresis (A) and IgE immunoblot analysis (B) of D. batatas extracts using the sera of the two patients. The IgE-binding 27-kDa protein exhibits several IgE-binding spots, the two largest of which (1 and 2) were selected for amino acid sequencing analysis.
Amino acid sequencing
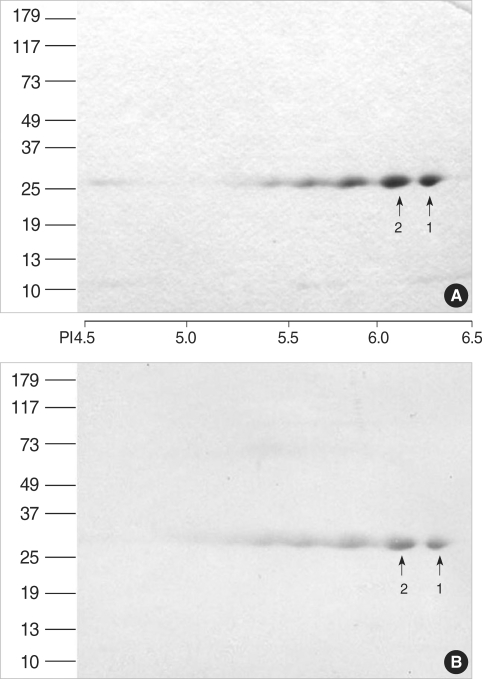
Fig. 4 shows the result of the amino acid sequencing analysis. The N-terminal amino acid sequence of the two largest spots in 27-kDa (pI 6.40 and 6.04, respectively) showed 80% of homology with a 268-amino acid tuber storage protein of D. polystachya.
Fig. 4.
Amino acid sequencing of the major spots from the D. batatas extracts. Gray boxes represent amino acid sequences identical to a 268-amino acid tuber storage protein of D. polystachya.
DISCUSSION
D. batatas is widely distributed in East Asia, including China, Japan, Taiwan, and Korea (7) and, from ancient times, it has been used as a health food to promote digestive functions. Recently, there have been reports that D. batatas has several beneficial anti-oxidative (8, 9), cholesterol-lowering (10), and anti-cholinerasterase activities (11).
In contrast, there have been several reports about the harmful effects of herbal materials including D. batatas, which may act as inhalant allergens to induce bronchial asthma in the form of a typical IgE-mediated response (1, 2, 4, 12-15). There has been no report on food allergy presenting as urticaria and angioedema after the ingestion of D. batatas. Therefore, we compared the immunologic findings between patients who had been sensitized through the oral or the inhalation route. Our results demonstrate that the same IgE-mediated mechanism underlies the development of allergic reactions in response to inhaled or ingested D. batatas, and these findings were verified by the presence in both patients of serum-specific IgE antibodies to a 27-kDa protein in the D. batatas extracts.
In a few studies, specific IgG antibodies have been implicated in the pathogenesis of occupational asthma (16). Some investigators have reported that these antibodies could be used as a marker of exposure, even though they have no clinically significant roles (17, 18). In contrast, we have shown previously the presence of serum-specific IgG4 antibodies in patients with occupational asthma caused by inhalation of various herbal materials, which suggests that serum-specific IgG and IgG4 antibodies have some pathogenic roles (1, 3). In the present study, high levels of serum-specific IgG4 antibodies were noted in the sera of patients with D. batatas-induced occupational asthma and food allergies. Further studies are needed to clarify the association between the presence of serum-specific IgG antibodies and clinical manifestations.
In the present study, we identified the major IgE-binding component in D. batatas extracts, which were found to share the same N-terminal amino acid sequence corresponding to DB3S (BAD18021) from D. batatas reported by Gaidamashvili et al. (19). Dioscorin was originally described as a major tuber storage protein that accounts for about 85% of the total protein content of the tuber of D. rotundata (20). Hou et al. (21) have shown that dioscorins purified from D. batatas are highly homologous to the deduced sequences of dioscorins from another yam species. Gaidamashvili et al. (19) have reported that the mannose-binding lectin of D. batatas is composed of a 66-kDa subunit (DB3L) and two 31-kDa subunits (DB3S). DB3S is composed of 268 amino acids, which was found to be identical to our newly found N-terminal amino acid sequences. However, given that there have been no reports on the allergenic components of D. batatas, this study is the first to identify the allergenic proteins in D. batatas extracts.
In conclusion, D. batatas exposure induces IgE-mediated allergic responses in patients sensitized via oral or inhalation route. We have first identified discorin from D. batatas (DB3S) as a causative allergen of D. batatas allergy in patients sensitized via the oral and inhalant routes.
Footnotes
This study was supported by a grant from the Korean Health 21 R&D Project of the Ministry of Health & Wealfare, Republic of Korea (A050571).
References
- 1.Park HS, Kim MJ, Moon HB. Occupational asthma caused by two herb materials, Dioscorea batatas and Pinellia ternata. Clin Exp Allergy. 1994;24:575–581. doi: 10.1111/j.1365-2222.1994.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee SK, Cho HK, Cho SH, Kim SS, Nahm DH, Park HS. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann Allergy Asthma Immunol. 2001;86:469–474. doi: 10.1016/S1081-1206(10)62498-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Lee YD, Bahn JW, Park HS. A case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng dusts. Allergy. 2006;61:392–393. doi: 10.1111/j.1398-9995.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Jeong H, Kim YK, Cho SH, Min KU, Kim YY. IgE-mediated occupational asthma induced by herbal medicine, Banha (Pinellia ternata) Clin Exp Allergy. 2001;31:779–781. doi: 10.1046/j.1365-2222.2001.01067.x. [DOI] [PubMed] [Google Scholar]
- 5.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Kim HM, Ye YM, Kim SH, Nahm DH, Park HS, Ryu SR, Lee BO. Evaluating the allergic risk of genetically modified soybean. Yonsei Med J. 2006;47:505–512. doi: 10.3349/ymj.2006.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shewry PR. Tuber storage proteins. Ann Bot (Lond) 2003;91:755–769. doi: 10.1093/aob/mcg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou WC, Lee MH, Chen HJ, Liang WL, Han CH, Liu YW, Lin YH. Antioxidant activities of dioscorin, the storage protein of yam (Dioscorea batatas Decne) tuber. J Agric Food Chem. 2001;49:4956–4960. doi: 10.1021/jf010606m. [DOI] [PubMed] [Google Scholar]
- 9.Hou WC, Hsu FL, Lee MH. Yam (Dioscorea batatas) tuber mucilage exhibited antioxidant activities in vitro. Planta Med. 2002;68:1072–1076. doi: 10.1055/s-2002-36356. [DOI] [PubMed] [Google Scholar]
- 10.Lee CL, Wang JJ, Kuo SL, Pan TM. Monascus fermentation of dioscorea for increasing the production of cholesterol-lowering agent-monacolin K and antiinflammation agent--monascin. Appl Microbiol Biotechnol. 2006;72:1254–1262. doi: 10.1007/s00253-006-0404-8. [DOI] [PubMed] [Google Scholar]
- 11.Oh MH, Houghton PJ, Whang WK, Cho JH. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine. 2004;11:544–548. doi: 10.1016/j.phymed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Cartier A, Malo JL, Pineau L, Dolovich J. Occupational asthma due to pepsin. J Allergy Clin Immunol. 1984;73:574–577. doi: 10.1016/0091-6749(84)90513-x. [DOI] [PubMed] [Google Scholar]
- 13.Subiza J, Subiza JL, Escribano PM, Hinojosa M, Garcia R, Jerez M, Subiza E. Occupational asthma caused by Brazil ginseng dust. J Allergy Clin Immunol. 1991;88:731–736. doi: 10.1016/0091-6749(91)90179-r. [DOI] [PubMed] [Google Scholar]
- 14.Moneret-Vautrin DA, Kanny G, Lagrange A. Occupational asthma caused by organic substances. Rev Med Interne. 1994;15(Suppl 2):216s–225s. doi: 10.1016/s0248-8663(05)82239-8. [DOI] [PubMed] [Google Scholar]
- 15.Golec M, Skorska C, Mackiewicz B, Gora A, Dutkiewicz J. Respiratory effects of exposure to dust from herbs. Ann Agric Environ Med. 2005;12:5–10. [PubMed] [Google Scholar]
- 16.Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 17.Tiikkainen U, Klockars M. Clinical significance of IgG subclass antibodies to wheat flour antigens in bakers. Allergy. 1990;45:497–504. doi: 10.1111/j.1398-9995.1990.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Suh CH, Nahm DH, Kim HY. Presence of specific IgG antibody to grain dust does not go with respiratory symptoms. J Korean Med Sci. 1999;14:39–44. doi: 10.3346/jkms.1999.14.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaidamashvili M, Ohizumi Y, Iijima S, Takayama T, Ogawa T, Muramoto K. Characterization of the yam tuber storage proteins from Dioscorea batatas exhibiting unique lectin activities. J Biol Chem. 2004;279:26028–26035. doi: 10.1074/jbc.M402139200. [DOI] [PubMed] [Google Scholar]
- 20.Harvey PJ, Boulter D. Isolation and characterization of the storage protein of yam tubers (Dioscorea rotundata) Phytochemistry. 1983;22:1687–1693. [Google Scholar]
- 21.Hou WC, Liu JS, Chen HJ, Chen TE, Chang CF, Lin YH. Dioscorin, the major tuber storage protein of yam (Dioscorea batatas decne) with carbonic anhydrase and trypsin inhibitor activities. J Agric Food Chem. 1999;47:2168–2172. doi: 10.1021/jf980738o. [DOI] [PubMed] [Google Scholar]