Abstract
We initiated this study to investigate whether combining Helicobacter pylori eradication with immunosuppressive therapy provides an additional benefit to patients with idiopathic thrombocytopenic purpura (ITP) that has relapsed or has not responded to steroid and/or danazol therapy in patients who have H. pylori infection. Thirty-four patients with chronic ITP that had relapsed or failed to steroid and/or danazol therapy were assessed for H. pylori infection. Of the 21 confirmed cases, 12 patients were given H. pylori eradication therapy alone (EA), while 9 patients received eradication therapy combined with immunosuppressive therapy (EI). The response rate was not significantly different between patients in the EA and those in the EI group (41.7% in the EA group vs. 66.7% in the EI group, p=0.345). The median platelet count at 6 months after therapy was higher in the EI group patients (75×109/L in the EI group patients vs. 18×109/L in the EA group patients, p=0.028). The median response duration was also longer in the EI group patients (9 months in the EI group patients vs. 3 months in the EA group patients, p=0.049). These results show that a significant benefit is gained by the use of H. pylori eradication combined with immunosuppressive therapy over the use of eradication therapy alone for patients with chronic ITP.
Keywords: Helicobacter pylori; Purpura, Thrombocytopenic, Idiopathic; Eradication
INTRODUCTION
Idiopathic thrombocytopenic purpura (ITP) is a disorder characterized by thrombocytopenia with a shortened platelet survival, normal to increased numbers of bone marrow megakaryocytes, and elevated levels of platelet-associated IgG (1, 2). Autoantibodies directed against platelet membrane glycoprotein and that lead to platelet destruction form the leading cause of ITP.
Helicobacter pylori has been considered a central etiologic agent for gastritis, peptic ulcer, and gastric adenocarcinoma for many years (3, 4). H. pylori infection has also been implicated in the pathogenesis of non-digestive diseases, such as vascular disease (coronary heart disease), autoimmune diseases (rheumatoid arthritis, Sjögren's syndrome, autoimmune thyroid diseases, and Henoch-Schönlein purpura), and certain skin diseases (chronic urticaria and rosacea) (5-7).
Recently, it has been suggested that H. pylori may contribute to the pathogenesis of ITP (8-14). Even though H. pylori eradication therapy has been shown to be effective in some studies (9-15), results have been inconsistent from study to study, and the pathogenetic mechanisms of H. pylori-associated ITP remain elusive. In one study, patients had a very low likelihood of effectiveness, and a response was seen 3 months after treatment (16). However, it should be noted that serious complications such as bleeding could happen within this 3-month period.
Therapy options for H. pylori-associated ITP may include both eradication therapy and classical therapy for ITP. Prednisolone is the drug of choice, but cyclophosphamide and vincristine have also been shown to be effective immunosuppressive agents in classic ITP therapy (17-19). Therefore, we hypothesized that adding immunosuppressive agents to eradication therapy would maximize the therapeutic effect. The purpose of this study was to assess the efficacy of H. pylori eradication therapy combined with immunosuppressive therapy.
MATERIALS AND METHODS
Patients
Between January 2003 and January 2006, 34 consecutive adult patients with chronic ITP were investigated in this study. The usual-first line therapy in patients with bleeding tendency and a low platelet count is treatment with prednisolone at a dose of 1 mg/kg/day.
Patients who had platelet counts of less than 30×109/L at least 3-6 months after steroid therapy were candidates for danazol and/or vincristine therapy. Patients with gum bleeding or a platelet count of less than 20×109/L while undergoing the above therapy received intravenous immunoglobulin therapy or platelet transfusion. Thirty-four patients with no response to this treatment schedule were enrolled to the present study. The diagnosis of ITP was made according to the criteria set forth by the American Society of Hematology (ASH) guidelines (8)-based thrombocytopenia unrelated to any underlying viral infection, collagen vascular disease, malignancy or medication. Patients were considered elligible if they presented during an acute initial or recurrent episodes of ITP, such that platelet counts did not increase to a safe level despite treatment with multiple modalities or if chronic ITP could not be managed with acceptable doses of corticosteroids.
This retrospective study was reviewed and approved by our institutional review board.
Response criteria
The clinical response to treatment was defined as follows. Complete response (CR) was defined as normalization of the platelet count (≥150×109/L) for more than 2 months with or without maintenance therapy. Partial response (PR) was defined as a platelet count between 50×109/L and 150×109/L for more than 2 months or when the platelet count increased above 50% compared to the pretreatment level with or without maintenance therapy. No response (NR) is defined as a platelet count below 50×109/L or when the platelet count did not increase to more than 50% of the pretreatment level with or without maintenance therapy.
Diagnosis of H. pylori infection
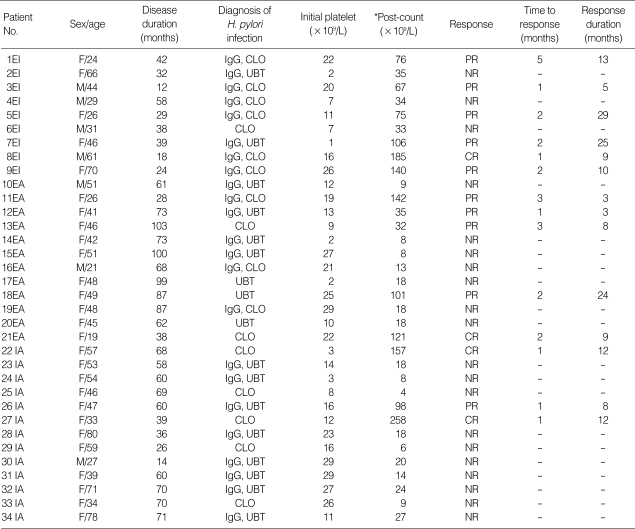
All patients were screened for H. pylori infection using a 13C-urea breath test (UBT), serum H. pylori immunoglobulin G, or a rapid urease test (CLO test) by an endoscopic biopsy (Table 1).
Table 1.
Clinical data and response to therapy in the patients
*Maximum platelet count after therapy was started.
IgG, Helicobacter pylori IgG; CLO, rapid urease test; UBT, 13C-urea breath test; EI, eradication therapy combined with immunosuppressive therapy; EA, H. pylori eradication therapy alone; CR, complete response; PR, partial respons; NR, no response; IA, immunosuppressive therapy alone; VA, vincristine; IVIG, intravenous immune globulin.
Treatment
H. pylori infection was treated with standard eradication therapy. For H. pylori-positive patients, therapy consisted of amoxicillin 1,000 mg, clarithromycin 500 mg, and a proton pump inhibitor (esomeprazole) 40 mg taken together twice a day for 2 weeks.
Cycles of immunosuppressive therapy were repeated every 3 weeks. Patients were treated with an immunosuppressive therapy consisting of oral cyclophosphamide (150 mg/m2/day taken orally on days 1 to 5 every 3 weeks), vincristine (1.4 mg/m2 taken intravenously on day 1 every 3 weeks), and prednisolone (40 mg/m2 taken orally on days 1 to 5 every 3 weeks) with a maximum of 6 cycles of therapy.
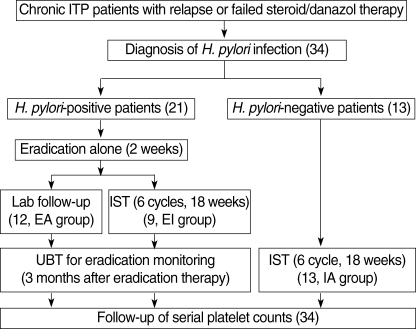
All of the 21 H. pylori-positive patients were treated with eradication therapy for 2 weeks, and 9 of the 21 patients were treated with additional immunosuppressive therapy immediately after receiving the eradication therapy. Monitoring of bacterial eradication using the UBT was performed at 3 months after the eradication therapy. H. pylori-negative patients received immunosuppressive therapy alone with the same schedule (Fig. 1).
Fig. 1.
Flow-chart of treatment indicating the three schedules for chronic ITP patients.
Round braskets, number of patients and treatment duration; EA, eradication alone; EI, eradication combined with immunosuppressive therapy; IA, immunosuppressive therapy alone.
Monitoring
Eradication or persistence of H. pylori was assessed after 3 months of eradication therapy using a UBT. The platelet count was assessed every week for a month and then on a monthly basis.
Statistical analysis
Numerical data were compared with the use of the Kruskal-Wallis H test and the Mann-Whitney U test for independent samples. Differences were considered to be statistically significant at a level of p<0.05. The event-free-response duration was assessed using the Kaplan-Meier method and compared between risk groups using the log-rank test. An event was defined as a platelet count below 20×109/L after initial response had been achieved. Data were analyzed using the Statistical Software Package for the Social Sciences (SPSS version 12.0 for Windows).
RESULTS
Patients
Of the 34 adult ITP patients, 7 were male and 27 were female; the median age was 45 yr (range, 19 to 70 yr). The median disease duration was 58 months (range, 12-103 months), and the median platelet count was 13.5×109/L (range, 1-29×109/L). All of the 34 patients were treated previously with steroid, danazol, and/or vincristine therapy. All of the patients transiently responded to steroid treatment for about 2 months, but relapsed. After relapse, all of the patients were treated with danazol and/or vincristine therapy, but without a durable response. H. pylori infection was confirmed in 21 of the 34 (61.7%) patients (Table 1).
H. pylori-positive patients
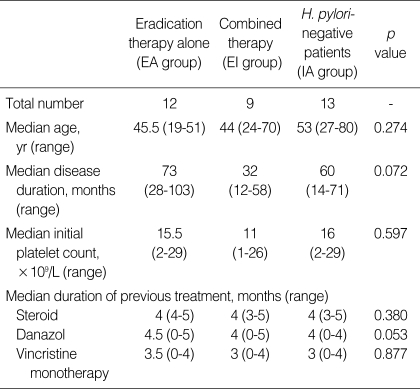
The 21 H. pylori-positive patients received eradication therapy alone (EA) or eradication therapy combined with immunosuppression (EI) according to the preference of the physicians. The median disease duration before eradication therapy was 73 months for patients in the EA group and 32 months for patients in the EI group. The initial mean platelet count was 15.5×109/L (range, 2-29×109/L) for patients in the EA group and 11×109/L (range, 126×109/L) for patients in the EI group. The median durations of previous steroid, danazol, and vincristine therapy were 4, 4.5, and 3.5 months for patients in the EA group, respectively, and 4, 4, and 3 months for patients in the EI group, respectively. The types of drugs used for treatment including steroid, danazol, and vincristine were not different between patients in the two groups. The demographic composition of the two groups was well balanced (Table 2).
Table 2.
Characteristics of the patients
EA, H. pylori eradication therapy alone; EI, eradication therapy combined with immunosuppressive therapy; IA, immunosuppressive therapy alone.
H. pylori-negative patients
The 13 H. pylori-negative patients were treated with immunosuppressive therapy alone (IA) and compared with the H. pylori-positive EI group of patients to evaluate the usefulness of eradication therapy. The median age was 53 yr and median disease durations before treatment was 60 months. The initial mean platelet count were 16×109/L (range, 2-29×109/L). The median durations of previous steroid, danazol and vincristine therapy were 4, 4, and 3 months for patients in the IA group (Table 2). The characteristics of the patients were not different from those of the H. pylori-positive patients.
Comparison of treatment response
In comparison of the EA and EI groups of patients, the time to response did not show a statistically significant difference.
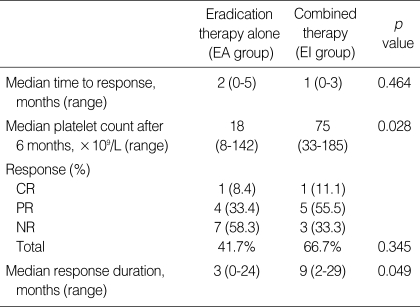
To investigate the predictive value of the response to treatment for the H. pylori-positive patients, several parameters including the mean platelet count at 6 months after treatment, the response duration, and the response rate were compared between the EA and EI groups of patients. The median platelet count at 6 months after treatment was 75×109/L for the EI group of patients compared with 18×109/L for the EA group of patients (p=0.028). The median response duration was 9 months for the EI group of patients, compared with 3 months for the EA group patients (p=0.049). Five patients (41.7%) in the EA group achieved a response (CR in one and PR in four patients), while 6 patients (66.7%) in the EI group also achieved a response (CR in one and PR in five patients). The response rate was not significantly different between the two groups (p=0.345) (Table 3).
Table 3.
Comparative results between H. pylori eradication therapy and immunosuppressive therapy of the patients
EA, H. pylori eradication therapy alone; EI, eradication therapy combined with immunosuppressive therapy; CR, complete response; PR, partial response; NR, no response.
Whether or not, H. pylori infection affects the pathogenesis of ITP and eradication results in a beneficial effect, the efficacy of treatment was compared between H. pylori-positive patients (EI group) and H. pylori-negative patients (IA group). The response rate for the two groups was not significantly different (p=0.096). The EI group of patients showed a higher median platelet count at 6 months after treatment (75×109/L for patients in the EI group and 18×109/L for patients in the EA group) and the median response duration (9 months for patients in the EI group and 3 months for patients in the EA group) was higher in the EI group than in the EA group (p=0.017 and 0.003, respectively). Three patients (23.1%) in the IA group achieved a confirmed response (CR in two and PR in one). The response rate was not significantly different between the EI and IA groups (Table 4).
Table 4.
Comparable results of immunosuppressive therapy between H. pylori-positive and -negative patients
EI, eradication therapy combined with immunosuppressive therapy; IA, immunosuppressive therapy alone; CR, complete response; PR, partial response; NR, no response.
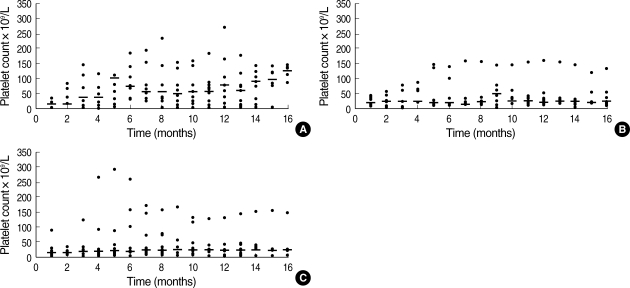
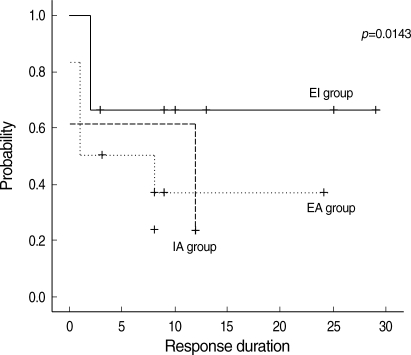
In a comprehensive evaluation, the serial platelet counts for patients in the EI group were highest among the three groups (Fig. 2). The median 2-yr response duration rates of the EI, EA, and IA groups were 66.7%, 41.7%, and 23.1%, respectively. However, the response duration was longer in the EI group of patients than in the other two groups (p=0.0143) (Fig. 3).
Fig. 2.
Distribution of serial platelet counts after treatment. Changes in the absolute value and median value at differential time points were measured. Serial platelet counts after therapy in the EI group (A), in the EA group (B) and in the IA group (C). -, Median platelet count at each time point.
Fig. 3.
Response duration according to therapeutic setting of ITP. The response duration was longer in the EI group than the other two groups (p=0.0143).
EA, eradication alone; EI, eradication combined with immunosuppressive therapy; IA, immunosuppressive therapy alone.
DISCUSSION
Recently, several studies have demonstrated that patients with ITP could demonstrate an increase in the platelet count after eradication of H. pylori infection (9-15). These results support the hypothesis that an H. pylori-associated variant can be identified among ITP patients. However, the results of other studies have failed to either demonstrate a significant improvement in the platelet counts after eradication of H. pylori infection or achieve a significant long-term benefit. Therefore, the pathogenetic mechanisms of H. pylori-associated thrombocytopenia remain elusive.
Recent studies in Italian and Japanese populations have suggested that H. pylori could induce ITP (8-14). This pathogenesis suggests that ITP is related to H. pylori infection due to an increased mutual coincidence of H. pylori and ITP in these groups versus the general population. This theory was strengthened by tests showing improvement in ITP by H. pylori eradication.
Takahashi et al. (20) reinforced the hypothesis of the link between H. pylori and ITP by showing that platelet-associated immunoglobulin can cross-react with the CagA protein. Franceschi and colleagues (9) contributed follow-up data from 8 reported ITP patients and showed the disappearance of anti-CagA antibodies in all 8 platelet responders whose H. pylori had been eradicated. These results suggest that the molecular mimicry between H. pylori CagA protein and a platelet antigen may mediate the autoimmunity in ITP patients. However, the CagA positivity of H. pylori varies according to the geographic location of the patients. In Japan, most H. pylori strains express CagA, whereas the population of CagA-positive strains in Western countries is lower (21). Furthermore, the amino-acid sequence of the H. pylori CagA protein is known to show a significant diversity among the East Asian and Western strains (22).
Another theory suggests that the expression of various Lewis (Le) antigens by H. pylori isolates and the subsequent production of anti-Le antibodies could play a role in ITP pathogenesis as platelets may absorb Lewis antigens from the serum (23). However, it is uncommon to achieve long-term benefits from the eradication of H. pylori, and several studies have shown a difficulty in demonstrating its efficacy (16, 24).
In the present study, unfortunately, the response duration in the EA group of patients was 2 months. Moreover, some patients in the EA group showed no response and almost all of them who showed a response eventually relapsed.
Eradication therapy for H. pylori-positive patients is acknowledged as being effective to some degree. In this study, comparative results in the EI and IA groups of patients showed that H. pylori eradication was likely to have a beneficial effect for ITP patients with an H. pylori infection. If the H. pylori infection did not affect ITP, the results would be similar in both groups of patients. However, disappointing results were shown for the EA group of patients compared to the EI group of patients. Therefore, we are sure that the pathogenesis of H. pylori-associated ITP is related not only to H. pylori infection but also to other concurrent mechanisms, including the pathogenesis of classic ITP. Therefore, the management of ITP associated with H. pylori infection is complex and may require additional immunosuppressive therapy.
Cyclophosphamide and vincristine are effective agents for the treatment of ITP. Figueroa et al. (17) recently reported responses in 8 out of 10 severely affected, highly refractory ITP patients treated with cyclophosphamide-based combined immunosuppressive therapy. In the present study, cyclophosphamide, vincristine, and prednisolone (CVP) were all administered to ITP patients who had failed to show a response from steroid therapy. Oral cyclophosphamide was given at a low dose since a high dosage is associated with greater degrees of drug-induced toxicity and lower patient compliance. Oral prednisolone was re-applied in an immunosuppressive regimen due to the synergistic effect when combined with other immunosuppressive agents. H. pylori eradication, both with and without immunosuppressive therapy, was performed, with superior results for the EI group of patients. The platelet count at 6 months after the initial treatment was higher in the EI group of patients than in the EA group of patients, while a longer response duration was achieved in the same group (Table 3).
The efficacy of immunosuppressive therapy in H. pylori-negative patients was evaluated to determine whether the results for the EI group of patients is the synergistic effect of the two-treatment arms or only the effect of the immunosuppressive therapy, regardless of eradication. The median platelet count at 6 months after treatment and the response duration were both superior in the EI group of patients than in the IA group of patients (Table 4). Both immunosuppressive therapy and eradication had a just limited effectiveness if used alone.
In this study, the effect of eradication alone therapy and eradication combined with immunosuppressive therapy was not compared, as all the H. pylori-positive patients received eradication therapy. Although the EI group did not show a superior response rate, the group achieved a longer response duration than the others. Therefore, in our opinion, there is insufficient evidence to include only eradication for ITP patients having an H. pylori infection.
CVP therapy is usually used for chronic ITP (17-19). However, because combination immunosuppressive therapy employs anticancer drugs, adverse risks including leukomogenic potential, teratogenicity, and sterility must be considered, especially when these drugs are used for patients with nonmalignant diseases. The most important side effect is secondary malignancy, particular acute leukemia. In patients with rheumatologic disorders or ITP treated with oral cyclophosphamide, the risk appears to be related to the cumulative cyclophosphamide dose and duration of therapy (25, 26). However, although a low cumulative dose of oral cyclophosphamide was administered during a short duration in this study than in previous studies, it did not show any remarkable adverse effects in this study.
Although a careful weighing of risk and benefit is required with the use of combination immunosuppressive therapy for refractory autoimmune diseases, the high mortality rate in patients with autoimmune diseases who experience disease relapse or fail to standard therapy warrants consideration of the strategy.
In conclusion, the current study was performed using a more practical approach for ITP patients showing evidence of H. pylori infection. In H. pylori-associated ITP patients, the median platelet count at 6 months after treatment and the response duration in the EI group of patients were superior to those of the other groups. However, since this study included only a small number of patients, a further investigation is required to confirm the findings of this study.
References
- 1.Karpatkin S. Autoimmune thrombocytopenic purpura. Blood. 1980;56:329–343. [PubMed] [Google Scholar]
- 2.McMillan R. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1981;304:1135–1147. doi: 10.1056/NEJM198105073041904. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: A model. Gastroenterology. 1997;113:1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 4.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric carcinoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 5.Zentillin P, Savarino V, Garnero A, Accardo S, Seriolo B. Is Helicobacter pylori infection a risk factor or disease severity in rheumatoid arthritis? Gastroenterology. 1999;116:503–504. doi: 10.1016/s0016-5085(99)70161-7. [DOI] [PubMed] [Google Scholar]
- 6.De Luis DA, Valela C, de la Calle H, Canton R, de Argila CM, San Roman AL, Boixeda D. Helicobacter pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol. 1998;26:259–263. doi: 10.1097/00004836-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Gasbarrini A, Franceschi F. Autoimmune diseases and Helicobacter pylori infection. Biomed Pharmacother. 1999;53:223–226. doi: 10.1016/S0753-3322(99)80092-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsang KW, Lam SK. Helicobacter pylori and extra-digestive diseases. J Gastroenterol Hepatol. 1999;14:844–850. doi: 10.1046/j.1440-1746.1999.01974.x. [DOI] [PubMed] [Google Scholar]
- 9.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 10.Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812–814. doi: 10.1182/blood.v97.3.812. [DOI] [PubMed] [Google Scholar]
- 11.Veneri D, Franchini M, Gottardi M, D'Adda M, Ambrosetti A, Krampera M, Zanetti F, Pizzolo G. Efficacy of Helicobacter pylori eradication in raising platelet count in adult patients with idiopathic thrombocytopenic purpura. Haematologica. 2002;87:1177–1179. [PubMed] [Google Scholar]
- 12.Emilia G, Luppi M, Morselli M, Potenza L, D'Apollo N, Torelli G. Helicobacter pylori infection and idiopathic thrombocytopenic purpura. Br J Haematol. 2002;118:1198–1199. doi: 10.1046/j.1365-2141.2002.363010.x. [DOI] [PubMed] [Google Scholar]
- 13.Kohda K, Kuga T, Kogawa K, Kanisawa Y, Koite K, Kuroiwa G, Hirayama Y, Sato Y, Niitsu Y. Effect of Helicobacter pylori eradication on platelet recovery in Japanese patients with chronic idiopathic thrombocytopenic purpura and secondary autoimmune thrombocytopenic purpura. Br J Haematol. 2002;118:584–588. doi: 10.1046/j.1365-2141.2002.03612.x. [DOI] [PubMed] [Google Scholar]
- 14.Hashino S, Mori A, Suzuki S, Izumiyama K, Kahata K, Yonezumi M, Chiba K, Kondo T, Ota S, Toyashima N, Kato N, Tanaka J, Imamura M, Asaka M. Platelet recovery in patients with idiopathic thrombocytopenic purpura after eradication of Helicobacter pylori. Int J Hematol. 2003;77:188–191. doi: 10.1007/BF02983220. [DOI] [PubMed] [Google Scholar]
- 15.Hino M, Yamane T, Park K, Takubo T, Ohta K, Kitagawa S, Higuchi K, Arakawa T. Platelet recovery after eradication of Helicobacter pylori in patients with idiopathic thrombocytopenic purpura. Ann Hematol. 2003;82:30–32. doi: 10.1007/s00277-002-0579-8. [DOI] [PubMed] [Google Scholar]
- 16.Michel M, Cooper N, Jean C, Frissora C, Bussel JB. Does Helicobater pylori initiate or perpetuate immune thrombocytopenic purpura? Blood. 2004;103:890–896. doi: 10.1182/blood-2003-03-0900. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa M, Gehlsen J, Hammond D, Ondreyco S, Piro L, Pomeroy T, Williams F, McMillan R. Combination chemotherapy in refractory immune thrombocytopenic purpura. N Engl J Med. 1993;328:1226–1229. doi: 10.1056/NEJM199304293281703. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Nakagawa Y, Sawanobori M, Suzuki K, Enomoto H. Combination chemotherapy (CVP) in a patient with refractory idiopathic thrombocytopenic purpura. Rinsho ketsueki. 1998;39:193–197. [PubMed] [Google Scholar]
- 19.Tamai Y, Takami H, Akagi T, Kawamura S, Munakata A. Combination chemotherapy in a patient with severe multiple systemic autoimmune disease. Clin Lab Haematol. 1998;20:315–316. doi: 10.1046/j.1365-2257.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Yujiri T, Shinohara K, Inonue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y, Nawata R, Oka Y, Shirai M, Tanizawa Y. Molecular mimicry by Helicobater pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Perez GI, Bhat N, Gaensbauer J, Fraser A, Taylor DN, Kuipers EJ, Zhang L, You WC, Elaser MJ. Country-specific constancy by age in cagA proportion of Helicobacter pylori infections. Int J Cancer. 1997;72:453–456. doi: 10.1002/(sici)1097-0215(19970729)72:3<453::aid-ijc13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Evans DJ, Jr, Evans DG. Helicobacter pylori CagA: analysis of sequence diversity in relation to phosphorylation motifs and implications for the role of CagA as a virulence factor. Helicobacter. 2001;6:187–198. doi: 10.1046/j.1523-5378.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 23.Veneri D, Gottardi M, Guizzardi E, Zanuso C, Krampera M, Franchini M. Idiopathic thrombocytopenic purpura, Helicobacter pylori infection and HLA class II alleles. Blood. 2002;100:1926–1927. doi: 10.1182/blood-2002-05-1348. [DOI] [PubMed] [Google Scholar]
- 24.Jarque I, Andreu R, Llopis I, De la Rubia J, Gomis F, Senent L, Jimenez C, Martin G, Martinez JA, Sanz GF, Ponce J, Sanz MA. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2001;115:1002–1003. doi: 10.1046/j.1365-2141.2001.03194.x. [DOI] [PubMed] [Google Scholar]
- 25.Baker GL, Kahl LE, Zee BC, Stolzer BL, Aganval AK, Medsger TA., Jr Malignancy following treatment of rheumatoid arthritis with cyclophosphamide. Am J Med. 1987;83:1–9. doi: 10.1016/0002-9343(87)90490-6. [DOI] [PubMed] [Google Scholar]
- 26.Krause JR. Chronic idiopathic thrombocytopenic purpura: Development of acute non-lymphocytic leukemia subsequent to treatment with cyclophosphamide. Med Pediatr Oncol. 1982;10:61–65. doi: 10.1002/mpo.2950100110. [DOI] [PubMed] [Google Scholar]