Abstract
A critical issue for clinical utilization of human ES cells (hESCs) is whether they can generate terminally mature progenies with normal function. We recently developed a method for efficient production of hematopoietic progenitors from hESCs by coculture with murine fetal liver-derived stromal cells. Large numbers of hESCs-derived erythroid progenitors generated by the coculture enabled us to analyze the development of erythropoiesis at a clone level and investigate their function. The results showed that the globin expression in the erythroid cells in individual clones changed in a time-dependent manner. In particular, embryonic ε-globin-expressing erythroid cells from individual clones decreased, whereas adult-type β-globin-expressing cells increased to ≈100% in all clones we examined, indicating that the cells undergo definitive hematopoiesis. Enucleated erythrocytes also appeared among the clonal progeny. A comparison analysis showed that hESC-derived erythroid cells took a similar differentiation pathway to human cord blood CD34+ progenitor-derived cells when examined for the expression of glycophorin A, CD71 and CD81. Furthermore, these hESC-derived erythroid cells could function as oxygen carriers and had a sufficient glucose-6-phosphate dehydrogenase activity. The present study should provide an experimental model for exploring early development of human erythropoiesis and hemoglobin switching and may help in the discovery of drugs for hereditary diseases in erythrocyte development.
Keywords: development, erythropoiesis, hemoglobin, primitive hematopoiesis
Hematopoiesis in humans is a dynamic process regulated both temporally and spatially. In the primitive hematopoiesis wave, blood islands in the yolk sac (YS) transiently generate nucleated RBCs that exclusively express those globins that are components of the embryonic Hbs: Gower-1 (composed of ξ- and ε-globins), Gower-2 (composed of α- and ε-globins), and Hb Portland (composed of ξ- and γ-globins). The second definitive hematopoiesis wave, which gives rise to transplantable hematopoietic stem cells, enucleated RBCs, and various other hematopoietic cells, takes place mainly in the fetal liver (FL) through midgestation, although there is some contribution by the YS. Definitive hematopoiesis finally shifts to bone marrow, the site of lifelong adult-type hematopoiesis. Fetal and adult-type definitive hematopoiesis exhibit different patterns of Hb expression. In the former, primarily α- and γ-globins, the components of fetal Hb (Hb F), are expressed, and in the latter, α- and β-globins, the components of adult-type Hb (Hb A), are expressed (1, 2).
Previously, however, early human hematopoiesis had been difficult to study because of ethical limitations in the use of human embryos. Recently established human ES cells (hESCs) provided an ideal tool for investigation of early human embryonic/fetal hematopoiesis (3). The in vitro generation of hematopoietic cells from hESCs has been reported by several studies (4–7). We also reported a method of hESC coculture with mouse FL-derived stromal cells (mFLSCs) that generated a large quantity of hematopoietic progenitors that could give rise to erythroid cells, providing a means to characterize hESC-derived erythropoiesis (8).
We show here that, in the coculture system, hESC-derived erythroid cells are fated mostly to definitive hematopoiesis. Tracing differentiation at a clonal level demonstrates that most hESC-derived erythroid colonies express adult-type Hb and its expression gradually increases to 100% over time. In addition, over time in culture, hESC-derived erythropoiesis generates erythrocytes that are not only enucleated but also functionally mature. Thus, we propose that hESC-derived erythroid cells can provide an experimental model for early human erythropoiesis, and, in particular, Hb switching. This model will also be useful for investigating pathogenesis and testing drug therapies for hereditary erythropoiesis-associated diseases.
Results
Generation of Erythroid Cells from hESCs by Coculture with mFLSCs.
In a previous study (8), we found that the production of erythroid progenitors from hESCs was greatly enhanced by coculture with mFLSCs. This experimental paradigm provides the opportunity to conduct large-scale investigations of hESC-derived erythropoiesis. In the cocultures, 1 × 104 undifferentiated hESCs routinely generate a total of 5 × 105 cells by day 6 and 1 × 106 cells by day 14, with cell numbers decreasing thereafter [supporting information (SI) Fig. S1A]. The hESC-derived cells were primarily nonfloating cells, whereas floating cells were <0.05% of the total at any time during the coculture (data not shown). Flowcytometric analysis revealed that CD34+ cells increased concomitantly with the total cell increase (Fig. S1 A and B). Although total cell numbers decreased after day 14, the number of CD34+ cells peaked on day 16 and decreased thereafter. There were no glycophorin A (GPA)+ erythroid cells before day 6 of coculture (Fig. S1 C and D). GPA+ cells appeared at days 8 to 10 and did not coexpress CD45 but contained a substantial proportion of CD71+ cells.
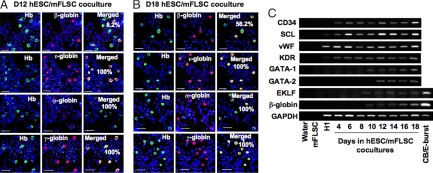
We examined the expression of various globins in the cocultures by using immunostaining (Fig. 1 A and B). Human Hb-expressing cells were observed as early as days 8–10 of coculture, consistent with the appearance of GPA+ cells. This early wave of hESC-derived Hb+ erythroid cells were all α-, ε-, and γ-globin-positive but β-globin-negative, indicating that they possessed the characteristics of embryonic and fetal erythrocytes. β-globin+ cells only appeared at days 11 and 12. Although the hESC-derived Hb+ erythroid cells expressed γ- and ε-globin at all times, the fraction of β-globin+/Hb+ cells gradually increased over time. By day 18, 50–60% of the hESC-derived erythroid cells were strongly positive for β-globin, indicating a gradual increase in adult-type erythropoiesis in the coculture.
Fig. 1.
Time-associated changes in expression of globins and hematopoiesis-related genes in hESC/mFLSC-derived erythroid cells. (A) Immunostaining of Hb, β-, ε-, α-, and γ-globins in cells from day-12 hESC/mFLSC cocultures. β-Globin was expressed only in 8.2% of the Hb+ erythroid cells, whereas ε-, α-, and γ-globins were expressed in 100% of Hb+ erythroid cells. (Scale bar: 25 μm.) (B) Immunostaining of Hb, β-, ε-, α-, and γ-globins in cells from day-18 hESC/mFLSC coculture cells. β-Globin+ cells increased to 56.2% of Hb+ erythroid cells, although ε-, α-, and γ-globins were still expressed in all Hb+ erythroid cells. (Scale bar: 25 μm.) (C) Time course of expression of early hematopoiesis-related genes and the definitive hematopoiesis β-globin gene during the hESC/mFLSC coculture detected by RT-PCR.
We investigated the expression of various genes regulating early hematopoiesis and erythroid lineage differentiation over the same time course as the analysis of globin expression by using RT-PCR (Fig. 1C). Genes for initial development of endothelial/hematopoietic cells were expressed early in the coculture, as reported in our previous work (8). The expression of β-globin could be detected by day 10, consistent with the immunostaining results. GATA-1, GATA-2, and EKLF expression paralleled that of β-globin.
Generation of Clonal Erythroid Progenitors from hESCs by Coculture with mFLSC.
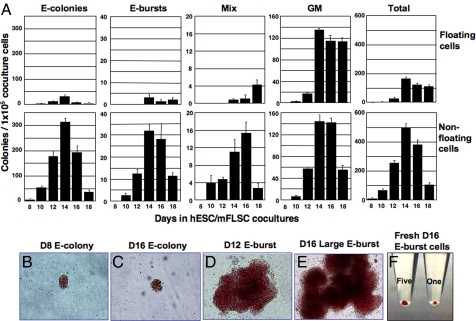
To further characterize hESC-derived erythropoiesis, we conducted hematopoietic colony assays of hESC/mFLSC coculture-derived cells. Before day 8 of coculture, there were few colony-forming cells (CFCs). The first erythroid-CFCs appeared on day 8, and various types of hematopoietic CFCs then rapidly increased in number, reaching a peak on day 14. As shown in Fig. 2A, most of the erythroid cell colonies [including erythroid (E) colonies, E bursts, and mixed-lineage (Mix) colonies] were in the nonfloating cell fraction, whereas a gradual increase in myeloid colonies among the floating cells was observed. At the peak (day 14), 1 × 105 nonfloating cells generated 499.3 ± 25.4 hematopoietic colonies, of which ≈75% were E colonies and E bursts (E colonies, 312.8 ± 14.3; E bursts, 31.8 ± 13.1; Mix colonies, 11.0 ± 2.8; myeloid colonies, 143.8 ± 11.9).
Fig. 2.
Generation of erythroid progenitors in hESC/mFLSC coculture. (A) Generation of CFCs from floating and nonfloating cells over time in hESC/mFLSC cocultures. (B–E) Micrographs of E colonies derived from day-8 (B) and day-16 (C) cocultures and E bursts from day-12 (D) and day-16 (E) cocultures. (F) Photo of harvested E burst cells from day-16 coculture, showing the red color of human erythroid cells. A total of 2 × 105 (Right) and 1 × 106 (Left) erythroid cells were collected from one and five E-bursts, respectively.
The size of the E colonies did not change with the time [132 ± 25.5 (n = 6) and 130.8 ± 29.1 (n = 6) cells per E colony on days 10 and 14, respectively, P = 0.96; Fig. 2 B and C]. E burst-forming cells (E-BFCs) appeared later that E-CFCs, on day 10, and the size of E bursts was relatively small at that time (Fig. 2D). However, large E bursts appeared by day 12 and continuously expanded in size through day 16 (Fig. 2E). On day 14, single large E-bursts had 1.97 ± 1.0 × 105 cells (n = 6). Fig. 3F shows 2 × 105 and 1 × 106 erythroid cells collected from one and five hESC-derived E bursts, respectively, on day 16.
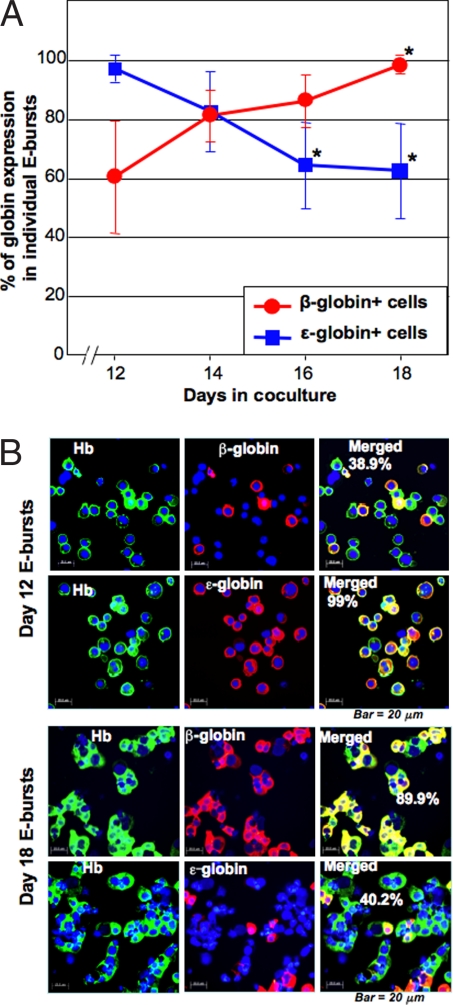
Fig. 3.
Clonal analysis of time-associated changes in globin expression during hESC/mFLSC coculture. (A) Globin expression in E bursts prepared after different amounts of time in coculture (n = 6 in each time point). Each E burst was individually picked from colony culture medium, and the globin expression was examined by immunostaining. The percentages were calculated from the ratio of β- or ε-globin+/human Hb+ cells. *, P < 0.01 when compared with the average expression of β- and ε-globins in day-12 cocultures. (B) Representative micrographs of Hb and β- and ε-globin immunostaining of E burst cells derived from E-BFCs at days 12 and 18 of coculture. (Scale bar: 20 μm.)
Thus, the large number of erythroid progenitors generated by coculturing allowed us to analyze the development of hESC-derived erythropoiesis at the clonal level.
Clonal Analysis of Globin Expression in hESC-Derived Erythropoiesis.
We first examined the globin expression in individual E bursts from colony cultures of E-BFCs transferred from hESC/mFLSC cocultures after 12, 14, 16, or 18 days in coculture. On day 15 of the colony culture, E bursts were randomly and individually picked from colony cultures started from the hESC/mFLSC cocultures, and expression of globins was analyzed by immunostaining. The fraction of β-globin-expressing cells increased from 60.3 ± 19.0% in E bursts (n = 6) derived from day-12 cocultures to 98.1 ± 1.1% in E bursts derived from day-18 cocultures, whereas the opposite trend was observed for ε-globin expression, 97.1 ± 4.3% of E bursts derived from day-12 cocultures decreasing to 62.4 ± 16.0% of E bursts derived from day-18 cocultures (Fig. 3). Thus, an up-regulation of β-globin expression and a down-regulation of ε-globin over time in hESC/mFLSC coculture was observed in all individual hESC-derived E bursts. Because at all coculture time points all E bursts simultaneously expressed α- and γ-globins at a rate of 100% (data not shown), these results indicate that at least one-third of the day-18 coculture-derived E bursts contained erythroid cells expressing adult-type Hb A and fetal-type Hb F, but not the embryonic-type Hbs Gower-1 and Gower-2.
To examine changes in globin expression in individual erythroid clones over time, we traced globin expression in single E bursts derived from day-15 coculture E-BFCs. On day 12 of clonal culture, individual E bursts were randomly selected, and 20% of the individual E burst cells were centrifuged onto glass slides while the remaining 80% were transferred into suspension cultures for an additional 6 days (referred to as 12+6 cultures) and β-globin expression was then examined. The 12 E bursts examined exhibited a significant increase in β-globin expression (from 26.4 ± 17.0% to 99.8 ± 0.6%, P < 0.001) and a corresponding decrease in ε-globin expression (from 95.6 ± 7.7% to 49.5 ± 15.4%, P < 0.001) (Table 1). These data indicate that individual E bursts undergo maturation, as measured by globin switching from the embryonic to adult type.
Table 1.
Progressive maturation of hESC/mFLSC coculture-derived erythroid cells
| E bursts no. | ε-globin+, % |
β-globin+, % |
||
|---|---|---|---|---|
| Day 12 | Day 12+6 | Day 12 | Day 12+6 | |
| 1 | 100 | 63.6 | 17.0 | 100 |
| 2 | 92.0 | 33.7 | 12.5 | 100 |
| 3 | 100 | 45.3 | 12.5 | 98.8 |
| 4 | 82.0 | 40.8 | 52.0 | 99.0 |
| 5 | 100 | 17.8 | 5.0 | 100 |
| 6 | 100 | 31.1 | 13.5 | 100 |
| 7 | 98.0 | 54.8 | 16.9 | 100 |
| 8 | 78.0 | 52.6 | 19.7 | 100 |
| 9 | 96.9 | 57.6 | 48.9 | 100 |
| 10 | 100 | 66.3 | 46.8 | 100 |
| 11 | 100 | 66.7 | 45.8 | 100 |
| 12 | 100 | 54.4 | 26.1 | 100 |
| Total | 95.6 ± 7.7 | 49.5 ± 15.4 | 26.4 ± 17.0 | 99.8 ± 0.6 |
Progressive maturation of hESC/mFLSC coculture-derived clonal erythroid cells. Cells were harvested on day 15 of hESC/mFLSC coculture and transferred to colony cultures. On day 12 of colony culture, 12 E bursts were randomly selected, and 20% of the individual clonal cells were centrifuged onto glass slides, and the remaining 80% were transferred to suspension culture. After an additional 6 days of suspension culture, globin expression in individual colonies was examined. The percentages were calculated from the ratio of β- or ε-globin+/human Hb+ cells.
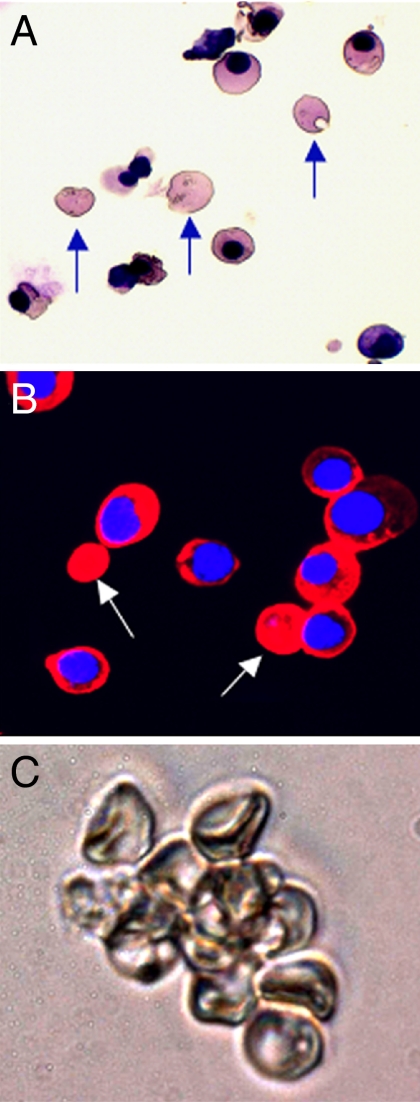
Interestingly, a substantial number of β-globin-expressing enucleated erythrocytes existed in day-12+6 erythroid cells (Fig. 4 A and B), whereas no enucleated erythrocytes were observed in day-12 clonal erythroid cells. We observed clusters of hESC-derived disk-shaped erythrocytes in the day-12+6 suspension culture (Fig. 4C).
Fig. 4.
Clonal analysis of progressive maturation of hESC/mFLSC coculture-derived erythroid cells. (A) Cytospin sample of hESC-derived erythroid cells from a day-12+6 suspension culture (May-Grunwald-Giemsa staining). Arrows indicate enucleated erythrocytes. (B) Immunostaining for β-globin expression in hESC-derived erythroid cells from the same suspension culture shown in A. Arrows indicate β-globin-expressing enucleated erythrocytes. (C) Cluster of enucleated erythrocytes derived from hESCs from the same suspension culture shown in A and B.
We also randomly selected 12 E colonies derived from day-10 hESC/mFLSC cocultures and 10 E-colonies derived from day-14 cocultures. All erythroid cells in the E colonies expressed α- and γ-globins (data not shown), whereas β-globin was expressed only in three day-10 coculture-derived E colonies (4.6 ± 8.7%, n = 12) and eight day-14 coculture-derived E-colonies (26.8 ± 26.2%, n = 10) [Table S1]. In contrast, ε-globin was expressed in 100% of the cells in all E colonies from both coculture time points (data not shown).
We further examined globin expression in Mix colonies derived from hESC/mFLSC cocultures. Using the clone-tracing method by which we analyzed E bursts, we confirmed that erythroid cells in Mix colonies underwent progressive maturation with increasing β-globin expression and development of enucleated erythrocytes (Table S2).
Finally, we analyzed globin gene expression in individual E bursts and Mix colonies by RT-PCR (Fig. S2). When day-15 hESC/mFLSC coculture-derived E bursts (n = 6) and Mix colonies (n = 6) were examined after 16 days in colony culture, none expressed OCT-4, nestin, α-fetoprotein, or brachyury. They all expressed α-, β-, ε-, and γ-globin, consistent with the immunostaining results, but ζ-globin was expressed only in five of six E bursts and three of six Mix colonies. These data indicate that the early embryonic Hbs Gower-1 and Hb Portland were completely absent from a fraction of the hESC-derived erythroid progenitors.
Thus, hESC-derived erythroid cells progressively matured in a time-dependent manner at the clonal level.
Flowcytometric Analysis of CD81, GPA, and CD71 Coexpression in hESC-Derived Erythroid Cells.
CD81 is a widely expressed surface protein that exists in almost all human tissues, with the exception of erythrocytes and megakaryocytes (9). Our previous report showed that human cord blood (CB)-derived GPA+ erythroid cells never expressed CD81 (10). To investigate the expression of CD81 in hESC-derived erythroid cells, we performed flowcytometric analysis and cell sorting to examine coexpression of CD81 with GPA and CD71.
As shown in Fig. S1D, GPA+CD45− erythroid cells appeared on days 8–10 in hESC/mFLSC coculture, and the proportion increased over time. All of the cells in this fraction coexpressed CD81, but most were negative for CD71 at days 10–14. However, CD81 was gradually down-regulated by days 16–18 concomitant with up-regulation of CD71.
We then analyzed expression of CD81 on erythroid cells in day-15 hESC/mFLSC coculture-derived E bursts on day 12 of colony culture. CD81 and CD71 were coexpressed in most GPA+ cells in the E bursts (Fig. S3A). By day 16 of colony culture, however, the expression of CD81 considerably decreased, and most GPA+ cells did not express CD81, whereas half of the GPA+ cells still expressed CD71. This expression pattern mimics that of human CB-derived E burst cells (Fig. S3B). Considering the increase in β-globin+ erythroid cells over the same time course, down-regulation of CD81 expression may mark the progression of maturation of hESC-derived erythroid cells. Therefore, we isolated GPA+ cells from E bursts on days 12 and 16 of clonal culture and analyzed their globin expression (Fig. S3C). On day 12 of clonal culture, fewer GPA+CD81high E burst cells than GPA+CD81low cells expressed β-globin (72.9% and 95.7%, respectively). On day 16, all GPA+CD81− cells expressed β-globin, whereas 6.1% of GPA+CD81+ cells still did not express β-globin (Table 2). These results indicate that the gradual down-regulation of CD81 on hESC-derived erythroid cells is correlated with progressive maturation to adult-type erythroid cells.
Table 2.
Time-related analysis of globin expression of sorted hESC-derived E burst cells defined by CD81 expression
| Globin | Day 12 hESC-E burst cells, % |
Day 16 hESC-E-burst cells, % |
CB-E burst cells | ||||
|---|---|---|---|---|---|---|---|
| CD81++ | CD81L | NS | CD81L | CD81− | NS | ||
| β-globin | 72.9 | 95.7 | 81.1 | 93.9 | 100 | 98.8 | 100 |
| ε-globin | 72.5 | 70.8 | 75.7 | 67.4 | 67.1 | 73.3 | 0.2 |
| γ-globin | 100 | 100 | 100 | 100 | 100 | 100 | 85 |
| α-globin | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Expression of various globins in the sorted hESC-derived erythroid cell fractions defined by the gates shown in Fig. S2C. Note that hESC-derived E-burst cells on day 16 of colony culture were 100% β-globin+ within the GPA+CD81− fraction. NS, no sorting.
Functional Assays of hESC-Derived Erythroid Cells.
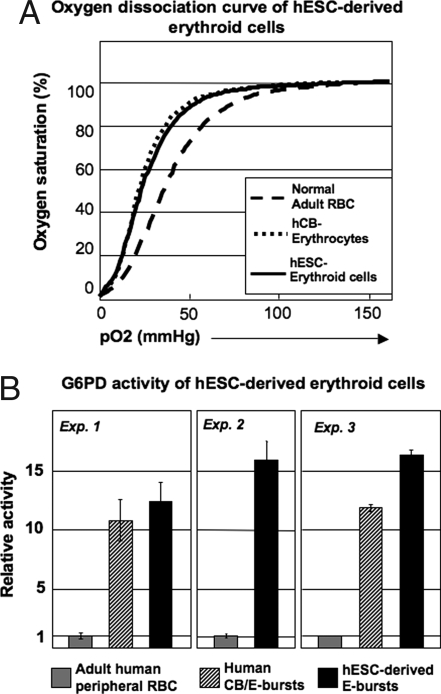
The large-scale generation of hESC-derived erythroid cells permitted us to examine their function in detail. We measured oxygen dissociation in hESC-derived E burst cells. As shown in Fig. 5A, hESC-derived erythroid cells displayed an oxygen dissociation curve similar to that of human CB, whereas human adult peripheral blood (PB) exhibited a slightly right-shifted curve. These data indicate that hESC-derived erythroid cells are functionally similar to fetal RBCs, rather than adult RBCs.
Fig. 5.
Functional assays of hESC-derived erythroid cells. (A) Oxygen dissociation curves of day-15 hESC/mFLSC coculture-derived clonal E burst erythroid cells at day 16 of colony culture, human CB, and adult PB. (B) G6PD activity of day-15 hESC/mFLSC coculture-derived clonal E burst erythroid cells at day 16 of the colony culture compared with human CB-derived E burst cells and adult PB.
Because the oxygen dissociation curve showed that hESC-derived erythroid cells are functional oxygen carriers, we examined glucose-6-phosphate dehydrogenase (G6PD) activity to confirm their ability to defend against oxidative stresses (Fig. 5B). Adult PB (5 × 106 RBCs per 5 μl) was used as a control. The hESC-derived erythroid cells (5 × 106) had higher G6PD activity than the control. Human CB-derived erythroid cells (5 × 106) also showed high G6PD activity, but comparatively lower than hESC-derived erythroid cells.
Discussion
In our previous report (8), hESC/mFLSC cocultures generated a large number of human hematopoietic progenitors, particularly erythroid and multipotential cells. In the current study, using these clonal erythroid cells, we demonstrated that most hESC-derived E colonies, E bursts, and Mix colonies contained adult-type β-globin-expressing erythroid cells, and the percentages of β-globin-expressing cells increased over time in culture to ≈100%, whereas embryonic ε-globin-expressing cells decreased concomitantly. Thus, we have provided evidence that hESC-derived hematopoiesis mainly generates erythroid cells of definitive property, given proper conditions (4–7).
Although it is not known whether primitive and definitive hematopoietic cells are derived from the same source, progenitors committed to primitive hematopoiesis are hypothesized to express only embryonic globins, and we did not find E bursts or Mix colonies that expressed only embryonic globins. There are several explanations for this result. One possibility is that the hematopoietic colony culture conditions do not support primitive progenitors or that mFLSCs are not able to stimulate the generation of primitive progenitors. Alternatively, primitive progenitors may be able to synthesize definitive globins in some hematopoietic environments. This hypothesis is supported by the present finding that embryonic (ε), fetal (γ), and adult-type (β) globins were coexpressed not only in single colonies but also in single cells, suggesting that switching of globin expression from primitive to definitive hematopoiesis is a sequential event occurring in individual cells. Therefore, an hESC-derived definitive hematopoiesis-fated erythroid cell may first coexpress both primitive and definitive globins and finally change to 100% definitive properties over time in culture. In murine experiments, successful transplantation of YS hematopoietic cells directly into adult recipients is difficult, indicating that the YS cells differ from adult-type hematopoietic cells. However, YS-derived hematopoietic stem cells transplantable to adult recipients can be generated both by in vitro coculture with murine aorta-gonad-mesonephros region-derived stromal cells (11) and in vivo transplantation into fetuses or FL (12–15). These data suggest that further stimulation of embryonic hematopoietic cells by the fetal environment may be critical to step up their potential for adult-type hematopoiesis. Our hESC/mFLSC coculture system may mimic such an environment.
The similarity between our culture system and hematopoietic conditions at midgestation is supported by a recent report by Chang et al. (7). Using an embryoid body (EB) formation method, the early stages of hESC-derived definitive hematopoiesis were retained, but further maturation was suspended. These results mimic human early YS hematopoiesis phenotypically and genetically (6). Therefore, because the environment lacked properties required to promote definitive hematopoiesis, these EB-derived erythroid cells may not progress toward further maturation. However, our hESC/mFLSC coculture system may stimulate the maturation of early hematopoietic cells. The present results in hESCs and nonhuman primate ESCs (16) show that ESC-derived erythroid progenitors capable of producing β-globin-expressing erythroid cells were predominantly within the nonfloating cell fraction in coculture with stromal cells, indicating that continuous association with the stromal layer is needed for the maturation of definitive progenitors.
The progressive maturation of hESC-derived erythropoisis could be confirmed not only by globin switching, but also by changes in surface marker expression. In our previous observations of human CB cells, CD81 was never expressed on GPA+ cells (10). In this study, however, CD81 was expressed exclusively on hESC-derived GPA+ erythroid cells at early times, but expression was gradually down-regulated by the time the colonies were 100% β-globin positive. Thus, coexpression of GPA and CD81 on hESC-derived erythroid cells may represent an early stage in development and suggests that CD81 could be used as a developmental marker in embryonic erythropoiesis.
In addition to providing insight into the mechanism of erythropoiesis in hESCs, the current study has important implications for clinical use of hESC-derived erythrocytes. The high G6PD activity in erythroid cells derived from hESCs and CB suggests that they are protected against oxidative damage, although the cultured cells may represent younger populations, with a higher percentage of reticulocytes and young RBCs that are known to express higher levels of G6PD. hESC-derived erythoid cells were also able to function as oxygen carriers, although they exhibited an oxygen dissociation pattern similar to CB rather than adult PB. In addition, hESC-derived erythroid cells showed no expression of genes associated with retention of ESC characteristics, indicating little possibility of their oncogenicity or differentiation into cells other than RBCs. Thus, the generation of hESC-derived mature erythrocytes suggests they might be a novel source of RBCs for transfusions in the clinical setting, but significant advances in bioprocess engineering are still needed to make clinical applications feasible. According to our in vitro culture system, 104 hESCs roughly amplify to 106 mature erythrocytes. Of note, a simple transfusion needs 1–2.5 × 1012 RBCs (500 ml of whole blood, at 5 × 109 RBCs per ml), and it would require a bulk cell culture of some 104 plates of hESCs. There will be interest in considering the further scale-up of the culture system for clinical transfusion.
Taken together, we have demonstrated the potential of hESC-derived erythroid cells to progressively mature to synthesize adult-type Hb, generate enucleated erythrocytes, and function as oxygen carriers. The large quantity and high purity of hESC-derived definitive erythroid cells generated in our coculture system can provide an experimental model for investigation of the early stages of human erythropoiesis, especially globin switching, and for examination of pathogenesis and therapeutic drugs for hereditary disorders of erythropoiesis. Because transplantable hematopoietic stem cells are exclusively derived from the definitive hematopoiesis, our system may also help in exploring the mechanism controlling the generation of these stem cells.
Materials and Methods
hESC Cultures.
The hESCs (line H1) were maintained and passaged weekly on irradiated mouse embryonic fibroblast feeder cells as described (3).
Establishment of Murine FL-Derived Stromal Cells.
mFLSCs were prepared by as described (8, 10). Briefly, FLs were removed from embryonic day 15 (E15) BCL/Black 6 mice. After trituration, free FL cells were washed once with PBS and treated with 0.05% trypsin/EDTA (Invitrogen) at 37°C for 10 min. Cells were then washed and plated in a 75-cm2 flask at a density of four FLs per flask. After 48 h in culture, floating cells were removed by washing, and fresh medium was added. The mFLSCs, which reached confluence by 4–5 days in culture, were treated with 0.05% trypsin/EDTA and replated. Cells thus maintained were harvested and stored in liquid nitrogen. Before use in coculture with hESCs, the frozen mFLSCs were thawed and plated at 1 × 105 cells per well in six-well plates, cultured for 2 days to reach confluence, and then irradiated (25 Gy).
Coculture of hESCs with mFLSCs.
Undifferentiated hESC colonies consisting of 0.5–1 × 103 cells per colony were physically picked up under a microscope by using a microtip. Harvested hESC colonies (20–30 per well) were plated onto irradiated mFLSCs prepared in gelatin-coated six-well culture plates in 3 ml of culture medium [15% FBS (HyClone), 1 mM glutamine, 1% nonessential amino acid solution (100×; Invitrogen), and α-MEM (Invitrogen)]. Plates were incubated at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was exchanged every 3 days by removing all of the supernatant from the culture dishes and adding new medium. On certain days, floating cells in the coculture were collected, and nonfloating cells were harvested by treating them with 0.05% trypsin/EDTA.
Hematopoietic Colony Culture and Suspension Culture.
Hematopoietic colony assays were performed as described (8, 10, 17, 18). Briefly, a 1-ml aliquot of culture mixture containing 1.2% methylcellulose (Shin-etsu Chemical), 30% FBS, 1% deionized fraction V BSA, 0.1 mM 2-mercaptoethanol (ME), αMEM, a human-cytokine mixture (100 ng/ml stem cell factor, 10 ng/ml IL-3, 100 ng/ml IL-6, 10 ng/ml thrombopoietin, 10 ng/ml granulocyte colony-stimulating factor, and 4 units/ml erythropoietin), and cells (hESC/mFLSC coculture cells or human CB mononuclear cells) was plated in a 35-mm culture dish (Becton Dickinson Labware). Cultures were then incubated in a humidified atmosphere containing 5% CO2 at 37°C under an inverted microscope. Erythroid cell colonies including E colonies, E bursts, and Mix colonies were characterized as containing bright red erythroid cells, which were confirmed by examining cytospin samples. E colonies, E bursts, and Mix colonies were defined as follows: E colonies, colonies that consist of <100 erythroid cells comprising a single subcolony and containing no other cell types; E bursts, colonies that consist of >200 erythroid cells, or exhibited two or more subcolonies, without other types of cells; Mix colonies, colonies that contained other cell types in addition to erythroid cells. The number of E colonies was determined on days 7–10 of the culture. The numbers of E bursts, Mix colonies, and myeloid colonies including granulocyte, macrophage, and granulocyte-macrophage colonies were determined on days 12–14 of the culture. In some experiments, individual colonies were picked from the methylcellulose culture, washed once with αMEM, and replated in a suspension culture composed of 15% FBS, 0.1 mM 2-ME, αMEM, and the cytokine mixture, similar to the previous method (8). For clonal analysis of Hb expression and RT-PCR, individual colonies were picked from the methylcellulose culture medium and centrifuged onto glass slides by using a Cytospin instrument (Shandon) or lysed in RNA-preparation buffer and frozen at −80°C.
Flowcytometry Analysis, Cell Sorting, and Antibodies.
Cocultured hESC/mFLSCs and hESC colony cells harvested after various times in culture were preincubated with normal rabbit serum to block nonspecific binding and then stained with various mAbs conjugated to FITC, phycoerythrin (PE), or allophycocyanin (APC). Stained cells were washed with PBS and analyzed by using a FACSCalibur flowcytometry system (BD Biosciences). Propidium iodide (PI)-stained dead cells were gated out. This analysis used mAbs against human CD45 (DakoCytomation), GPA (BD Pharmingen), CD71 (Beckman Coulter), and CD81 (BD). Recorded data were analyzed by using the Flowjo software (Tomy Digital Biology). In some experiments, hESC-derived colony cells were stained with anti-CD81-PE, anti-GPA-FITC, and anti-CD45-APC and fractionated by sorting on a FACSAria sorter (BD).
RT-PCR.
To detect early stages of hESC-derived hematopoiesis and globin expression, RT-PCR was used. Total RNA was prepared from hESC/mFLSC cocultures, individual hESC-derived E burst and Mix colony cells, and human CB-derived E burst cells by using the RNA subtract kit (Promega). Single-stranded cDNA was synthesized from total RNA using a SuperScript first-strand synthesis system for RT-PCR (Invitrogen). PCR conditions were the same as reported (8, 16). Human gene-specific primers were used throughout our experiments to avoid interference from mFLSCs (8, 16, 19). For semiquantitative comparisons of gene expression, amounts of cDNA template were standardized against the relative expression of GAPDH in each sample.
Morphological Observation and Immunochemical Staining.
For morphological observation, clonal cells or cells in suspension culture were centrifuged onto glass slides and stained with a May-Grunwald-Giemsa solution. For immunochemical staining using fluorescently labeled Abs, glass slide samples of hESC/mFLSC cocultures, hESC-derived colony cells, suspension cultures, and human CB-derived colony cells were fixed in 4% paraformaldehyde (PFA) and permeabilized with PBS containing 5% skim milk and 0.1% Triton X-100 for 30 min. Slides were then incubated with primary anti-human Abs [goat anti-human Hb polyclonal Ab (pAb); Bethyl Laboratories; rabbit anti-human Hb pAb; MP Biomedicals; mouse anti-human α-, β-, and γ-globin mAbs; Santa Cruz Biotechnology; and mouse anti-human ε-globin mAb; CortexBiochem] overnight at 4°C, washed three times with PBS containing 5% skim milk, and incubated with FITC- or carbocyanin (Cy) 3-conjugated secondary Abs (Jackson ImmunoResearch) for 30 min at room temperature. Nuclei were labeled with Hoechst 33342 (Molecular Probes). After three washes with PBS, samples were observed with a fluorescence microscope. With the exception of E colony cells, the percentage of positive cells was determined from examination of 200–400 cells.
Functional Assays for hESC-Derived Erythroid Cells.
To detect G6PD activity, an assay kit using a water-soluble tetrazolium salt (WST-8) was used according to the manufacturer's protocol (Dojindo) (20). The oxygen binding ability of hESC-derived erythroid cells, human CB, and adult PB was measured with a Hemox analyzer, as reported (21, 22).
Statistical Analysis.
Data are presented as mean ± SD. Statistical significance was determined by using Student's t test. P < 0.05 is considered significant.
Acknowledgments.
We thank Drs. Y. Cui, N. Watanabe, and Y. Ishii for their help. This work was supported by Japan Society for the Promotion of Science Grants 18591217 (to F.M.), 19591277 (to Y.E.), and 17390297 (to K.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802220105/DCSupplemental.
References
- 1.Orkin S-H, Zon L-I. Hematopoesis and stem cells: Plasticity versus developmental heterogeneity. Nat Immunol. 2002;3:323–328. doi: 10.1038/ni0402-323. [DOI] [PubMed] [Google Scholar]
- 2.Palis J, Segel G-B. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. doi: 10.1016/s0268-960x(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 3.Thomson J-A, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman D-S, Hanson E-T, Lewis R-L, Auerbach R, Thomson J-A. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L-S, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Zambidis E-T, Peault B, Park T-S, Bunz F, Civin C-I. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K-H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma F, et al. Novel method for efficient production of multipotential hematopoietic progenitors from human embryonic stem cells. Int J Hematol. 2007;85:371–379. doi: 10.1532/IJH97.06203. [DOI] [PubMed] [Google Scholar]
- 9.Levy S, Todd S-C, Maecker H-T. CD81 (TAPA-1): A molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Ma F, et al. Development of human lymphohematopoietic stem and progenitor cells defined by expression of CD34 and CD81. Blood. 2001;97:3755–3762. doi: 10.1182/blood.v97.12.3755. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka S, et al. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98:6–12. doi: 10.1182/blood.v98.1.6. [DOI] [PubMed] [Google Scholar]
- 12.Toles J-F, Chui D-H-K, Belbeck L-W, Starr E, Barker J-E. Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci USA. 1989;86:7456–7459. doi: 10.1073/pnas.86.19.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischman R-A, Custer R-P, Mintz B. Totipotent hematopoietic stem cells: Normal self-renewal and differentiation after transplantation between fetuses. Cell. 1982;30:315–359. doi: 10.1016/0092-8674(82)90233-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoder M-C, Hiatt K. Engraftment of embryonic hematopoietic cells in conditioned newborn recipients. Blood. 1997;89:2176–2183. [PubMed] [Google Scholar]
- 15.Palis J, Yoder M-C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 16.Umeda K, et al. Sequential analysis of α- and β-globin gene expression during erythropoiesis differentiation from primate embryonic stem cells. Stem Cells. 2006;24:2627–2636. doi: 10.1634/stemcells.2006-0199. [DOI] [PubMed] [Google Scholar]
- 17.Sui X, et al. Erythropoietin-independent erythrocyte production: Signals through gp130 and c-Kit dramatically promote erythropoiesis from human CD34+ cells. J Exp Med. 1996;183:837–845. doi: 10.1084/jem.183.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakahata T, Ogawa M. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J Clin Invest. 1982;70:1324–1328. doi: 10.1172/JCI110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma F, et al. Direct development of functionally mature tryptase/chymase double positive connective tissue-type mast cells from primate ES cells. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0348. [DOI] [PubMed] [Google Scholar]
- 20.Tantular I-S, Kawamoto F. An improved, simple screening method for detection of glucose-6-phosphate dehydrogenase deficiency. Trop Med Int Health. 2003;8:569–574. doi: 10.1046/j.1365-3156.2003.01055.x. [DOI] [PubMed] [Google Scholar]
- 21.Sakai H, Cabrales P, Tsai A-G, Intaglietta M, Tsuchida E. Oxygen release from low and normal P50 Hb-vesicles in transiently occluded arterioles of the hamster window model. Am J Physiol. 2005;288:H2897–H2903. doi: 10.1152/ajpheart.01184.2004. [DOI] [PubMed] [Google Scholar]
- 22.Shirasawa T, et al. Oxygen affinity of hemoglobin regulates O2 consumption, metabolism, and physical activity. J Biol Chem. 2003;278:5035–5043. doi: 10.1074/jbc.M211110200. [DOI] [PubMed] [Google Scholar]