Abstract
Objectives
To explore the increased incidence of intravenous immunoglobulin (IVIG) resistance among San Diego County Kawasaki disease (KD) patients in 2006 and to evaluate a scoring system to predict IVIG-resistant patients with KD.
Study design
We performed a retrospective review of patients with KD treated within 10 days of fever onset. Using multivariate analysis, independent predictors of IVIG-resistance were combined into a scoring system.
Results
In 2006, 38.3 % of patients with KD in San Diego County were IVIG-resistant, a significant increase over previous years. IVIG-resistance was not associated with a particular brand or lot of IVIG. Resistant patients were diagnosed earlier, had higher % bands, and higher concentrations of C-reactive protein, alanine aminotransferase, and γ-glutamyl transferase (GGT). They also had lower platelet counts and age-adjusted hemoglobin (zHgb) concentrations and were more likely to have aneurysms (p=0.0008). A scoring system developed to predict IVIG-resistant patients using illness day, % bands, GGT, and zHgb, had a sensitivity of 73.3% and specificity of 61.9%.
Conclusions
An unexplained increase in IVIG-resistance was noted among patients with KD in San Diego County in 2006. Scoring systems based on demographic and laboratory data were insufficiently accurate to be clinically useful in our ethnically diverse population.
Keywords: coronary artery aneurysm, vasculitis, pediatrics
Kawasaki disease (KD), the leading cause of pediatric acquired heart disease in the United States and Japan, is an acute, systemic vasculitis. Treatment with a single dose of intravenous immunoglobulin (IVIG) and high-dose aspirin results in resolution of fever in most patients and significantly reduces the rate of coronary artery aneurysms (1). Despite this success, 10–20% of children will have persistent or recrudescent fever after their first infusion of IVIG (2–5). These patients are at increased risk of developing coronary artery abnormalities (5, 6). Additional therapies used in these patients include retreatment with IVIG, immunomodulatory agents such as infliximab, high dose methylprednisolone, cyclophosphamide, and plasmapheresis. (7–11). Identification of patients who are likely to be IVIG-resistant would allow the use of additional therapies early in the course of their illness when prevention of coronary artery damage might still be possible.
A number of recent studies from Asia have identified demographic and laboratory characteristics, including age, illness day, platelet count, erythrocyte sedimentation rate (ESR), and concentrations of hemoglobin, C-reactive protein (CRP), lactate dehydrogenase, and alanine aminotransferase (ALT) as predictors of IVIG-resistance (2, 12–14).
We noted an increase in the numbers of IVIG-resistant patients with KD in San Diego County in 2006 as compared to previous years. This prompted us to examine the epidemiology of IVIG-resistance in our population. We tested the hypothesis that comparison of KD patient characteristics and their response to IVIG could lead to a scoring system that predicted IVIG-resistance.
Methods
All patients met the standard case definition for KD and had fever and at least 4 of the 5 standard KD clinical criteria (rash, conjunctival injection, cervical lymphadenopathy, changes in the oral mucosa, and changes in the extremities) or fever and 3 criteria plus coronary artery abnormalities (dilatation or aneurysm) documented by echocardiography (15). Only patients diagnosed within the first 10 days after fever onset were analyzed. Patients were enrolled at two clinical sites (Rady Children’s Hospital San Diego and the University of California San Diego [UCSD]) after obtaining parental informed consent and subject assent, as appropriate. The study protocol was reviewed and approved by the Institutional Review Boards at both study sites. Demographic and clinical data including sex, ethnicity, race, age, illness day (first day of fever= illness day 1), response to IVIG therapy, and coronary artery status were recorded for all subjects. Laboratory testing at hospital admission and prior to IVIG administration included white blood cell count with differential, platelet count, hemoglobin, hematocrit, ESR, CRP, ALT, and γ-glutamyl transferase (GGT). IVIG brand and lot data and response to IVIG therapy were also analyzed for patients with KD treated within the first 10 days after fever onset at Children’s Hospital Boston.
IVIG-resistance was defined as persistent or recrudescent fever (T ≥ 100.4°F rectally or orally) at least 48 hours but not longer than 7 days after completion of the first IVIG infusion (2 g/kg). Patients were classified as having normal (<2.5 standard deviation units (z score) from the mean, normalized for body surface area), dilated (2.5 ≤ z score <4.0), or aneurysmal (focal or diffuse dilatation of a coronary artery segment with z score≥4.0) coronary arteries based on the maximal internal diameters of the right coronary artery and left anterior descending artery measured by echocardiography at the time of diagnosis and at 2–4 weeks after onset of fever (15).
The analysis of the epidemiological data and the development of a scoring system were performed with the data sets available at the time each component of the study was initiated. For the epidemiological evaluation of IVIG-resistance in San Diego County, data from subjects admitted between January 1, 1998, and December 31, 2006, were evaluated to determine the annual incidence of KD in San Diego County. Demographic and pre-IVIG laboratory data collected between October 1998 and September 2006 from subjects who presented within the first 10 days of illness were used to develop the scoring system to predict IVIG-resistant subjects. Pharmacy records from Rady Children’s Hospital, San Diego and Children’s Hospital Boston were retrospectively reviewed to retrieve IVIG brand and lot data for patients treated in 2006. These data were not available for previous years for Rady Children’s Hospital. To test whether there were differences in clinical presentation in years with high or low IVIG-resistance rates, we compared clinical criteria for KD between patients diagnosed in 2005 and 2006. Greater than 90% of the patients during these two years were evaluated during their acute illness by a single physician (JCB), thus reducing the potential for interobserver variability.
The scoring system to identify IVIG-resistant patients by Egami et al was applied to our cohort of patients with KD in San Diego County (October 1998 to September 2006) to determine its predictive value in an ethnically diverse population (12). In order to create a new scoring system that might better identify children at risk for IVIG-resistance in a multiethnic population, we first performed univariate analysis to identify variables significantly associated with IVIG-resistance (p<0.05). Because hemoglobin concentrations are age dependent, we analyzed age-adjusted hemoglobin concentrations (zHgb) using the following formula: ([observed hemoglobin] – [mean hemoglobin for age])/standard deviation for age (16). The standard deviations were estimated as one-quarter of the reported range of normal hemoglobin concentrations for each age interval. We then performed a multivariate logistic regression analysis with backward elimination to identify independent predictors of IVIG-resistance. The model was simplified by retaining those variables with a p<0.2 in the scoring system. Results were expressed as an odds ratio with a 95% confidence interval. Analyses were performed using NCSS version 2007 (NCSS, Kaysville, Utah) and SAS Learning Edition 2.0 (SAS Institute, Inc., Cary, North Carolina). Continuous variables were converted to dichotomous variables by choosing a break point based on receiver-operator characteristic (ROC) curves and the upper or lower quartile for each independent predictor identified by the multivariate logistic regression. Variables and break points that were tested included age (≤6 and ≤12 months), illness day (≤4 days), platelet count (≤30,000 and ≤150,000 cells/mm3), ALT (≥ 80 IU/L), GGT (≥60 and ≥ 100 IU/L), CRP (≥8 mg/dL), % bands (≥20), and zHgb (≤−1.0, ≤−1.5, and ≤−2.0). Different scoring systems were developed using the odds ratio to determine the weight of each variable, giving a weight of two points for those with the highest odds ratios. Even though most variables were given 1 point, illness day ≤4 days, GGT ≥60 IU/L, and % bands ≥20 were weighted with both 1 and 2 points to evaluate the effects on the scoring system. The scores were calculated for each patient, with a final risk score consisting of two risk strata, low-risk (score 0–1) or high-risk (score 2–5). The sensitivity, specificity, and positive and negative predictive values for each scoring system were calculated.
The t-test and Wilcoxon rank-sum test were used to compare normally distributed and skewed continuous laboratory values, respectively, between IVIG-resistant and – responsive patients. Categorical variables were compared by a 2-sided chi-square test and Fisher exact test as appropriate.
Results
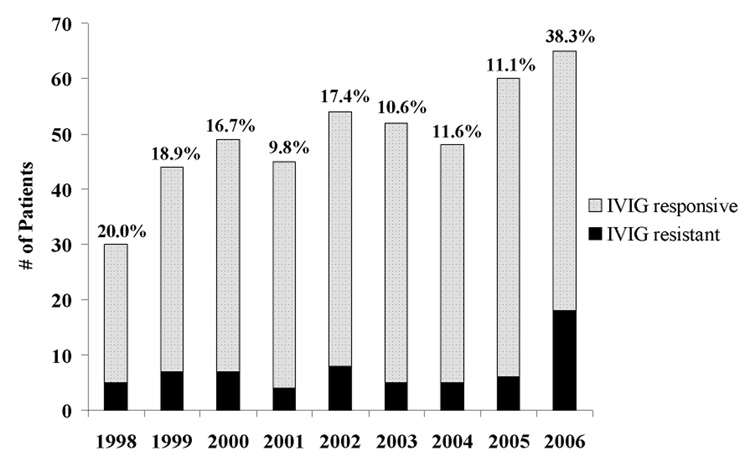
From January 1, 1998, to December 31, 2006, 362 children with a median age of 2.3 years were admitted and treated for KD at UCSD and Rady Children’s Hospital, San Diego. The percentage of IVIG-resistant patients with KD from 1998 to 2005 ranged from 9.8–20% (Figure). In 2006, the incidence of IVIG-resistance increased to 38.3% (p=0.009). To explain this increase in IVIG-resistance, we postulated that either the disease or the IVIG had changed.
Figure. IVIG Resistance in San Diego.
IVIG-responsive and –resistant KD patients in San Diego County (1998–2006) Number of patients and percentages of IVIG-responsive and –resistant KD patients for San Diego County from 1998 to 2006 (p = 0.009 for 2006 versus other years, overall chi-square)
To test the first hypothesis, we evaluated the frequency of the classical clinical signs associated with KD between 2005, when the incidence of IVIG-resistance was only 11.1%, and 2006 when the incidence was 38.3%. No difference was noted in the frequency of the classical clinical signs between the two years (data not shown).
Because of our concern about the possibility of a change in the IVIG product, IVIG-resistant patients from 2006 were reported to the Food and Drug Administration through the MedWatch system and the Pharmacovigilance Division of Baxter Pharmaceuticals was contacted. The lots of IVIG used to treat these resistant patients were investigated by Baxter Healthcare Corporation. The investigation included review of batch record, release data, manufacturing changes, temperature deviations during shipment, and relevant stability data. Furthermore, the IgG content (monomer/dimer/polymer/fragments), anticomplement activity, and trypsin/chymotrypsin exposure time of the IVIG batches used were within the historical trend for these measures. In addition, Baxter verified that no significant changes in the donor pool or collection sites had occurred between 2005 and 2006. In our investigation in San Diego, we found no difference in the distribution of brands and lots used to treat IVIG-responsive and – resistant patients in 2006, although our power to detect a difference was limited by the small sample size. (Supplemental Table I; available at www.jpeds.com). We also obtained data from Children’s Hospital Boston on the IVIG response of 25 patients with KD treated with the same brands and lots as the San Diego patients (Supplemental Table I). Whereas 13 of 29 San Diego patients (45%) were IVIG-resistant when treated with 3 specific IVEEGAM lots, only 1 of 23 Boston patients (4%) treated with these same lots was IVIG-resistant. The rate of IVIG-resistance in Boston in 2006 was 8%. To confirm that IVIG-resistant patients actually received the prescribed dose, the serum IgG levels were analyzed in a subset of patients (n=20). Levels were not significantly different from those published in the first trial of single-infusion (2g/kg) IVIG (1).
IVIG-resistance was strongly associated with an increased aneurysm rate. Analysis in 362 patients with KD from 1998 to 2006 revealed that aneurysms developed in 9 of 60 (15%) IVIG-resistant patients as compared with 9 of 302 (3%) IVIG-responsive patients (p = 0.0008) (Supplemental Table II; available at www.jpeds.com).
Multiple scoring systems for predicting IVIG-resistance in patients with KD have been published from other countries (2, 12–14). However, the only scoring system for which we had all of the variables in our database was published by Egami et al from Japan (12). This scoring system includes age ≤6 months (1 point), illness day ≤4 day (1 point), platelet count ≤30,000 (1 point), ALT ≥80 IU/L (2 points), and CRP ≥8 mg/dL (1 point). When applied to our patient population, this model missed over 60% of IVIG-resistant patients (Table VI[H2]). When applied only to Asian patients in the San Diego County cohort, the Egami score had a specificity of 89.3% and a sensitivity of 33.3%.
A new scoring system was developed using data from our cohort of patients with KD diagnosed between October 1998 and September 2006. Comparison of the demographic and clinical characteristics of IVIG-resistant and -responsive patients with KD revealed that IVIG-resistant patients tended to be younger (p = 0.06) and presented earlier (p = 0.002) (Supplemental Table III; available at www.jpeds.com). Univariate analysis of the laboratory data revealed that % bands, absolute band count, and concentrations of CRP, ALT, and GGT were significantly higher in IVIG-resistant patients (Table I). Platelets and zHgb were significantly lower in IVIG-resistant patients.
Table 1.
Univariate analysis comparing laboratory values between IVIG-resistant and - responsive KD patients
| N^ | Resistant* | Responsive* | p† | |
|---|---|---|---|---|
| WBC, ×103/mm3 | 60:301 | 12.8 (10.0–17.3) | 14.0 (11.2–18.0) | >0.05 |
| [6–17] | ||||
| % Polys | 58:295 | 50 (34–59) | 51 (38–60) | >0.05 |
| ANC, cells/mm3 | 58:295 | 9600 (6606–12730) | 9027 (6716–12000) | >0.05 |
| % Bands | 58:295 | 24 (13–41) | 14 (5–23) | 0.00001 |
| ABC cells/mm3 | 58:295 | 2816 (1670–5291) | 1852 (691–3356) | 0.0002 |
| Platelets, ×103/mm3 | 60:299 | 362 (288–444) | 404 (309–491) | 0.050 |
| [150–350] | ||||
| zHemoglobin# | 60:300 | −1.9 (−3.0, −0.8) | −1.3 (−2, −0.6) | 0.014 |
| ESR, mm/hr | 58:298 | 59 (47–80) | 65 (46–86) | >0.05 |
| [4–20] | ||||
| CRP, mg/dL | 50:227 | 11.9 (6.0–18.7) | 7.4 (4.5–14.1) | 0.041 |
| [0–1] | ||||
| ALT, IU/L | 51:271 | 56 (35–137) | 31 (19–87) | 0.002 |
| [13–45] | ||||
| GGT, IU/L | 52:286 | 84 (37–161) | 32 (16–92) | 0.0001 |
| [0–23] |
WBC, white blood cell; Polys, polymorphonuclear leukocytes; ANC, absolute neutrophil count; ABC, absolute band count; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ALT, alanine aminotransferase; GGT, γ glutamyl transferase.
Number of subjects available for comparison, IVIG-resistant:IVIG-responsive
Expressed as median (25th–75th percentiles)
zHemoglobin, standard deviation units from the mean based on age-adjusted normal values
Wilcoxon rank sum test
[] Normal values(1)
1. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 16th ed. Philadelphia: Mosby; 2002.
Multivariate logistic regression analysis found illness day at diagnosis, % bands, GGT, and zHgb to be independent predictors of IVIG-resistance (Table II). In the final scoring system, dichotomous variables were weighted as follows: Illness day at diagnosis ≤ 4 days (1 point), % bands ≥ 20 (2 points), GGT ≥ 60 IU/L (1 point), and zHgb ≤ −2.0 (1 point). This scoring system had a sensitivity of 73.3% and a specificity of 61.9% (Table III). If this scoring system were used prospectively to identify patients with KD who are likely to be resistant to IVIG and therefore candidates for additional therapy, 31.8% of patients (false positives) would receive additional therapy when not needed and 26.7% of patients (false negatives) would be IVIG-resistant but would not have received additional treatment.
Table 2.
Multivariate predictors of IVIG-resistance in KD patients
| Variables | Logisitic Coefficient (β) | Standard Error | Odds Ratio (95%CI) † | p† | Points |
|---|---|---|---|---|---|
| Illness day ≤ 4 | 0.61 | 0.35 | 1.84 (0.91–3.72) | 0.09 | 1 |
| % Bands ≥ 20 | 1.02 | 0.34 | 2.76 (1.42–5.37) | 0.003 | 2 |
| GGT ≥ 60 IU/L | 0.80 | 0.34 | 2.22 (1.15–4.31) | 0.02 | 1 |
| zHgb ≤ −2 | 0.71 | 0.33 | 2.04 (1.06–3.93) | 0.03 | 1 |
CI, confidence interval limits
Chi-square test
Table 3.
Ability of scoring systems to predict IVIG-resistance and coronary artery aneurysms in KD patients in the San Diego County cohort
| IVIG-resistance | Aneurysms | ||
|---|---|---|---|
| Egami Score (%) | San Diego Score (%) | San Diego Score (%) | |
| Sensitivity | 38.3 | 73.3 | 72.2 |
| Specificity | 83.8 | 61.9 | 57.6 |
| PPV | 31.9 | 27.7 | 8.2 |
| NPV | 87.2 | 92.1 | 97.5 |
| Missed* | 61.7 | 26.7 | 27.8 |
PPV = positive predictive value; NPV = negative predictive value
Missed = Percentage of IVIG-resistant subjects that were not identified by this scoring system
When our cohort was stratified by ethnicity, the scoring system showed decreasing sensitivity for detecting IVIG-resistance as follows: Caucasians > Hispanics > Asians (81.3%, 68.2%, and 66.7%, respectively). When patients were stratified by coronary artery status (normal or dilated versus aneurysm), our scoring system had a sensitivity of 72.2% and a specificity of 57.6% for predicting the development of aneurysms.
Discussion
The cause of the unusual increase in IVIG-resistance among patients with KD treated in San Diego County in 2006 was investigated. No change in the clinical presentation of KD or an association of resistance with particular IVIG brands or lots was found, although the small sample size limited our ability to detect a difference. The same lots associated with IVIG-resistance in San Diego were used at other sites across the United States without apparent changes in their IVIG-resistance rates (personal communication, Donald Baker, Baxter Healthcare Corporation). Given that the mechanism of action of IVIG as well as the cause of KD remain unknown, constructing testable hypotheses to explain what might have led to the increase in IVIG-resistance is difficult. We publish this report to alert clinicians to examine the rate of IVIG-resistance at their institutions and to track IVIG brand and lot use in these patients.
Rates of IVIG-resistance at institutions across the United States, including Hawaii, varied by year and by institution but rates as high as 30% were sporadically observed. In published series of IVIG-resistance, the rates vary from a low of 9.4% in Iran to a high of 23% in the United States (3, 4, 6, 17, 18).
Because IVIG-resistant patients are at higher risk for coronary artery aneurysms, it is important to identify these patients who might benefit from more aggressive initial therapy. In our study, because of differences in the type of clinical and laboratory data recorded for our cohort, we were only able to test one of the four previously-published scoring systems. The poor performance, particularly the low sensitivity of the Egami score in our population of ethnically diverse children (18% Asian, predominantly Korean and Filipino), may be because the Egami scoring system was created and validated only in cohorts of Japanese children. Despite robust statistical evaluation of available demographic, clinical, and laboratory data, our own scoring system would miss more than one quarter of IVIG-resistant patients. Because genetics likely play an important role in determining disease severity and outcome, genetic differences between cohorts may affect the predictive value of different scoring systems. In the future, scoring systems might incorporate polymorphic alleles to create a more robust predictor of disease outcome.
Limitations of this study include the small sample size, the availability of IVIG brand and lot data only for 2006, and the collection of limited laboratory data that precluded the testing of other scoring systems. We were unable to test our scoring system in an independent cohort because measurement of GGT concentrations and reporting of % bands were not routine at other US institutions with large cohorts of patients with KD.
Surveillance for IVIG-resistance by a broader network of institutions would be helpful in defining the epidemiology and providing clues to the cause of IVIG-resistance. As new therapies become available, identification of patients with KD who would be candidates for more aggressive therapy becomes increasingly important. Infliximab, which binds the pro-inflammatory cytokine tumor necrosis factor a, was evaluated in a retrospective case review of IVIG-resistant patients with KD. No infusion reactions or complications attributed to infliximab administration were seen in this patient population (11). A randomized trial in IVIG-resistant patients with KD has recently been completed (JCB, personal communication). To improve the identification of patients with KD who are candidates for more aggressive therapy, we need a better understanding of the mechanism of action of IVIG, the pathogenesis of aneurysms, and the contribution of host genetics to disease outcome.
In summary, although we were unable to explain the sudden increase in IVIG-resistance in San Diego County in 2006, it is critical that clinicians be aware that increases in IVIG-resistant KD may occur in their community. In addition, scoring systems based on demographic and laboratory data were insufficiently sensitive to be clinically useful in our ethnically diverse population. A better understanding of the pathogenesis and host genetics in KD will help identify key factors that may be important to include in future scoring systems.
Supplementary Material
Acknowledgments
The authors thank Joan Pancheri, R.N. and Annette Baker, R. N. for assistance with data collection. We are indebted to the families and children who have agreed to participate in this study.
Financial Support: This work is supported in part by grants from National Heart, Lung, and Blood Institute to JCB (HL69413 and K24 HL074864), from the National Institute for Child Health and Human Development, Pediatric Pharmacology Research Unit to BMB (5U10 HD031318), and the Ciaranello Family Fund at Children’s Hospital Boston to JWN.
Abbreviations
- zHgb
Age-adjusted hemoglobin
- ALT
alanine aminotransferase
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- GGT
γ-glutamyl transferase
- IVIG
intravenous immunoglobulin
- KD
Kawasaki disease
- UCSD
University of California, San Diego
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: The authors have no conflicts of interest to report.
Bibliography
- 1.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324(23):1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 2.Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003;24(2):145–148. doi: 10.1007/s00246-002-0216-2. [DOI] [PubMed] [Google Scholar]
- 3.Han RK, Silverman ED, Newman A, McCrindle BW. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch Pediatr Adolesc Med. 2000;154(7):694–699. doi: 10.1001/archpedi.154.7.694. [DOI] [PubMed] [Google Scholar]
- 4.Kashef S, Safari M, Amin R. Initial intravenous gamma-globulin treatment failure in Iranian children with Kawasaki disease. Kaohsiung J Med Sci. 2005;21(9):401–404. doi: 10.1016/S1607-551X(09)70141-X. [DOI] [PubMed] [Google Scholar]
- 5.US/Canadian Kawasaki Syndrome Study Group. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. Pediatr Infect Dis J. 1998;17(12):1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sittiwangkul R, Pongprot Y, Silvilairat S, Phornphutkul C. Management and outcome of intravenous gammaglobulin-resistant Kawasaki disease. Singapore Med J. 2006;47(9):780–784. [PubMed] [Google Scholar]
- 7.Mori M, Imagawa T, Katakura S, Miyamae T, Okuyama K, Ito S, et al. Efficacy of plasma exchange therapy for Kawasaki disease intractable to intravenous gamma-globulin. Mod Rheumatol. 2004;14(1):43–47. doi: 10.1007/s10165-003-0264-3. [DOI] [PubMed] [Google Scholar]
- 8.Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43(3):211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 9.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993;123(4):657–659. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 10.Chiyonobu T, Yoshihara T, Mori K, Ishida H, Nishimura Y, Yamamoto Y, et al. Early intravenous gamma globulin retreatment for refractory Kawasaki disease. Clin Pediatr (Phila) 2003;42(3):269–272. doi: 10.1177/000992280304200311. [DOI] [PubMed] [Google Scholar]
- 11.Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146(5):662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Fukunishi M, Kikkawa M, Hamana K, Onodera T, Matsuzaki K, Matsumoto Y, et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr. 2000;137(2):172–176. doi: 10.1067/mpd.2000.104815. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113(22):2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 15.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 16.Nathan D, Oski F, editors. Hematology of Infancy and Childhood. Philadelphia: WB Saunders; 1998. [Google Scholar]
- 17.Durongpisitkul K, Sangtawesin C, Khongphatthanayopthin A, Panamonta M, Sopontammarak S, Sittiwangkul R, et al. Epidemiologic study of Kawasaki disease and cases resistant to IVIG therapy in Thailand. Asian Pac J Allergy Immunol. 2006;24(1):27–32. [PubMed] [Google Scholar]
- 18.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105(6):E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.