Abstract
We examined whether alterations in vascular endothelial function and early structural changes in atherosclerosis are associated with microvascular complications in patients with type 1 diabetes mellitus (DM). Flow-mediated dilation (FMD) of the brachial artery and carotid intima-media thickness (IMT) measurement were performed in 70 young adults (aged 19 to 35 yr), 48 with type 1 DM, and 22 normal controls. Patients with diabetes had a lower peak FMD response (7.8±3.9 vs. 11.1±1.9%, p<0.001) and increased IMT (0.51±0.10 vs. 0.42±0.07 mm, p<0.001) compared with controls. Twenty (41.7%) of the patients had microvascular complications including neuropathy, nephropathy, or retinopathy. In these complicated diabetic patients, we found a lower FMD response (6.1±2.5 vs. 9.9±3.5%, p=0.001) compared with diabetics without microvascular complications. The presence of microvascular complications was also associated with older age and longer duration of the disease. However, no differences were observed in IMT, body size, blood pressure, HbA1c, C-reactive protein, low-density lipoprotein or high-density lipoprotein cholesterol levels between complicated and non-complicated patients. Endothelial dysfunction and early structural atherosclerotic changes are common manifestations in type 1 DM, and endothelial dysfunction is thought to be an early event in the atherosclerotic process and important in the pathogenesis of microvascular complications.
Keywords: Vascular Endothelial, Atherosclerosis, Microangiopathy, Diabetes Mellitus
INTRODUCTION
It is widely accepted that endothelial dysfunction precedes and may cause diabetic microangiopathy in type 1 diabetes mellitus (DM) receiving standard insulin therapy (1, 2). However, no clinical studies have been reported, to confirm the relationship between the functional studies of vascular endothelium and the presence of microangiopathy. In the present study, we examined whether alterations in vascular endothelial function and early structural changes in atherosclerosis are associated with the presence of the microvascular complications in young adults with type 1 DM.
MATERIALS AND METHODS
Study populations
The study population consisted of 48 young adults with type 1 DM, all of whom were diabetic for at least 6 yr and were taking insulin. There were 25 males and 23 females (mean age, 24.8±3.6 yr [range, 20 to 33.5 yr]). The duration of their diabetes was 13.8±4.3 yr (range, 6.8 to 24.8 yr). Twenty two age- and gender-matched subjects (age 23.8±5.4 yr [range 19 to 35 yr]; 12 males and 10 females) were recruited for the healthy control subjects after informed consent. We excluded subjects with arterial hypertension, hyperglycemia, hyperlipidemia, microalbuminuria, obesity (body mass index >25 kg/m2), a history of smoking or significant passive exposure to smoking, a family history of premature vascular disease, and those taking any medication. Ten of the diabetic, patients were medicated with angiotensin-converting enzyme inhibitors (ACEi) for more than 3 months. ACEi were medicated for the purpose of renal protection and afterload reduction in the patients who had diabetic nephropathy with or without systemic arterial hypertension.
Study design
At the outpatient clinic, a physical examination was performed, and blood samples were obtained by venipuncture in the morning after 8 to 12 hr of fasting. The serum levels of creatinine, glycosylated hemoglobin (HbA1C), C-reactive protein (CRP), total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol were checked. All type 1 diabetic patients were assessed for subclinical evidence of microvascular complications and abnormal neurologic function. To evaluate the presence of nephropathy, albuminuria was assessed on 24 hr of urine collection. Microalbuminuria was defined as 30-300 mg/24 hr or 20-200 µg/ min in 2 out of 3 consecutive tests taken within 2-3 months. The glomerular filtration rate was estimated from the levels of serum and urine creatinine (3, 4). The presence of retinopathy was assessed by fundus photography, which was interpreted by ophthalmologists. Peripheral nerve function was assessed by the measurement of nerve conduction velocity (NCV) (4, 5).
The vascular study was performed between 11 AM to 2 PM with the usual morning dose of insulin for diabetic subjects and a light, low-fat breakfast for which subjects received specific instructions. For the flow-mediated vasodilation (FMD) of the brachial artery, patients fasted for ≥4 hr before the study. ACEi were discontinued for at least four times of the expected half life of captopril or enalapril in each diabetic patient. Caffein intake and cigarette smoking were prohibited for 24 hr before the study.
After arrival, the subjects rested in a quiet, temperature-controlled room for 15 min in the supine position, and then conventional transthoracic echocardiogram was performed before the procedure of vascular function test. Quantitative analysis in the form of two-dimensional (2D) and Doppler echocardiography was performed to determine ventricular function, aortic insufficiency, and atrioventricular valve regurgitation. We excluded patients with moderate to severe ventricular dysfunction, aortic insufficiency, or atrioventricular valve regurgitation.
The study protocol was approved by the Ethics Committee of Seoul National University (Seoul, Korea), and informed consent was obtained before the study from all patients and/or their parents after a detailed description of the procedure.
FMD and intima media thickness (IMT)
All studies were performed with a Vivid 7 ultrasound machine (GE-Vingmed, Horten, Norway) using 12-MHz linear array transducer. FMD of the brachial artery was assessed according to the standard protocols (6). After recording baseline image and Doppler signal, a blood pressure cuff was placed at the distal forearm and inflated to supra-systolic pressure (180-200 mmHg) for 5 min. Then the cuff was rapidly deflated and Doppler signal was recorded for 15 sec. The vessel was continuously scanned from baseline until 3 min after cuff deflation. Digital loops were stored on the hard disk of the echocardiography machine, and transferred to a workstation (EchoPAC PC, General Electric, Horten, Norway) for offline analysis. The brachial artery diameter was determined in end diastole, indicated by the R wave of the electrocardiogram. Reactive hyperemia (RH) was calculated as the percentage change in blood flow from baseline. Blood flow (F) was calculated from mean velocity of blood (V) and vessel diameters (D) during baseline (V1, D1) and maximal reactive hyperemic period (V2, D2) as follows: RH (%)=100(F2-F1)/F1=100 (V2× πD22-V1× πD12)/ V1× D12.
Maximal obtained diameter during ischemia-induced hyperemia was used for the calculation of FMD. FMD was expressed as percent changes in the diameter ([maximum diameter-baseline diameter]/baseline diameter×100%).
Carotid IMT was measured at common carotid artery, 10 mm proximal to the bifurcation. The far wall IMT was identified as the region between the lumen- intima interface and the media-adventitia interface (7). Imaging was performed by two experienced sonographers who were blinded to the clinical or laboratory profile of the study patient. Offline measurements were taken by two physicians who also had no knowledge of the clinical or laboratory profile of the study subjects. Data from at least 3 measurements were averaged for the calculation of FMD and IMT. The intra- and inter-observer variability expressed as coefficients of variation were 6.8 and 7.1% for FMD, and 5.4 and 5.8% for IMT, respectively. The results of the measurements of FMD and IMT were highly reproducible.
Statistical analysis
All data were stored and analyzed using the SPSS statistical package 12.0 (SPSS Inc., Chicago, IL, U.S.A.). Descriptive data are expressed as mean±standard deviation (SD). For the comparison of quantitative data of the 2 groups, the Student's t test or chi-square test was applied. A p value less than 0.05 was considered statistically significant.
RESULTS
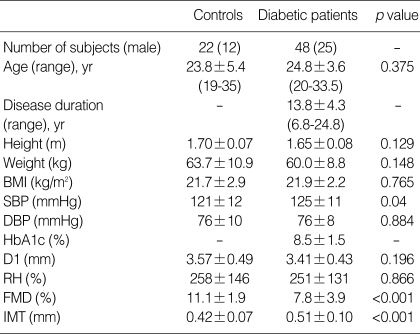
The clinical characteristics of the type 1 diabetic patients and controls are given in Table 1. There were no significant differences in age, gender distribution, body weight, height, body mass index, and diastolic blood pressure between the groups. However, mean systolic blood pressure was significantly higher in diabetic patients. Twelve (25%) of the diabetic patients had systemic hypertension.
Table 1.
Clinical characteristics and results of the ultrasound studies in controls and type 1 diabetic patients
Data are presented as the mean value±SD.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; D1, baseline brachial artery diameter; RH, reactive hyperemia; FMD, flow-mediated vasodilation; IMT, intima-media thickness.
The results of ultrasound studies are shown in Table 1. There were no significant differences between the two groups in brachial artery baseline diameter or increase in blood flow during reactive hyperemia. Patients with type 1 DM had lower peak FMD response (7.8±3.9 vs. 11.1±1.9%, p<0.001) and increased IMT (0.51±0.10 vs. 0.42±0.07 mm, p<0.001) compared with controls. Thirty-six diabetic patients without hypertension also showed reduced FMD (7.2±3.2 vs. 11.1 ±1.9%, p<0.001) and increased IMT (0.51±0.11 vs. 0.42 ±0.07 mm, p=0.001) compared with controls.
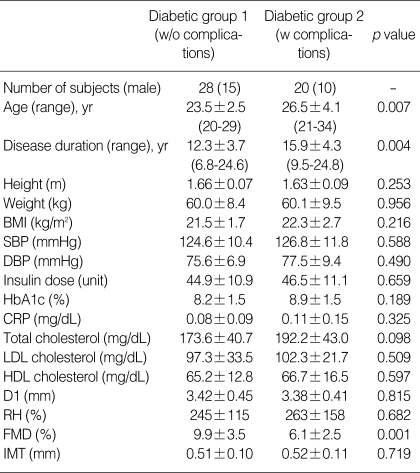
Twenty (41.7%) of the patients had one or more microvascular complications including neuropathy, nephropathy, or retinopathy. Thirteen patients had nephropathy, eight patients had retinopathy, and two patients had neuropathy. Two of the patients who had diabetic nephropathy also had retinopathy concomitantly. In these complicated diabetic patients, we found lower FMD response compared with diabetics without microvascular complications (6.1±2.5 vs. 9.9±3.5%, p=0.001) (Table 2). The presence of microvascular complications was also associated with older age and longer duration of the disease. However, no differences were observed in IMT, body size, systolic or diastolic blood pressure, HbA1c, CRP, LDL or HDL cholesterol levels between complicated and non-complicated patients.
Table 2.
Clinical characteristics and results of the laboratory and ultrasound studies in non-complicated (diabetic group 1) and complicated (diabetic group 2) type 1 diabetic patients
Data are presented as the mean value±SD.
w/o, without; w, with; CRP, C-reactive protein; HDL and LDL, high- and low-density lipoprotein; other abbreviations are same as in Table 1.
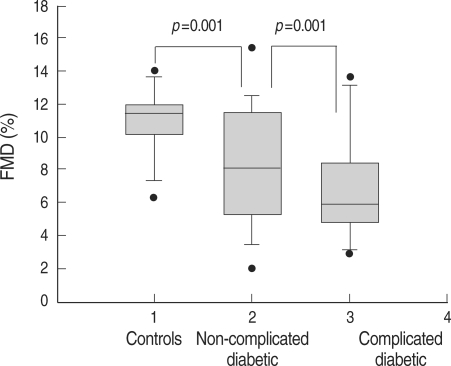
Non-complicated diabetic patients showed significantly lower FMD (9.9±3.5 vs. 11.1±1.9%, p=0.001) and higher IMT (0.51±0.09 vs. 0.42±0.07 mm, p=0.013) compared with controls. Fig. 1 shows the peak FMD values across 3 groups (controls and diabetic patients with and without microvascular complications).
Fig. 1.
Comparison of the results of flow-mediated vasodilation (FMD) in three study groups (controls, non-complicated and complicated type 1 diabetic patients).
DISCUSSION
Morbidity and mortality in diabetes mellitus are caused chiefly by its vascular complications, both in the microcirculation and in large vessels. Microangiopathy of the vasa nervorum is important in the pathogenesis of diabetic neuropathy, and diabetic retinopathy and nephropathy are the hallmarks of microangiopathy. Diabetic nephropathy is strongly associated with other complications, including neuropathy, retinopathy, and atherosclerosis (8). Considerable laboratory and clinical evidence indicates that endothelial dysfunction is a critical part of the pathogenesis of microvascular and macrovascular complications in type 1 DM (9-11). However, the literature on endothelial function in diabetes is complex in part because endothelial function can be measured in many ways and in many vascular beds (1).
The FMD, endothelium-dependent vasodilation during reactive hyperemia, is predominantly modulated by local release of nitric oxide (12). Impaired local availability of nitric oxide and endothelium-dependent vasodilation may result from either short- or long-term exposure to several factors. With recurrent or persistent exposure to the risk factors, there is a state of persistent endothelial dysfunction and altered vascular wall milieu that promotes structural changes of atherosclerosis (13). In the present study, the patients with type 1 DM had lower peak FMD response and increased IMT compared with controls. Several studies have reported that endothelial dysfunction is common in children and young adults with type 1 DM of short duration (5, 13-15). Endothelial dysfunction in large arteries is paralleled by endothelial dysfunction in resistance vessels and metabolically important capillary beds that contributes to the development of the metabolic syndrome (16). Impaired FMD response is associated with increased carotid artery IMT. Endothelial dysfunction in children with type 1 DM may predispose them to the development of early atherosclerosis (14). Increased IMT is believed to represent structural atherosclerosis and is a proven risk factor for myocardial infarction and stroke in older adults (17). Our result is consistent with the previous study (18), which found a significant, albeit small, increase in carotid IMT in diabetic children compared with control subjects.
The mean FMD values in controls and diabetic patients in the present study were higher than those observed in the previous studies (14, 15). And the mean IMT values in controls and diabetic patients in the present study were lower than those observed in the previous study (14). Compared with the diabetic children of these former studies, those included in the present study were older, had a longer duration of diabetes, and many (41%) of the patients had diabetic microvascular complications. Significant differences in genetic and environmental cardiovascular risk factors (lifestyle, diet, or other risk factors) between the study populations may explain the discrepancy.
The mechanisms of endothelial dysfunction and accelerated atherosclerosis in diabetes are multifactorial and have not been fully characterized. In this study, the glycemic control in patients was suboptimal, with a mean glycosylated hemoglobin value of 8.5±1.5% (range, 5.4 to 12%; normal value, 4.0 to 6.0% [19]). It is known that hyperglycaemia and its immediate biochemical sequelae directly alter endothelial function or influence endothelial cell functioning indirectly by the synthesis of growth factors, cytokines, and vasoactive agents in other cells (1, 20, 21). In vitro, endothelial cells exposed to high glucose show an increased production of extracellular matrix components, such as collagen and fibronectin, and of procoagulant proteins, such as von Willebrand factor and tissue factor, and decreased proliferation, migration, and fibrinolytic potential (22). Many of these features are also apparent in vivo, especially in patients with micro- or macroalbuminuria (23). Glycosylation of LDL apolipoprotein B through nonenzymatic linkages and production of advanced glycation end products are characteristic to diabetic hyperglycemia (24). Recent experimental studies have shown glycated LDL to induce various atherogenic events, including endothelial dysfunction (25). The components of the metabolic syndrome can also affect endothelial function in diabetes (26). Regardless of the presence of diabetes, chronic low-grade inflammation is closely associated with endothelial dysfunction (27, 28).
In type I DM, patients who have had diabetes for more than 5-10 yr are characterized by subtle increases in blood pressure and autonomic dysfunction (29). In this study, diabetic patients showed higher systolic blood pressure than controls. Because hypertension itself causes endothelial activation as indicated by elevated levels of soluble adhesion molecules (30) and impaired NO availability (1), hypertension should be another factor that resulted in endothelial dysfunction in diabetic patients. Hypertension is a confounding variable that can render the data interpretation problematic. Nevertheless, there were 36 patients with type 1 DM without hypertension who showed abnormal FMD and IMT.
In the present study, non-complicated diabetic patients showed significantly lower FMD compared with controls. It is consistent with the previous study, which reported that type 1 diabetic children without diabetic complications had attenuated endothelial function compared with controls (15). Diabetic patients with microvascular complications showed more severely impaired FMD than diabetic patients without these complications. NG-Monomethyl-L-arginine (an inhibitor of endothelium-derived relaxing factor/nitric oxide biosynthesis) did not influence nitroprusside (an endothelium-independent vasodilator) responses but reduced carbachol (an endothelium-dependent vasodilator) responses in control subjects and normoalbuminuric diabetic patients but not in microalbuminuric diabetic patients. These results provided evidence of abnormal nitric oxide biosynthesis in insulin-dependent diabetic patients with microalbuminuria (31). On the contrary, Donaghue et al. reported that the changes in large vessel endothelial function were not related to detectable microvascular disease or autonomic dysfunction (5). Differences in the study populations may explain this disparity. The subjects enrolled in the previous study (5) were younger, had a shorter duration of diabetes compared to the present study. The small patient population in the previous study may be insufficient to reveal the association between endothelial function and the presence of microangiopathy.
Endothelial dysfunction, as estimated by plasma vWF concentration, preceded the development of microalbuminuria in type 1 DM (2). Other prospective studies have shown that high vWF concentrations are associated with an increased risk of developing microalbuminuria, an increased progression of microalbuminuria, the occurrence of diabetic retinopathy and neuropathy and, an increased risk of cardiovascular events and death (32-34). Endothelial dysfunction can contribute to the pathogenesis of albuminuria both directly, by causing increased glomerular pressure and the synthesis of a leaky glomerular basement membrane, and indirectly, by influencing glomerular mesangial and epithelial cell function in a paracrine fashion (1). Chronic low-grade inflammation can be both the cause and consequence of endothelial dysfunction, and it is one candidate to explain the association between (micro) albuminuria and extrarenal complications (35, 36). Genetic or environmental factors are also likely to play a role in determining who among type 1 diabetic patients go on to develop aggressive angiopathy and who do not (1).
The IMT measurements in diabetic patients were significantly greater than those in the control population. However, we found no differences in carotid IMT between complicated and non-complicated diabetic patients. In a recent study, it was shown that abnormal FMD occurs within the first decade of the disease in children with type 1 DM and precedes the increase in IMT (13). Although endothelial dysfunction is not atherosclerosis, it may be an important pathophysiological precursor (37, 38). The increase in the carotid IMT would occur after a considerably longer exposure to the diabetic milieu (13), and it is the likely explanation for the findings in our study. The relative timing of these events is important in the evaluation of strategies to prevent progression of atherosclerosis and other vascular complications in this patient population (13).
In conclusion, this study shows that young adults with type 1 DM have signs of large vascular endothelial dysfunction and early atherosclerosis. Although the present study is a cross-sectional study and only a small number of young adults were investigated, our findings indicate that changes in systemic vascular endothelial function predispose to microvascular complications. Our results emphasize the importance of early detection and control of risk factors of endothelial dysfunction in these patients. The assessment of FMD responses may provide a valuable tool for risk stratification of the young patients with type 1 DM.
Some limitations should be noted in this study. First, the size of the patient population was small compared with that of large studies of type 2 DM. Second, it was a cross-sectional study that assessed the impairment of subclinical vascular endothelial function and the presence of microangiopathy in young adults with type 1 DM, so further longitudinal studies would be needed to clarify whether an improvement in endothelial function would reduce the development of microvascular complications in the young type 1 diabetic patients. Third, although the patients with moderate to severe ventricular dysfunction were excluded from this study, there must be some interactions between ventricles and peripheral vessels. The influences of ventricles on vessels were not analyzed in the present study.
References
- 1.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 2.Stehouwer CD, Fischer HR, van Kuijk AW, Polak BC, Donker AJ. Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes. 1995;44:561–564. doi: 10.2337/diab.44.5.561. [DOI] [PubMed] [Google Scholar]
- 3.Bennett PH, Haffner S, Kasiske BL, Keane WF, Mogensen CE, Parving HH, Steffes MW, Striker GE. Screening and management of microalbuminuria in patients with diabetes mellitus: Recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107–112. doi: 10.1016/0272-6386(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 4.Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ. 2006;333:475–480. doi: 10.1136/bmj.38922.650521.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaghue KC, Robinson J, McCredie R, Fung A, Silink M, Celermajer DS. Large vessel dysfunction in diabetic adolescents and its relationship to small vessel complications. J Pediatr Endocrinol Metab. 1997;10:593–598. doi: 10.1515/jpem.1997.10.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy R, Case C, Fathi R, Johnson D, Isbel N, Marwick TH. Does renal failure cause an atherosclerotic milieu in patients with end-stage renal disease? Am J Med. 2001;110:198–204. doi: 10.1016/s0002-9343(00)00695-1. [DOI] [PubMed] [Google Scholar]
- 8.Stehouwer CD, Schaper NC. The pathogenesis of vascular complications of diabetes mellitus: one voice or many? Eur J Clin Invest. 1996;26:535–543. doi: 10.1046/j.1365-2362.1996.1780527.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen RA. Dysfunction of vascular endothelium in diabetes mellitus. Circulation. 1993;87:V67–V76. [Google Scholar]
- 10.Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997;34:55–68. doi: 10.1016/s0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- 11.Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000;43:1205–1223. doi: 10.1007/s001250051515. [DOI] [PubMed] [Google Scholar]
- 12.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 13.Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41:661–665. doi: 10.1016/s0735-1097(02)02894-2. [DOI] [PubMed] [Google Scholar]
- 14.Jävisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimäki T, Rönnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 15.Wiltshire EJ, Gent R, Hirte C, Pena A, Thomas DW, Couper JJ. Endothelial dysfunction relates to folate status in children and adolescents with type 1 diabetes. Diabetes. 2002;51:2282–2286. doi: 10.2337/diabetes.51.7.2282. [DOI] [PubMed] [Google Scholar]
- 16.Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes. 1997;46(Suppl 2):S9–S13. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- 17.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 18.Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, Celermajer DS, Raitakari OT. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 19.Shivalkar B, Dhondt D, Goovaerts I, Gaal L, Bartunek J, Van Crombrugge P, Vrints C. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol. 2006;97:77–82. doi: 10.1016/j.amjcard.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 21.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzi M. Glucose toxicity in the vascular complications of diabetes: the cellular perspective. Diabetes Metab Rev. 1992;8:85–103. doi: 10.1002/dmr.5610080202. [DOI] [PubMed] [Google Scholar]
- 23.Stehouwer CD, Donker AJ. Urinary albumin excretion and cardiovascular disease risk in diabetes mellitus: is endothelial dysfunction the missing link? J Nephrol. 1993;6:72–92. [Google Scholar]
- 24.Brownlee M. Lilly Lecture 1993: glycation and diabetic complications. Diabetes. 1994;43:836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 25.Posch K, Simecek S, Wascher TC, Jurgens G, Baumgartner-Parzer S, Kostner GM, Graier WF. Glycated low-density lipoprotein attenuates shear stress-induced nitric oxide synthesis by inhibition of shear stress-activated L-arginine uptake in endothelial cells. Diabetes. 1999;48:1331–1337. doi: 10.2337/diabetes.48.6.1331. [DOI] [PubMed] [Google Scholar]
- 26.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 27.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S25–S28. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]
- 28.Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis. 2002;161:233–242. doi: 10.1016/s0021-9150(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 29.van Ittersum FJ, Spek JJ, Praet IJ, Lambert J, IJzerman RG, Fischer HR, Nikkels RE, Van Bortel LM, Donker AJ, Stehouwer CD. Ambulatory blood pressures and autonomic nervous function in normoalbuminuric type I diabetic patients. Nephrol Dial Transplant. 1998;13:326–332. [PubMed] [Google Scholar]
- 30.Boulbou MS, Koukoulis GN, Makri ED, Petinaki EA, Gourgoulianis KI, Germenis AE. Circulating adhesion molecules levels in type 2 diabetes mellitus and hypertension. Int J Cardiol. 2005;98:39–44. doi: 10.1016/j.ijcard.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Elliot TG, Cockcroft JR, Groop PH, Viberti GC, Ritter JM. Inhibition of nitric oxide synthesis in forearm vasculature of insulin dependent diabetic patients: blunted vasoconstriction in patients with microalbuminuria. Clin Sci (Lond) 1993;85:687–693. doi: 10.1042/cs0850687. [DOI] [PubMed] [Google Scholar]
- 32.Verrotti A, Greco R, Basciani F, Morgese G, Chiarelli F. von Willebrand factor and its propeptide in children with diabetes. Relation between endothelial dysfunction and microalbuminuria. Pediatr Res. 2003;53:382–386. doi: 10.1203/01.PDR.0000049509.65496.BF. [DOI] [PubMed] [Google Scholar]
- 33.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. Prognostic implications of retinopathy and a high plasma von Willebrand factor concentration in type 2 diabetic subjects with microalbuminuria. Nephrol Dial Transplant. 2001;16:529–536. doi: 10.1093/ndt/16.3.529. [DOI] [PubMed] [Google Scholar]
- 34.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:3071–3078. doi: 10.1161/01.atv.19.12.3071. [DOI] [PubMed] [Google Scholar]
- 35.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 36.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2002;22:593–598. doi: 10.1161/01.atv.0000013786.80104.d4. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105:2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 38.Harrison DG, Armstrong ML, Freiman PC, Heistad DD. Restoration of endothelium-dependent relaxation by dietary treatment of atherosclerosis. J Clin Invest. 1987;80:1808–1811. doi: 10.1172/JCI113276. [DOI] [PMC free article] [PubMed] [Google Scholar]