Abstract
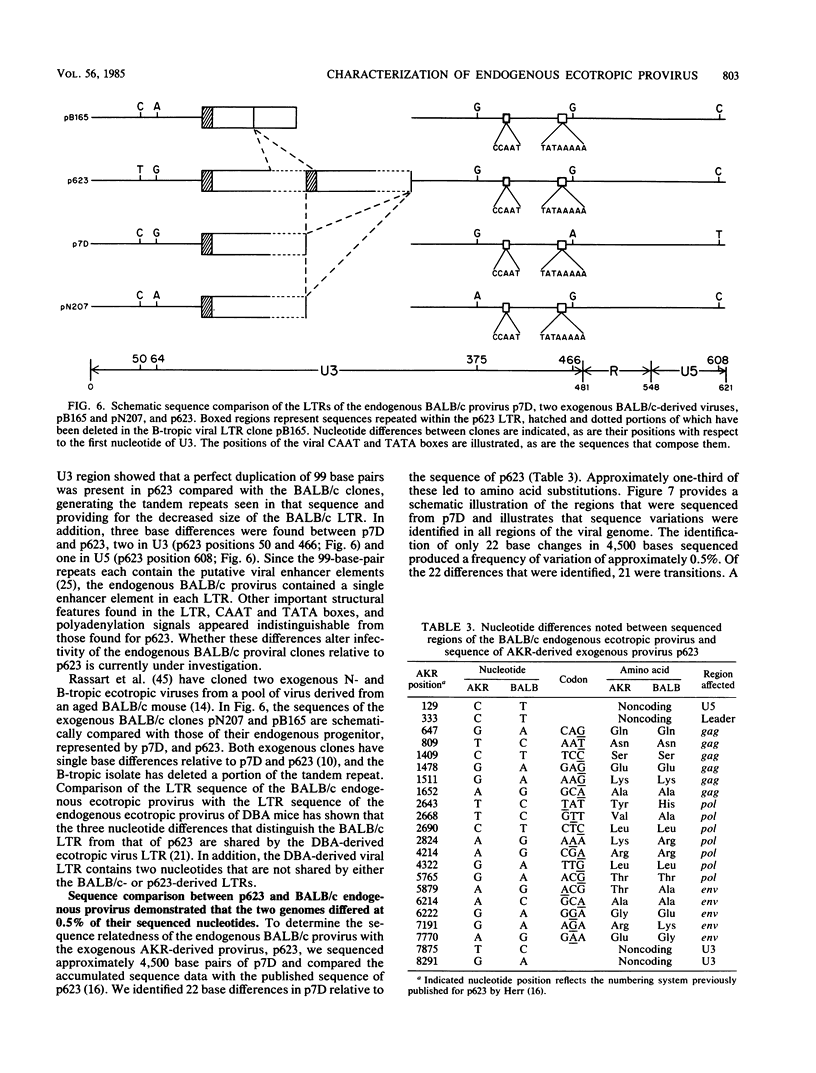
We have isolated two identical molecular clones of the single, endogenous ecotropic provirus of BALB/c mice. The BALB/c clones are approximately 1/10 as infectious as an exogenous proviral clone derived from AKR mice, p623. Transfection of mouse cells with each BALB/c proviral clone yielded XC-negative, N-tropic, ecotropic virus. Cotransfection of subgenomic fragments of p623 and the BALB/c provirus did not increase infectivity to the level observed for p623; however, a 292-base-pair fragment of the p623 env gene was found to rescue XC-plaque formation. Sequence analysis showed that the XC-negative BALB/c provirus differed from the XC-positive AKR-derived provirus at a single nucleotide at the junction of the gp70 and p15E envelope proteins. Extensive sequence analysis of the BALB/c endogenous provirus showed that it differed from the sequence of the AKR-derived provirus at approximately 0.5% of 4,500 sequenced nucleotides. In addition, the BALB/c long terminal repeat contains a single copy of the enhancer-containing sequences that are repeated twice in p623. The limited variation between the ecotropic proviruses of BALB/c mice and AKR mice suggests that few cycles of reverse transcription separate these viral genomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Rowe W. P., Teich N. M., Lowy D. R. Definitive evidence that the murine C-type virus inducing locus Akv-1 is viral genetic material. Proc Natl Acad Sci U S A. 1975 Mar;72(3):906–910. doi: 10.1073/pnas.72.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Wilson A. C. Evidence from mtDNA sequences that common laboratory strains of inbred mice are descended from a single female. Nature. 1982 Jan 14;295(5845):163–165. doi: 10.1038/295163a0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Atchley W. R. Evolution in inbred strains of mice appears rapid. Science. 1985 Jun 7;228(4704):1169–1175. doi: 10.1126/science.4001935. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Copeland T. D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984 Nov;52(2):492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Germ-line MuLV reintegrations in AKR/J mice. Nature. 1982 Apr 29;296(5860):865–868. doi: 10.1038/296865a0. [DOI] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Besmer P., DeLeo A. B., Law L. W. High-frequency cotransfer of the transformed phenotype and a tumor-specific transplantation antigen by DNA from the 3-methylcholanthrene-induced Meth A sarcoma of BALB/c mice. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7555–7559. doi: 10.1073/pnas.78.12.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Jolicoeur P. Variants of N-tropic leukemia virus derived from BALB/c mice. J Virol. 1975 Oct;16(4):991–999. doi: 10.1128/jvi.16.4.991-999.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. A locus that enhances the induction of endogenous ecotropic murine leukemia viruses is distinct from genome-length ecotropic proviruses. J Virol. 1982 Dec;44(3):950–957. doi: 10.1128/jvi.44.3.950-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison K. W., Copeland N. G., Jenkins N. A. Dilute-coat-color locus of mice: nucleotide sequence analysis of the d+2J and d+Ha revertant alleles. Mol Cell Biol. 1984 Dec;4(12):2899–2904. doi: 10.1128/mcb.4.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Domotor J. J., Jr Genetic linkage of C3H/HeJ and BALB/c endogenous ecotropic C-type viruses to phosphoglucomutase-1 on chromosome 5. Science. 1979 Apr 6;204(4388):71–73. doi: 10.1126/science.219476. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Horowitz J. M., Risser R. Structure and expression of endogenous ecotropic murine leukemia viruses in RF/J mice. J Exp Med. 1982 Nov 1;156(5):1461–1474. doi: 10.1084/jem.156.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Activation of nonexpressed endogenous ecotropic murine leukemia virus by transfection of genomic DNA into embryo cells. J Virol. 1983 Mar;45(3):950–955. doi: 10.1128/jvi.45.3.950-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Allelism and linkage studies of murine leukemia virus activation genes in low leukemic strains of mice. J Exp Med. 1982 Apr 1;155(4):1233–1238. doi: 10.1084/jem.155.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in induction of endogenous murine leukemia virus from low leukemic mice. Cell. 1982 Apr;28(4):881–888. doi: 10.1016/0092-8674(82)90067-8. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in the spontaneous production of endogenous murine leukemia virus in low leukemic mouse strains. J Exp Med. 1982 Aug 1;156(2):337–349. doi: 10.1084/jem.156.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. High-efficiency ligation and recombination of DNA fragments by vertebrate cells. Science. 1983 May 6;220(4597):606–609. doi: 10.1126/science.6301012. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F., Doyle T., Linkhart S., Medeiros E., Pickering R. Oncornaviruses produced by murine leukemia cells in culture. Virology. 1977 Sep;81(2):363–370. doi: 10.1016/0042-6822(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Hartley J. W., Spahn G. J., Rabstein L. S., Whitmire C. E., Turner H. C., Huebner R. J. Prevalence of the group-specific (gs) antigen and infectious virus expressions of the murine C-type RNA viruses during the life span of BALB-cCr mice. Int J Cancer. 1972 Sep 15;10(2):283–289. doi: 10.1002/ijc.2910100208. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Rabstein L. S., Spahn G. J., Madison R. M., Huebner R. J. Incidence of spontaneous neoplasms in breeding and retired breeder BALB-cCr mice throughout the natural life span. Int J Cancer. 1972 Sep 15;10(2):273–282. doi: 10.1002/ijc.2910100207. [DOI] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Bremner T. Genetic mapping of a murine leukemia virus-inducing locus of AKR mice. Science. 1972 Nov 24;178(4063):860–862. doi: 10.1126/science.178.4063.860. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. doi: 10.1128/jvi.29.2.555-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]