Abstract
Wounding corneal epithelium establishes a laterally oriented, DC electric field (EF). Corneal epithelial cells (CECs) cultured in similar physiological EFs migrate cathodally, but this requires serum growth factors. Migration depends also on the substrate. On fibronectin (FN) or laminin (LAM) substrates in EF, cells migrated faster and more directly cathodally. This also was serum dependent. Epidermal growth factor (EGF) restored cathodal-directed migration in serum-free medium. Therefore, the hypothesis that EGF is a serum constituent underlying both field-directed migration and enhanced migration on ECM molecules was tested. We used immunofluorescence, flow cytometry, and confocal microscopy and report that 1) EF exposure up-regulated the EGF receptor (EGFR); so also did growing cells on substrates of FN or LAM; and 2) EGFRs and actin accumulated in the cathodal-directed half of CECs, within 10 min in EF. The cathodal asymmetry of EGFR and actin staining was correlated, being most marked at the cell–substrate interface and showing similar patterns of asymmetry at various levels through a cell. At the cell–substrate interface, EGFRs and actin frequently colocalized as interdigitated, punctate spots resembling tank tracks. Cathodal accumulation of EGFR and actin did not occur in the absence of serum but were restored by adding ligand to serum-free medium. Inhibition of MAPK, one second messenger engaged by EGF, significantly reduced EF-directed cell migration. Transforming growth factor β and fibroblast growth factor also restored cathodal-directed cell migration in serum-free medium. However, longer EF exposure was needed to show clear asymmetric distribution of the receptors for transforming growth factor β and fibroblast growth factor. We propose that up-regulated expression and redistribution of EGFRs underlie cathodal-directed migration of CECs and directed migration induced by EF on FN and LAM.
INTRODUCTION
Directed cell movements are fundamental in tissue construction and reconstruction. DC electric fields (EFs) exist where cell migrations occur: in embryonic development and during wound healing of skin and cornea (Jaffe and Vanable, 1984; Chiang et al., 1992; Shi and Borgens, 1995; Robinson and Messerli, 1996). EFs equivalent to the field strength in vivo induce directed movement of cultured cells. Consequently, a physiological EF may be one guidance cue used by migrating cells (Robinson, 1985; Nuccitelli, 1988; McCaig and Zhao, 1997).
After corneal wounding, growth factor and cytokine levels increase in tear fluid and in stroma layers, and a provisional ECM is elaborated (Gipson and Inatomi, 1995; Virtanen et al., 1995; Sheardown and Cheng, 1996; Tervo et al., 1997; Vesaluoma et al., 1997a,b). How EFs interact with growth factors and different substratum components in directing cell migration is unknown but relevant, because growth factors alone have chemotactic and chemokinetic effects on cells, and EFs may create extracellular gradients of relevant molecules (Messerli and Robinson, 1997).
Cell–substratum adhesion is important in cell migration (Palecek et al., 1997). For instance, the ECM influences EF-directed migration of human keratinocytes (Sheridan et al., 1996). Fibronectin (FN) normally is not present beneath corneal epithelial cells (CECs) but appears after injury and persists until migration is complete (Fujikawa et al., 1984). Local FN also increases rapidly after human photorefractive keratectomy (Virtanen et al., 1995) and experimental corneal wounding (Cai et al., 1993; Espaillat et al., 1994; Vitale et al., 1994). Some data suggest that FN stimulates corneal epithelial migration in vivo and in vitro (Nishida et al., 1983, 1984; Mooradian et al., 1993; Gundorova et al., 1994; Gipson and Inatomi 1995), but not all reports agree (Newton et al., 1988). Laminin (LAM) may not be essential for migration during corneal wound healing (Fujikawa et al., 1984) but is important in CEC–substrate adhesion (Ohji et al., 1993) and is expressed in corneal epithelial wound healing in vitro (Kurpakus et al., 1990). Integrins are expressed on CECs. Among them, α2β1, α3β1, and ανβ1 bind LAM, collagen, and FN. Epithelial expression of β1 integrins increased as FN in the wound increased and decreased as wound healing was completed (Murakami et al., 1992; Elner and Elner, 1996). α6β4 is synthesized and redistributed in wound healing (Kurpakus et al., 1991) and binds LAM (Sonnenberg et al., 1993), whereas LAM promotes reorganization of filamentous actin (F-actin) in CECs (Svoboda, 1992; Khoory et al. 1993). In addition, the ECM contains a complex array of fixed charges and the ionic charge of a substrate modulates corneal cell integrin expression, cell spreading, and cell migration. For example, the expression of α6 and β4 integrin proteins and their mRNAs on CECs is down-regulated by hydrogel surfaces lacking positively charged amine moieties (Wu and Trinkaus-Randall, 1997), whereas corneal epithelia and fibroblast show differential spreading behavior on differently charged surfaces (Bergethon et al., 1989). Because EFs, LAM, FN, and growth factors coexist after corneal injury, they may interact to modulate cell motility. We show that bovine CECs cultured on FN or LAM in a physiological EF migrated faster and more directly cathodally. Both responses were serum dependent.
Epidermal growth factor (EGF) stimulated CEC motility (Tao et al., 1995), and overexpression of EGF receptor (EGFR) enhanced keratinocyte motility (McCawley et al., 1997). Cultured bovine and human CECs migrated cathodally in a physiological EF and required serum or EGF, basic fibroblast growth factor (bFGF), or transforming growth factor β1 (TGF-β1) in serum-free medium to do so (Zhao et al., 1996a,b, 1997). Therefore, signal transduction through growth factor receptors may underlie EF-directed CEC migration, although the mechanisms are not clear.
EF-induced reorganization of charged surface receptors and of the cytoskeleton seems to be involved (Poo et al., 1979; Onuma and Hui, 1988; Brown and Loew, 1994; McCaig and Zhao, 1997). EF-induced extracellular gradients of growth factors or FN or LAM also may be important (Robinson and Messerli, 1996; Messerli and Robinson, 1997). Thus reorganization of cell surface receptors or extracellular molecules to induce a gradient of either receptor or ligand, respectively, is likely an initial event, which activates cells asymmetrically and drives subsequent cytoskeletal reorganization and directed cell migration.
We tested whether EGFR expression and distribution were regulated by an EF or by ECM proteins in a manner that could underlie directed CEC migration. Flow cytometry was used to monitor membrane expression of EGFRs. Not only did EF increase expression of EGFRs, so also did a substratum of FN or LAM. Using semiquantitative confocal microscopy, we have asked whether actin and the receptors for the three growth factors (EGF, TGF-β1, and FGF), which restored field-directed migration in serum-free medium (Zhao et al., 1996a,b), become asymmetrically distributed. Actin and all three receptors accumulated cathodally after EF exposure, with the EGFR and actin showing patterned colocalization at the cell–substrate interface and early cathodal asymmetry, consistent with a role in directing cell migration. We also used PD98059, which prevents the phosphorylation of MAPK kinase (MEK; MAPK is an element of the EGF signaling pathway), and this reduced directed migration of CECs in EFs plus EGF. We propose that EF-directed migration of CECs uses the well-recognized ligated EGFR as a proximal element, with downstream signaling, and that up-regulated expression and redistribution of EGFRs and coordinate redistribution of actin underlie cathodal-directed migration. The intracellular location and markedly slower field-induced asymmetry of the FGF receptor and the TGF receptor II (TGFR II) suggest that they may be less important in instigating field-directed CEC migration.
MATERIALS AND METHODS
Antibodies, Growth Factors, and Reagents
The monoclonal mouse anti-human EGFR (catalog number E2760), FITC- and TRITC-conjugated secondary antibodies (catalog numbers F2883 and T6778), and normal rabbit and sheep serum were from Sigma (Poole, United Kingdom). Rhodamine phalloidin and fluorescein-conjugated EGF (fluorescein EGF; E3478) were from Molecular Probes Europe (Leiden, The Netherlands). Polyclonal rabbit anti-chicken FGF receptor (FGFR) antibody (catalog number 06-177), which reacts with bovine FGFR, and polyclonal rabbit anti-human TGF-β type II receptor (catalog number 06-277) were from Upstate Biotechnology (TCS Biologicals, Buckingham, United Kingdom). Cy5-labeled goat anti-rabbit immunoglobulin G (IgG; catalog number PA45004) was from Amersham (Arlington Heights, IL). PD98059 (MEK1 inhibitor; 9900S) was from New England Biolabs (Beverly, MA).
Cell Cultures and EF Stimulation
Primary cell cultures of bovine CECs were prepared by standard methods (Zhao et al., 1996a). For immunofluorescence study the chamber base was an acid-washed glass coverslip, or slide, stuck down with silicone grease (MS4; Dow Corning, Auburn, MI) in a plastic culture dish. Cells were cultured at a cell density of ∼24 × 104 cells/ml for migration and immunofluorescence experiments and at ∼100 × 104 cells/ml for flow cytometry experiments. FN (McIntosh et al., 1988), and LAM (from mouse sarcoma, Sigma) were diluted using PBS. One milliliter of diluted solution was pipetted into the galvanic culture chamber (see Zhao et al., 1996a, their Figure 1), left for 2 h (37°C, 5% CO2), washed off, and rinsed three times with PBS. Cells adhered to the base for 16∼24 h (37°C; 5% CO2), before a roof of number 1 cover glass (Chance Propper, Warley, England) was added and sealed with silicone grease. Final dimensions of the chamber, through which current was passed, were 22 × 10 × 0.2 mm for migration and immunofluorescence microscopy and 64 × 10 × 0.2 mm for flow cytometry experiments.
For experiments labeling EGFR in live cells, slide chambers were mounted directly on the microscope stage immediately after preparing the cells and adding the coverslip roof.
Quantification of Cell Behavior
Cells were tracked and analyzed with an image analyzer (Zhao et al., 1996a). Mean migration rate and directedness were quantified over 5 h (Gruler and Nuccitelli, 1991; Zhao et al., 1996a). The angle that each cell moved with respect to the imposed EF vector was measured. The cosine of this angle is 1 if the cell moved directly along the field lines toward the cathode, 0 if the cell moved perpendicular to the EF vector, and −1 if the cell moved directly toward the positive pole of EF. Averaging the cosines (Σ iCOS θ/N, where θ is the angle between the field vector and the cellular translocation direction, and N is the total number of cells) yields average directedness of cell movement.
Inhibition of MEK Activation and Phosphorylation
Cells were washed and incubated for 1 h with 50 μM PD98059 diluted in serum-free medium before EF application. Immediately before EF exposure, serum-free medium with 25 ng/ml EGF and 50 μM PD98059 was substituted into the EF chamber. Control cultures lacked PD98059.
Flow Cytometry Analysis
Cells were exposed to EF at 37°C, 5% CO2 for 12∼16 h or for ∼3 h in room air for FN or LAM experiments. After EF exposure, cells were washed thoroughly with PBS (Ca2+ and Mg2+ free), then 4 ml of Versene solution (Dow Chemical, Midland, MI) was added and incubated for 10–20 min at 37°C. In some experiments trypsin (final concentration, 0.05%) was added. Harvested cells were washed twice in cold serum-free DMEM. Cells were suspended in 5–10 ml of serum-free DMEM and revitalized for 15 min at 37°C. Harvested samples included no less than 106 cells/ml. Cells were washed twice with ice-cold FACS buffer. All subsequent staining was done at 4°C or on ice. Cells were incubated with antibodies against EGFRs for 30 min, washed twice with FACS buffer, and incubated with FITC-conjugated secondary antibody (1:128) for 30 min. After two washes, the pellet was suspended with 400 μl of FACS buffer for immediate flow cytometry or with 400 μl of FACS fix (1% formaldehyde) when flow cytometry was performed the next day. Appropriate isotype control included OX21 to determine the level of nonspecific binding of IgG mAbs to CECs. Additional controls included cells alone and FITC-conjugated secondary antibody. Cell viability was determined either by trypan blue or with propidium iodide (Sigma) after treatment with FACSperm (Becton Dickinson, San Jose, CA) after secondary antibody incubation. Cell viability was consistently 85–95% for flow cytometric analysis. Cytofluorometric analysis was performed using a FACScaliber (Becton Dickinson) flow cytometer equipped with a 488-nm argon line. A linear forward scatter versus linear side scatter display was used to set a broad gate that eliminated small debris and large aggregates for collection of fluorescent list mode data. The data were collected and computer analyzed using CellQuest analysis software (Becton Dickinson). For each sample a minimum of 10,000 gated events were analyzed, and mean fluorescence intensity of three subpopulations was determined.
Confocal Fluorescence Microscopy Analysis
Cells were washed three time with PBS, fixed with acetone (4°C for 10 min), air dried at room temperature for 30 min, and rehydrated with PBS (with 1% BSA). All antibodies were diluted in PBS with 1% BSA and used as follows: monoclonal anti-EGFR at 1:80; polyclonal anti-FGFR, 1:50; polyclonal anti-TGFR II, 10 μg/ml; monoclonal anti-FGFR, 1:20; and monoclonal anti-TGFR, 1:20. Dilutions for secondary antibodies were: sheep anti-mouse IgG FITC conjugate (Fab′), 1:250; goat anti-rabbit IgG TRITC conjugate, 1:300; and goat anti-rabbit IgG Cy5 conjugate, 30 μg/ml. For triple staining, cells were incubated with primary antibodies against EGFR in a humid chamber at 37°C for 30 min and washed twice with PBS, 10 min each. The cells were incubated with FITC-conjugated sheep anti-mouse IgG (Fab′) for 30 min and then washed twice with PBS. Cells were incubated with anti-TGFR or anti-FGFR for 30 min and washed twice (the last time with 10% normal goat serum). Cy5-conjugated anti-rabbit IgG was mixed with rhodamine-phalloidin (5 U/ml) and added to the cells for 30 min at 37°C. For double labeling, only one of anti-EGF, or anti-TGFR or anti-FGFR and appropriate secondary antibodies was used. This staining was used also in some experiments with triple staining to make sure the triple-staining results were not caused by excessive cross-reaction. For negative control staining, only the secondary antibodies were incubated with the cells. No positive staining was observed with secondary antibodies alone. When monoclonal anti-TGFR or anti-FGFR was used, anti-mouse IgM (μ-chain specific) FITC conjugate (Sigma) was used with rhodamine-phalloidin. After washing twice with PBS, slides were mounted in Vectorshield (Vector Laboratories, Peterborough, United Kingdom) and viewed with a Bio-Rad (Hercules, CA) MRC 1024 confocal microscope.
For live cell labeling, antibodies were diluted in serum-free DMEM. After two washes (10 min each) with serum-free DMEM, cells were incubated with primary antibodies at 37°C for 30 min, washed twice, and incubated with secondary antibodies. A coverslip roof was attached, and appropriate medium replaced the washing solution (as described above, Cell Cultures and EF Stimulation). Slides were mounted on the confocal microscope, and EF was applied as before.
For fluorescein-conjugated EGF labeling, cells were washed with ice-cold PBS after 3 h of EF application (150 mV/mm) and kept on ice or in an ice-cold environment thereafter. Cells were incubated with ice-cold serum-free medium with fluorescein EGF (100 ng/ml) for 1 h, washed with ice-cold PBS, and immediately viewed on an ice-cold stage with fluorescent and confocal microscopes.
To quantify the asymmetric distribution of growth factor receptors or F-actin, fluorescence intensity was measured for cathodal- and anodal-facing sides. Well-spread, positively stained cells showing asymmetric distribution of staining were selected. The images were typically three to five frame averaged. A cell projection or a section of cell was divided into left (cathodal) and right (anodal) halves by a vertical line through the center of the cell or center of the nucleus perpendicular to the EF vector. In cells with well-defined cathodal patches of fluorescence, the polygon selection function was used, and the complete, positively stained area was measured. Measurements also were made using the same polygons from the anodal half, opposite from the cathodal patches (along the axis of the EF). Polygons were drawn with Bio-Rad LaserSharp 2.1a and avoided including areas near cell boundaries, where fluorescence intensity was near background. Freehand polygonal areas were drawn also to determine membrane–juxtamembrane region labeling, as opposed to intracellular labeling. An asymmetric index was calculated: Ai = (Cfi − Afi)/(Cfi + Afi) for each cell or cell section, where Cfi represents mean cathodal side fluorescence intensity, and Afi is mean anodal side fluorescence intensity. Acellular areas in the same image were measured as background and subtracted from cellular measurements. This method avoids potential artifacts attributable to differential expression and detection of given proteins within different cells. A cell with uniform fluorescence staining will have an Ai of 0. A cell with fluorescence staining totally restricted to the cathodal half will have an Ai of 1, whereas a cell stained only in the anode-facing half will have an Ai of −1. Therefore, Ai values >0 indicate cathodal accumulation of fluorescence staining, with stronger cathodal staining giving Ai values progressively closer to 1. The average Ai from a group of cells gives an objective estimate of fluorescence staining asymmetry across the group.
Statistical analyses were made using unpaired, two-tailed Student’s t test or Welch’s unpaired t test when SDs were significantly different from each other. Data are expressed as mean ± SEM, unless stated otherwise.
RESULTS
FN and LAM Enhance Cathodally Directed Migration of CECs
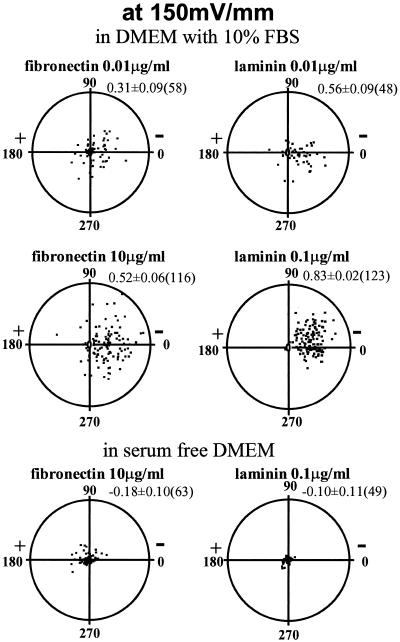
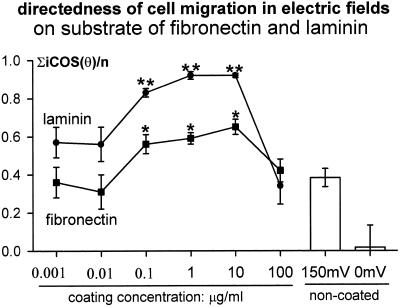
CECs cultured without applied EFs migrated randomly, with directedness near 0 (Zhao et al., 1996a). In EFs, both single cells and sheets of cells moved cathodally (Zhao et al., 1996a,b). Cathodal-directed motility increased on FN or LAM (Figure 1; compare with Zhao et al., 1996a, their Figure 3) and was concentration dependent. Concentrations >100 and <0.01 μg/ml did not enhance cathodal directedness (Figure 2). At most concentrations, LAM more effectively stimulated directedness than FN (Figures 1 and 2). Directional migration of CECs required serum (Zhao et al., 1996a). CECs on a range of FN or LAM concentrations in serum-free DMEM (150 mV/mm) lost all cathodal directedness (Figure 1).
Figure 1.
Typical translocation of bovine CECs over 5 h in EF (150 mV/mm). Substrate and serum dependency are illustrated. Each point represents a single cell, located initially (0 h) at the center of the circular graph with final location plotted after 5 h in EF. The radius of each circle is 200 μm, and average cell length is ∼20 μm. The average cosine (directedness) of the distribution ± SEM and the number of cells plotted are indicated at upper right of each distribution. (for distributions for unstimulated cells and cells exposed to EF alone, see Zhao et al., 1996a).
Figure 2.
FN or LAM significantly increased cathodal directedness of migration. The enhancement was concentration dependent. **, p < 0.01; *, p < 0.05 compared with that on the noncoated substratum in same EFs (shown at right). Directedness of 0 indicates random migration; directedness of 1 indicates all cells migrated directly cathodally; and −1 indicates direct anodal migration of all cells along the field vector. For methods of determining directedness, see Zhao et al. (1996a). Number of cells for each point, 43∼221.
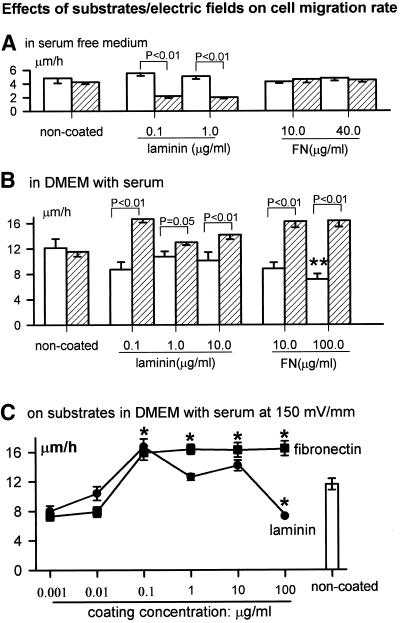
LAM and FN Increased the Rate of Cell Migration in EF
FN or LAM alone did not alter cell speed (Figure 3A), which remained low, ∼5 μm/h in serum-free medium. Serum increased cell speed on uncoated plastic and on FN or LAM substrates (Figure 3B), although high concentrations of FN (100 μg/ml) suppressed the migration rate in serum (Figure 3B). Cells on noncoated dishes in EF moved at 11.6 ± 0.8 μm/h (n = 183) but moved faster on FN or LAM in the same EF (150 mV/mm; Figure 3, B and C). This also was concentration dependent. On FN (0.1–100 μg/ml), enhanced rates were constant, ∼16 μm/h, although at 100 μg/ml enhanced cathodal directedness was lost (compare Figures 2 and 3C). LAM also increased the migration rate at 0.1 μg/ml, but higher concentrations were less effective. Only when FN or LAM and EF were present together did migration rate increase significantly (Figure 3).
Figure 3.
Migration rate of CECs cultured in: serum-free DMEM (A) without EF (□) and with EF at 150 mV/mm (▨) and DMEM plus 10% FBS (B) without EF (□) and with EFs at 150 mV/mm (▨). **, p < 0.01 compared with that on the noncoated substratum without EF. (C) Coating concentration dependency of migration rate in serum-containing DMEM at 150 mV/mm. *, p < 0.05 compared with noncoated substratum in same EFs (shown at right). Number of cells for each point, 38∼221.
The synergistic effect of ECM and EF on migration rate also depended on serum. Cells on FN or LAM in EF but no serum migrated slowly, like those with no EF, no substrate coating, and no serum (Figure 3, compare A and B).
Inhibition of MEK Significantly Reduced EF-directed Cell Migration
The MAPK inhibitor PD98059 significantly reduced both the migration rate and directedness of CECs in EF. Migration rate dropped from 6.6 ± 0.4 μm/h in serum-free medium plus EGF to 4.5 ± 0.3 μm/h (n = 170–198; four experiments; p < 0.0001) after drug exposure. Drug-treated cells therefore migrated at the same rate as those in serum-free medium plus EFs (Zhao et al., 1996a; 4.8 ± 0.8 μm/h), indicating that the effect of adding EGF on the migration rate of CECs in EFs was completely abolished. Directedness also dropped significantly from 0.78 ± 0.02 to 0.60 ± 0.04 (n = 170–198; four experiments; p < 0.001) in PD98059 but remained substantial. Treatment with PD98059 also reduced the migration rate of CECs on LAM or FN significantly (p < 0.001, two experiments) from control of 16.7 ± 0.54 μm/h (n = 123) to 11.8 ± 1.18 μm/h (n = 45) on FN and 16.3 ± 0.98 μm/h to 8.33 ± 0.8 μm/h (n = 90) on LAM (coating concentration, 0.1 and 10 μg/ml, respectively). However, the directedness remained unchanged for cells on both substrata exposed to EFs (our unpublished results).
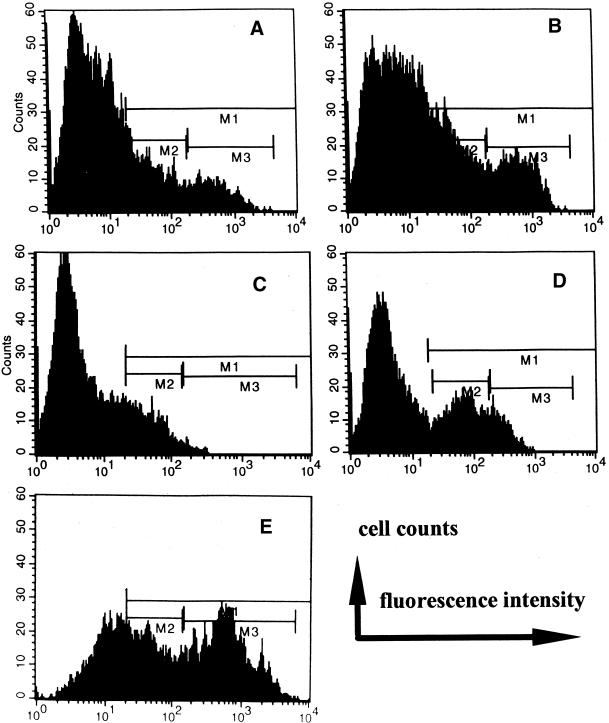
EF Exposure Increased Expression of EGFR
With negative isotype and second antibody control as background, positively stained cells were recognized as total expression of EGFR and gated as M1 (with background gated events set to <1% of total population). Within gate M1, two peaks of EGFR-labeled CECs were distinguished (Figure 4A) and further divided and gated as M2 and M3. Gate M2 represents a lower amount of EGFR expressed on the cell surface, recognized by dim fluorescence, which we defined as EGFRLow. Gate M3 represents a high amount of EGFR expressed on the cell surface, recognized by bright fluorescence, which we defined as EGFRHigh. The cells for these experiments were cultured on plastic for ∼16 h before EF application. After a further ∼16 h in a physiological EF (100–150 mV/mm; 37°C, 5% CO2, 95% humidity), the percentage of membrane EGFR-positive cells increased significantly (Figure 4B). The EGFRLow-expressing cells increased most (p < 0.05), from control value of 16.1 ± 1.4% (n = 5) to 22.0 ± 1.8% (n = 8 at 100–150 mV/mm), making a significant (p < 0.05) increase in the total percentage of positive cells, from control value of 25.3 ± 1.6% (n = 5 with no EFs) to 34.3 ± 2.8% (n = 8 at 100–150 mV/mm). However, average EGFRHigh remained unchanged (11.1 ± 1.3%; n = 8; at 100–150 mV/mm versus 9.3 ± 1.5%; n = 5; with no EFs), although a few experiments showed a dramatic increase in the percentage of EGFRHigh cells. Mean fluorescence intensity, corresponding to the number of EGFRs per cell, did not increase (our unpublished results). Interestingly, up-regulation did not occur in cells exposed to higher EFs (200–300 mV/mm; n = 4): percents of EGFR total, EGFRLow, and EGFRHigh were 25.7 ± 2.1, 19.0 ± 1.2, 7.1 ± 0.7%, respectively. Serum starvation might be expected to increase the membrane expression of EGFR, but we found no significant increase (EGFR total, 29.0 ± 2.0%; EGFRLow, 16.4 ± 1.2%; EGFRHigh, 12.6 ± 1.5%; n = 7).
Figure 4.
Representative fluorescent intensity flow cytometric histograms show the effect of LAM or FN with or without EF on membrane expression of EGFR on CECs. Isotype-matched negative control, OX21 as primary mAb, background fluorescence was similar with secondary Ab as negative control. M1 (positively stained cells) was set where the expression of gated events was <1% in isotype-matched negative control. (A) Control (without substratum coating, no EF) cells showed a population of positively stained cells, region M1, which can be further divided into a low peak (region M2) representing a subpopulation of low EGFR expression (EGFRLow) and a higher peak (region M3) representing a subpopulation of high EGFR expression (EGFRHigh) expression. (B) After 12 h in EF (100 mV/mm), positively stained cells increased, as shown by gated events in M1 as total, M2 as EGFRLow expression cells (p < 0.01), and M3 as EGFRHigh expression cells. The cells were cultured on plastic without substratum coating. (C) Withdrawal of serum from culture medium and exposed to EF (100 mV/mm) for 13 h shows a significant decrease in EGFR expression (p < 0.05 for EGFRHigh). The cells were cultured on plastic without substratum coating. (D) Addition of EGF (25 ng/ml) in serum-free medium and EF (100 mV/mm) for 13 h; EGFRLow expression was significantly up-regulated; compare with C. Also see text for detailed numerical data. (E) Significant increases in total EGFR expression and more dramatically in EGFRHigh were evident for CECs cultured on substratum of LAM (1 μg/ml) followed by 3 h of EF exposure at 100 mV/mm.
Culturing CECs in serum-free medium plus an EF (100–150 mV; n = 3; 200–300 mV; n = 4) did not induce EGFR up-regulation (Figure 4C; our unpublished results). To test whether receptor up-regulation by a small EF depends on the ligand, cells in serum-free medium with added EGF were assessed. Up-regulation of membrane expression of EGFR by EF (100 mV/mm) plus specific ligand was virtually identical to that seen in EF plus 10% fetal calf serum: total expression, 33.14 ± 0.46% (n = 2; p = 0.09); up-regulation was seen largely for EGFRLow: 22.07 ± 0.02% (p < 0.01) (Figure 4D); whereas EGFRHigh remained unchanged (11.48 ± 0.48%) compared with serum-free medium and 0 mV/mm. Addition of EGF alone to serum-free medium (no EF) did not increase EGFR expression (p = 0.91).
FN or LAM Substratum Increased Expression of Membrane EGFR
The effects of FN or LAM alone or in combination with EFs in modulating EGFR expression on CECs also were tested. To minimize possible effects of secreted ECM, which occurs 18∼24 h after seeding CECs (Sugrue and Hay, 1982; Ohji et al., 1993), CECs cultured for ∼15 h on plastic or on substratum of FN or LAM were exposed to EF for 3 h (100 mV/mm) in separate experiments.
Membrane EGFR expression of CECs cultured on plastic for ∼15 h followed by 3 h of EF exposure remained unchanged compared with that of the cells not stimulated with EF (Table 1).
Table 1.
Effects of EF and fibronectin or laminin on membrane expression of EGF receptors on corneal epithelial cells
| EF strength | No coating
|
Substratum
|
||||
|---|---|---|---|---|---|---|
| Fibronectin (1 μg/ml)
|
Laminin (1 μg/ml)
|
|||||
| 0 mV | 100 mV | 0 mV | 100 mV | 0 mV | 100 mV | |
| In DMEM with 10% FCS | ||||||
| Total expression (%) | 43.13 ± 5.35 (n = 4) | 43.46 ± 4.56 (n = 4) | 49.91 ± 3.79a (n = 8) | 56.22 ± 6.04ab (n = 4) | 48.87 ± 2.86c (n = 6) | 52.66 ± 3.24ab (n = 6) |
| Low expression (%) | 32.71 ± 8.50 | 35.83 ± 2.34 | 29.95 ± 8.80 | 32.07 ± 6.45 | 30.14 ± 10.60 | 31.57 ± 12.00 |
| High expression (%) | 10.45 ± 4.67 | 7.78 ± 2.78 | 17.79 ± 11.34 | 24.52 ± 8.27ab | 19.02 ± 12.59 | 25.40 ± 13.17ab |
| In serum-free DMEM | ||||||
| Total expression (%) | 38.97 ± 6.76 (n = 2) | 44.73 ± 5.86 (n = 2) | 51.51 ± 3.16(4)a (n = 4) | 51.59 ± 3.12c (n = 3) | 50.47 ± 2.91ad (n = 6) | 56.40 ± 7.79ad (n = 7) |
| Low expression (%) | 25.23 ± 0.75 | 31.60 ± 7.40 | 32.46 ± 10.05 | 27.74 ± 10.50 | 29.51 ± 88 | 27.10 ± 12.73 |
| High expression (%) | 13.90 ± 6.04 | 13.44 ± 1.42 | 21.31 ± 7.15 | 30.18 ± 10.00 | 21.28 ± 10.34 | 33.41 ± 9.63ab |
EF exposure time, 3 h; values are mean ± SD; FN and laminin coating concentration was 1 μg/ml. The cells were first cultured in DMEM with 10% FCS. Only during EF exposure was the serum-free medium used.
p < 0.05 when compared with that from cells cultured on noncoated dishes without applied EFs.
p < 0.05 when compared with that from cells cultured on noncoated dishes with 100 mV/mm applied EFs.
p = 0.056∼0.058 when compared with that from cells cultured on noncoated dishes without applied EFs.
p = 0.09 when compared with that from cells cultured on noncoated dishes with 100 mV/mm applied EFs.
CECs on FN or LAM alone (1 μg/ml coating concentration) for ∼18 h, however, did show significantly higher total expression of membrane EGFR even without applied EFs. There were no significant changes in EGFRLow or EGFRHigh for those cells (Table 1).
FN or LAM (∼15 h) plus 3 h of EF exposure in medium with or without serum did not further up-regulate total membrane EGFR expression. (Cells were cultured in DMEM with 10% serum for ∼15 h before EF exposure and serum-free exposure. Only during the 3-h EF exposure was serum-free DMEM used.) However, analyzing EGFRLow and EGFRHigh subpopulations in the cells revealed an interesting issue. EGFRHigh tended to be higher for the cells cultured on FN or LAM than on plastic whether EFs were applied (Table 1). When those cells on substrata of FN or LAM were further subjected to 3 h of 100 mV/mm, significant increases in EGFRHigh became evident. This was particularly true for the cells cultured on FN followed by 3 h in serum-containing medium at 100 mV/mm and cells cultured on LAM followed by 3 h at 100 mV/mm in a medium with or without serum (Table 1). A dramatic increase in EGFRHigh was evident for the cells cultured on LAM followed by 3 h of exposure to 100 mV/mm in serum-free medium (Figure 4E).
Mean receptor numbers per cell (mean fluorescence intensity) did not change, with EF, FN or LAM, or EF plus FN or LAM (our unpublished results).
Three Growth Factor Receptors and Actin Are Redistributed by a Physiological EF
Mean fluorescence intensity of probes for growth factor receptors and for actin on the cathodal- and anodal-facing halves of CECs was assessed, and asymmetric indices (Ai) for 10–20 single cells were calculated (see MATERIALS AND METHODS).
Early EF-induced Redistribution of EGFR and Actin Are Serum Dependent
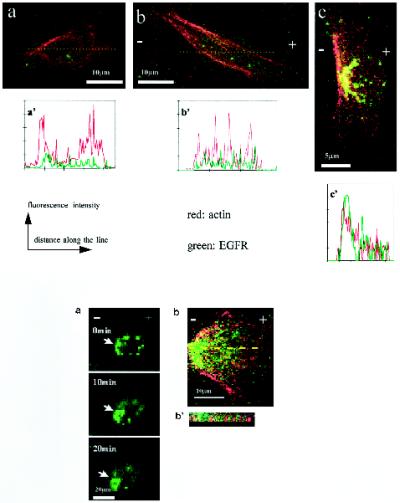
With no EF, most cells showed no asymmetry of EGFRs or actin staining (Figure 5, a and a′). After exposure to an EF (150 mV/mm) in serum-containing medium, EGFR and actin staining redistributed and accumulated at the cathode-facing side of the cells (Figure 5, c and c′) (38% of 587 cells showed clear asymmetry of receptors). Figure 6 shows the dynamic cathodal accumulation of EGFR in a live cell. After 10 min in an EF (300 mV/mm), EGFR had accumulated cathodally. The increase of cathodal EGFR staining was more obvious at 20 min with a concomitant decrease in anodal staining. In serum-free medium, however, no cathodal accumulation of EGFR or actin was observed for cells exposed for 3 h to EFs of 150 mV/mm (Figure 5b) or for live cells exposed to an EF of 300 mV/mm (our unpublished results).
Figure 5.
Confocal images of CECs double labeled for actin filaments (red) and EGFR (green). Colocalization is indicated by yellow. (a) Cell cultured without EF, no obvious asymmetric distribution of EGFR or F-actin. (b) Cell cultured in serum-free medium at 150 mV/mm, no obvious asymmetric distribution of EGFR or F-actin. (C) Cell cultured in EF in serum. Obvious accumulation of EGFR and F-actin cathodally was evident. (a′–c′) Fluorescence intensity in arbitrary units along a line drawn across the cells in a–c, respectively. Asymmetry of both actin and EGFR is evident both visually and digitally only for the cell in c. Figure 6. (A) Confocal images of a live CEC labeled with EGFR antibodies, showing dynamic accumulation of EGFR at the cathode facing side (arrows) when exposed to 300 mV/mm for a 20-min duration, cultured in DMEM with 10% FBS. Bar, 20 μm. (B) Asymmetry of EGFR and F-actin in a cell exposed to 150 mV/mm for 3 h. (B′) Representative vertical section (through the line drawn across the cell in B) shows actin and EGFR staining in an interdigitating pattern in punctate spots near the cell–substratum interface.
Ai was used to quantify EGFR and actin asymmetry (see MATERIALS AND METHODS for details). An Ai value close to 0 indicates symmetric distribution. Increasing asymmetric accumulation is indicated by a higher value of Ai from 0 to 1. There are two important points. 1) EF-induced asymmetry was voltage dependent; there was a higher Ai at higher field strength. Cells exposed to EF for 3 h had Ai values for EGFR of 0.19 ± 0.06 at 150 mV/mm and 0.30 ± 0.09 at 250 mV/mm, both significantly higher than the control: −0.06 ± 0.05 (p < 0.05; average of 10–20 cells). 2) EF-induced asymmetry was serum dependent. In serum-free medium, the Ai value for EGFR was −0.01 ± 0.04 at 150 mV/mm, showing no obvious asymmetry. Even exposure to 250 mV/mm did not increase the Ai of EGFR significantly (0.16 ± 0.08; p = 0.08 compared with no field control). Ai for actin was 0.34 ± 0.07 (n = 10) when cultured in 150 mV/mm with 10% FBS, whereas Ai failed to rise when cultured in serum-free medium (Ai = 0.06 ± 0.26; n = 21) at the same field strength. When exposed to 300 mV/mm in 10% FCS, Ai for EGFR in live cells (n = 7) was −0.02 ± 0.01 at 0 min, 0.07 ± 0.05 at 10 min, and 0.37 ± 0.13 at 20 min (p < 0.05 when compared with that at 0 min), whereas cells cultured in serum-free medium did not show significant changes in Ai at the same field strength. To test whether growth factors might facilitate cathodally directed growth factor receptor accumulation, we added TGF-β1 to serum-free culture medium (1.5 pg/ml). This is one of the growth factors that partially restored EF-induced directional migration of CECs in serum-free medium (Zhao et al., 1996a). Clear cathodal accumulation of EGFR and TGFR and rhodamine-phalloidin staining for actin filaments were observed (our unpublished results). We have not done the equivalent experiment with EGFR ligand.
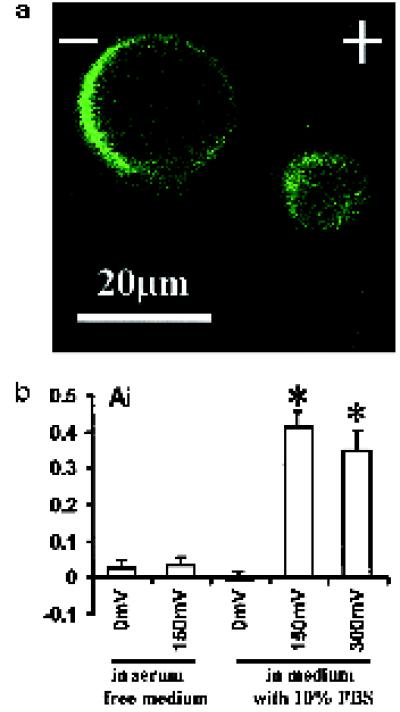
Redistributed receptors were capable of binding EGF, because there was marked cathodal accumulation of EGFR–fluorescein EGF complexes after 3 h of EF exposure (Figure 7a). The Ai values also show voltage and serum dependency of the cathodal accumulation of this ligand–receptor complex (Figure 7b).
Figure 7.
Marked cathodal accumulation of EGFR on live CECs revealed by fluorescein EGF binding. (A) Cells were exposed to EF for 3 h at 150 mV/mm and labeled as described in MATERIALS AND METHODS. (B) Ai of EGFR (in 10–20 cells for each group) as revealed by fluorescein EGF binding (see MATERIALS AND METHODS for details). An Ai value >0 indicates higher fluorescence intensity on the cathodal facing side; values close to 1 indicate extreme cathodal asymmetry of EGFR. *, p < 0.001 when compared with that of the cells in serum-free medium or cells not exposed to EF.
Membrane and Intracellular Asymmetry of EGFR and F-actin
Cathodal asymmetry was also analyzed using horizontal and vertical optical sections to reveal the subcellular distribution of receptor and actin staining. Qualitative observations and Ai calculations of single horizontal sections from base to top together indicate that well-spread cells showed greater cathodal asymmetry of EGFR in basal layers, whereas for less-spread cells, a higher Ai was evident in the middle or top layers. EGFRs accumulated cathodally both at the leading edge and intracellularly; the latter was largely perinuclear. We analyzed these separately. Intracellular and membrane–juxtamembrane regions were selected (see MATERIALS AND METHODS), and Ai values were calculated. The most marked cathodal asymmetry was found for membrane-associated staining for EGFR and for F-actin. The greatest asymmetry of membrane EGFR occurred in serum-containing medium at 150 mV/mm (Ai = 0.40 ± 0.06 compared with −0.03 ± 0.06 for control cells [no EF]; n = 10; p = 0.0002). EF exposure also induced asymmetry of EGFR staining intracellularly (Ai = 0.18 ± 0.04; no EF = 0.03 ± 0.04; n = 10; p = 0.04). Membrane F-actin (at the leading edge) also accumulated cathodally after EF exposure, (Ai = 0.28 ± 0.03; no EF = 0.08 ± 0.06; p < 0.01). Intracellular Ai for F-actin also suggested cathodal asymmetry (0.23 ± 0.05) but was not statistically different from no EF control values of 0.07 ± 0.12.
Close Colocalization of EGFR and F-actin
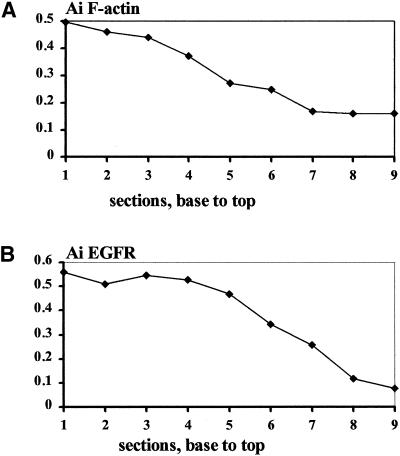
Both F-actin and EGFRs accumulated cathodally and frequently were observed colocalized or adjacent to each other (Figure 5, c and c′). These observations were quantified by optically sectioning 10 cells (EF 150 mV/mm; 3 h) and assessing the Ai for both EGFR and for actin in parallel in each of 96 optical sections. The mean Ai was very similar for each probe (0.38 ± 0.07 for EGFR, 0.34 ± 0.07 for actin). The paired values were highly correlated (R = 0.72; p < 0.001, two-tailed Pearson correlation test), indicating a high degree of colocalization. A representative Ai as a function of position (base to top) in one cell is shown in Figure 8. A similar pattern with a high correlation between Ai for F-actin (Figure 8A) and Ai for EGFR (Figure 8B) was observed in 9 of 10 cells. EGFR and actin therefore were asymmetric to a similar extent in similar locations. This asymmetry in cathodal- and anodal-facing half cells was further refined. Asymmetrical distribution of EGFR and actin associated with either membrane, or intracellular locations also were assessed and showed marked correlation, perhaps indicating causal relationships. The Ai for membrane EGFR was highest and was highly correlated with the Ai for intracellular EGFR and for membrane F-actin and intracellular F-actin (R = 0.32, 0.62, and 0.53, respectively; p < 0.004). The Ai for membrane-associated F-actin also correlated well with that for intracellular EGFR and for intracellular F-actin (R = 0.23 and 0.61, respectively; p < 0.05).
Figure 8.
Ai of F-actin and EGFR from serial optical sections through a single cell, showing close correlation of the subcellular localization of F-actin (A) and EGFR (B). An Ai value >0 indicates higher fluorescence intensity on the cathodal facing side; therefore, values close to 1 indicate extreme cathodal asymmetry of F-actin or EGFR. Each section is 0.5 μm thick.
At the cell–substratum interface, EGFR and actin staining frequently were colocalized and interdigitated, to form “tank track”–like repeats of red (actin filament) and green (EGFR) staining (Figure 6, B and B′). This intriguing interdigitated pattern of EGFR and actin staining close to the cell–substrate interface was obvious in 9 of 10 cells.
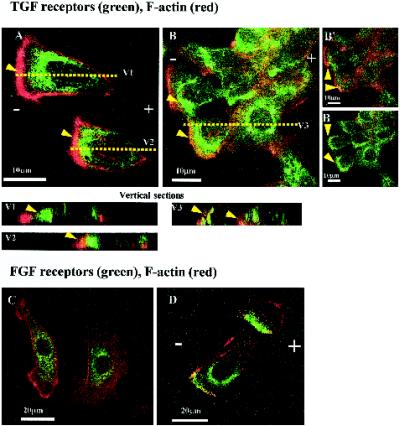
EF-induced Asymmetry of FGFR and TGFR II
Like EGF, bFGF and TGF-β1 also partially restored cathodally directed migration of CECs in serum-free medium (Zhao et al., 1996a). Perhaps the receptors for these growth factors also redistribute and accumulate cathodally. CECs in EF for 3 h (150 mV/mm) did not show a clear asymmetry of FGFR or TGFR, although a weak cathodal asymmetry of TGFR was seen after contrast enhancement (our unpublished results). After 12–16 h in EF (37°C), however, both TGFR and FGFR showed pronounced EF-induced cathodal redistribution (Figure 9). Asymmetry of TGFRs occurred in single cells (Figure 9A, vertical sections, V1 and V2) and grouped cells (Figure 9B, V3). Marked perinuclear asymmetry of TGFR is evident in basal (Figure 9B′) and middle (Figure 9B") optical sections of the cells. Long (∼16-h) EF exposure also induced marked cathodal asymmetry of FGFR (Figure 9D), whereas control cells (no EF) showed no obvious asymmetry of FGFR (Figure 9C). Longer EF exposure therefore revealed cathodal perinuclear asymmetry for both TGFR and FGFR.
Figure 9.
Confocal images of CECs exposed to EF of 150 mV/mm for 12–16 h and double labeled with rhodamine-phalloidin for F-actin (red) and antibodies against growth factor receptors (green): TGFR (top panel) or FGFR (lower panel). Colocalization is indicated by yellow. (A) Two single cells showing stronger staining at the cathodal side for both TGFR and F-actin. (V1 and V2) Vertical sections along the line V1 and V2 in A. (B) Grouped cells also show accumulation of TGFR staining at the cathodal side (arrows). (V3) Vertical section cut along line V3 in B shows asymmetric accumulation of TGFR at the cathodal facing side. (B′ and B") TGFR and F-actin asymmetry in the basal and middle sections of B. (C) Two control cells (no EF exposure) show F-actin and FGFR staining. (D) Striking asymmetric distribution of the FGFR (green) and actin (red) observed in two cells after 16 h in EF of 150 mV/mm.
Asymmetry of TGFR and FGFR also was quantified. After 16 h of EF exposure at 150 mV/mm, the Ai for TGFR increased to 0.17 ± 0.08 (n = 9), significantly higher (p = 0.013) than no EF control, 0.05 ± 0.11 (n = 13). A striking increase in Ai for FGFR staining was found after 16 h of exposure to EF of 150 mV/mm: 0.61 ± 0.08 (n = 14); p < 0.01 compared with control. However, a short duration (3 h) of EF exposure did not increase Ai of FGFR: Ai values for FGFR were 0.02 ± 0.05 for the control; −0.01 ± 0.05 for the cells exposed to 150 mV/mm in DMEM with 10% FCS; 0.03 ± 0.06 for the cells exposed to 250 mV/mm in DMEM with 10% FCS; and 0.06 ± 0.04 for the cells exposed to 150–250 mV/mm in serum-free medium (n = 10–20 cells).
DISCUSSION
Corneal wounding induces secretion of EGF and FN, the latter acting as a transient matrix for epithelial migration (Gipson and Inatomi, 1995). Endogenous, laterally oriented EFs also are induced after corneal injury (Chiang et al., 1992), and cultured CECs migrate cathodally in similar, physiological EFs (Zhao et al., 1996a,b). (We used field strengths two- to threefold greater than those measured in vivo [42 mV/mm], but these are underestimates [Chiang et al., 1992]. The theoretical maximum in vivo is as much as 500 mV/mm.) When these elements were combined, both the speed and the extent of cathodal directedness increased on FN or LAM, and both events were serum dependent. Because EGF restored cathodal-directed migration in serum-free medium, we tested the hypothesis that EGFR activation may be an early event in EF-directed CEC migration. EF, FN, or LAM each up-regulated membrane expression of EGFRs (all enhance cathodal directedness), whereas EGFRs and polymerized actin redistributed to accumulate cathodally, with a time course and a pattern of colocalization consistent with a role in directing cell migration cathodally. Additionally, up-regulation of EGFRs and asymmetric redistribution of EGFRs and actin all required serum.
Enhancement of Migration Speed: Serum Dependency
FN or LAM with or without serum and FN or LAM with EF but no serum did not increase rates of cell movement. Only cells grown on FN or LAM with EF and serum showed an increased speed of migration. Cells may migrate in response to chemotactic (growth factor) and/or haptotactic (ECM protein) stimuli, which may be spatially distributed in concentration gradients. However, multiple stimuli such as FN or LM with EF and growth factors alter speed of migration as well as directionality (see below). This may indicate that the coordinated interaction of these stimuli alters the kinetics of the migratory response signaling pathway, perhaps via intracellular regulation of second messenger responses, e.g., via rates of phosphorylation and dephosphorylation of receptors. Such interactions seem likely. For instance, human CECs migrate on FN only in the presence of EGFR activation, and migration on FN is inhibited by antibodies to EGF (Maldonado and Furcht, 1995). Similarly, anti-FN antibodies inhibit EGF-stimulated migration of rabbit CECs, indicating that migration may require activation of both growth factor receptors and the integrin receptors for FN (Nishida et al., 1990). FN significantly potentiates activation of MAPK by EGF in fibroblast cells (Miyamoto et al., 1996) and endothelial cells (Short et al., 1998) and only does so if the integrins are both aggregated and occupied by ligand (Miyamoto et al., 1996). ECM and EGF may interact to activate MAPK at the level of integrin aggregation, integrin occupancy, and growth factor binding (Miyamoto et al., 1996). The present study shows that additionally there may be regulation of EGFRs by ECM (see Up-regulation of EGFR by FN and LAM). Serum dependency of faster, ECM- and field-induced cell speed may represent a requirement for EGF and the induction of a shift to more optimal levels of cell–substratum adhesiveness (Palecek et al., 1997).
There may be changes also in the total number of receptors expressed on migrating cells, making more receptors available to bind excess ligand. Adding serum to cultured fibroblasts increased gene expression for FN, FN receptors, actin, and tropomyosin within 15 min (Ryseck et al., 1989). EGF increased synthesis and secretion of FN and may control integrin expression in CECs, because α2β1, α3β1, and ανβ1 integrins all increased in wounded CECs (Elner and Elner, 1996). Additionally, growth factors, FN, and a single electrical pulse (at a field strength 100- to 250-fold stronger than used here; Pazmany et al., 1995) all induced early activation of growth-related genes. Whether immediate early gene expression is increased by the much smaller and constant physiological EF used here is not known.
Enhancement of Cathodal Directedness
LAM or FN did not induce directed migration. On FN or LAM plus EF, migration was more sharply focused directly toward the cathode. LAM was more effective than FN, although below and above a range of concentrations, enhancement of directedness was lost (Figures 1 and 2). LAM plus EF also enhanced migration rate, and similar concentrations were effective, suggesting common mechanisms (compare Figures 2 and 3C). By contrast, FN- plus EF-enhanced migration rates persisted at concentrations that no longer enhanced cathodal directedness (100 μg/ml; Figures 2 and 3C), suggesting separate underlying mechanisms.
It is perhaps not surprising that the MAPK inhibitor PD98059 did not reduce the directedness of CECs cultured on FN or LAM in EFs to the same extent as it reduced the migration rate. MEK1 is a downstream element in EGF signaling. It is quite likely that the asymmetry of EGFR serves as a steering wheel, whereas the motor machinery activated through MAPK-phosphorylating myosin moves the cells on ECM (Klemke et al., 1997).
Potential Mechanisms: Up-regulation of EGFR by EF
The EGFR is up-regulated by its ligand EGF and by TNF and down-regulated in vitro by increasing cell density (Holley et al., 1977; Earp et al., 1986; Palombella et al., 1987). Additionally, LAM down-regulates both the EGFR and insulin-like growth factor I receptor on rat enterocytes (Wolpert et al., 1996). Up-regulation of EGFRs by an EF or by FN or LAM is a novel observation and adds to the tight regulation of this receptor. The mechanisms underlying EGFR up-regulation by EF or by ECM proteins in CECs are unclear. However, because EGFR expression was determined 12–16 h after EF or ECM exposure, there would be time for nuclear transcription and new protein synthesis to occur, as well as recruitment of preexisting receptors. DC fields do regulate gene expression, e.g., c-fos, jun, and c-myc (Lin et al., 1994; Pazmany et al., 1995; Sauer et al., 1997). In addition, EGFR up-regulation was voltage dependent, occurring only at EF close to the physiological wound field strength. Higher EFs were ineffective. Wounding cornea or skin also induced and up-regulated expression of EGFR (Wenczak et al., 1992; Murata et al., 1993) and induced laterally oriented DC EF (Chiang et al., 1992). Perhaps the EF is causal in up-regulating the EGFR in vivo.
Interestingly, EF did not up-regulate EGFRs in serum-free medium, but this did occur if the ligand EGF was present. This implicates the ligated EGFR as a proximal element in EF-induced receptor up-regulation. EGFR regulation probably involves ligand–receptor interaction and EF-induced, ligand-bound receptor clustering (see Redistribution of EGFR and Actin below).
Up-regulation of EGFR by FN and LAM
CECs on FN or LAM also increased membrane expression of EGFR (Table 1). We do not know whether the EGFR is actively synthesized in our experimental system. If this is the case, one potential mechanism is that ECM may stimulate TGF-β1 gene transcription (Streuli et al., 1993), which in turn up-regulates EGFR transcription and expression in fibroblasts (Thompson et al., 1988). Integrin receptors and growth factor receptors (such as EGF) act synergistically by common signaling pathways (Schlaepfer et al., 1994; Miyamoto et al., 1996), and expression of integrin receptors in CECs is specifically regulated by ECM ligands (Grushkin-Lerner et al., 1997). Perhaps EGFRs and integrin receptors are up-regulated in parallel by FN, LAM, or EF. Additionally, enhanced exportation from intracellular pools of EGFR to the cell membrane cannot be excluded.
The discovery that both EF and FN up-regulated EGFR may be significant given that both stimuli are early responses to corneal wounding. However, their effects on EGFR up-regulation were not additive, suggesting that they operate by a common and saturable mechanism.
Redistribution of EGFR and Actin
Asymmetric redistribution of EGFR was demonstrated in both live and fixed CECs exposed to EFs using either antibody against EGFR (Figures 5, 6, and 8B) or the physiological ligand for the EGFR (Figure 7). The dynamics of receptor redistribution in live cells also was demonstrated (Figure 6A).
EFs also induced cathodal accumulation and colocalization of EGFR and actin. Both AC and DC fields redistribute cell surface receptors (including the EGFR) and the actin cytoskeleton (Poo and Robinson, 1977; Luther et al., 1983; Giugni et al., 1987; McCaig and Dover, 1991; Cho et al., 1994, 1996; Brown and Loew, 1996), although the mechanisms differ, because redistribution in an AC field is not along the field vector (Cho et al., 1996). We propose that EGFR redistribution cathodally leads to localized actin polymerization at the EGFR–plasma membrane interface and that these events respectively trigger and mediate cathodal-directed CEC migration. The EGFR is an actin-binding protein (den Hartigh et al., 1992), and EGF induces rapid remodeling of the actin cytoskeleton. In human epidermal carcinoma cells, actin polymerization is localized to activated EGFRs in the plasma membrane and is not associated with internalized EGFRs (Rijken et al., 1995). Moving the EGFR cathodally, therefore, may be a key event, with downstream consequences of asymmetry of actin polymerization. Both receptor and actin asymmetries were serum dependent. Ligated EGFRs may have considerably greater lateral mobility within the plasma membrane than unbound receptors. The IgE–Fcε receptor complex accumulated strongly cathodally on leukemic rat basophils, whereas ligand-free Fcε receptors showed minimal cathodal accumulation in DC fields 10 times those used here (McCloskey et al., 1984). Because neither EGFR nor F-actin accumulated cathodally in serum-free medium, EGFR redistribution might be both sufficient and necessary for subsequent actin asymmetry. (We cannot exclude the possibility that cathodal redistribution of some other receptor also might induce actin accumulation cathodally.) Intracellular EGFRs accumulated cathodally also, perhaps reflecting initial asymmetry of membrane EGFRs and asymmetric internalization. Significantly, EGFRs also accumulated cathodally in cell sheets, and sheets of cells migrate cathodally (Zhao et al., 1996b). This is important because corneal wound healing involves migration of cell sheets, which maintain intercellular linkages (Dua and Forrester, 1989; Gipson and Sugrue, 1994). EF-induced F-actin redistribution to the leading edge of single cells also may facilitate actin cable formation at the leading edge of healing cornea, a process that underlies the coordinated movement of cell sheets (Danjo and Gipson, 1998).
In mouse fibroblasts, EGFR activation disassembles focal contacts, and the extent of this is correlated with increasing migration speed (Xie et al., 1998). EF-directed, faster migration of CECs on FN or LAM also may involve polarized focal contact disassembly and integrin receptor redistribution (see above). We have no data on integrin expression in migratory CECs, although the α5β1 FN receptor redistributed on fibroblasts in a DC EF, with smaller aggregates accumulating cathodally and larger aggregates accumulating anodally (Brown and Loew, 1996). Also, the LAM receptor α6β4 redistributes and spreads out in wounded corneal epithelium (in an endogenous, wound-induced EF) to break up and decrease focal adhesion in preparation for cell migration (Gipson and Inatomi, 1995). Additionally, AC field-directed migration of human macrophages was inhibited by antibodies to the β2 but not the β1 integrin subunit (Cho et al., 1997). Irrespective of the integrins involved, close colocalization of EGFR, actin polymerization, and integrin receptor turnover at dynamic substrate contact points are likely to underpin cathodal migration. An interesting pattern of interdigitated, colocalized EGFR clusters and F-actin was observed (Figure 6B′), particularly cathodally at the cell–substrate interface in CECs. The relationship of this to directed cell migration or focal adhesion disassembly is not known.
Our data indicate asymmetries both on the membrane and intracellularly. The intracellular asymmetry of EGFR and F-actin suggests a causal relationship between the asymmetries of membrane EGFR and membrane-associated F-actin and those of intracellular EGFR and F-actin. Live cells showed cathodal accumulation of EGFR within 10 min in an EF (Figure 6A). Binding with EGF (Figure 7A) may initiate asymmetric cell signaling and EF-directed cell migration. As activated membrane EGFRs, the internalized receptors that remain associated with EGF are also capable of phosphorylating endogenous substrates (Sorkin, 1996). Therefore, signals from activation of EGFRs may be expected from both membrane and intracellular EGFR–EGF complexes, and both are distributed asymmetrically in EFs. Asymmetry of intracellular proteins will not result directly from EF exposure (because of the high resistance of the cell membrane); therefore, the new observations of cathodal accumulation of intracellular EGFR probably indicate asymmetric internalization of EGFR. One signaling pathway used by the EGFR to engage cell migration involves MAPK (Klemke et al., 1997). The MAPK inhibitor PD98059 significantly reduced EF-directed cell migration, further supporting the notion of asymmetric signaling triggered by asymmetric receptor activation.
The TGFR and FGFR also accumulated cathodally but less strikingly and more slowly than the EGFR (Figure 9). TGF and bFGF partially restored EF-directed migration in serum-free medium over 5 h (Zhao et al., 1996a). Whether the mechanism involves receptor accumulation cathodally is unclear.
Physiological Significance
The basic mechanism of EF-directed cell motility and its relevance in corneal wound healing are key issues. EF-directed CEC migration may begin with cathodal redistribution of EGFRs and culminate with asymmetric polymerization of the actin cytoskeleton. Manipulations that enhanced cell speed and cathodal directedness, EF and EF plus FN or LAM, each up-regulated the EGFR and redirected both EGFR and actin cathodally. Higher, nonphysiological EFs, which did not up-regulate the EGFR, resulted in slower speed, although directedness was maintained (Zhao et al., 1996a,b). Because growth factor receptor asymmetry can induce directed cell movement, perhaps enhanced directedness requires enhanced receptor asymmetry. The EGFR is widely expressed and tightly regulated and appears to control many aspects of cell motility (Xie et al., 1998; Ware et al., 1998). It is satisfying and significant that a physiological EF may initiate directed cell movement by using EGFR signaling.
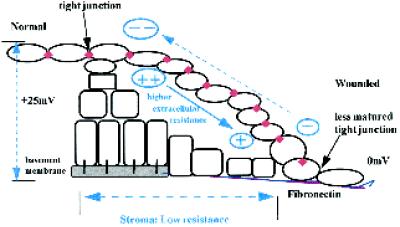
Wounding collapses the transcorneal potential difference (PD) and creates a laterally directed EF (Chiang et al., 1992). CECs cultured in a similar EF migrate directionally. However, there is a question of polarity (Figure 10). The inside positive transcorneal PD drives current (flow of positive charge) below the epithelial layers, out the wound with a return path in the thin tear fluid film (Chiang et al., 1992). Direct measurements of the resultant lateral fields indicate that the higher resistance path, and the one across which the greater voltage drop is measured, is the tear fluid. In that layer, the wound is positive relative to distant sites (Figure 10); yet in culture CECs migrate cathodally. At subepithelial stromal levels, the resistance to current flow was much lower, establishing only a very small lateral fields, wound site negative (Chiang et al., 1992). However, what has not been determined experimentally is the more relevant lateral fields immediately below the upper epithelial layer, where the tight junctons exist and across which the transcorneal PD is established (Klyce, 1972; Wolosin, 1988). Immediately below the migrating surface epithelia, cells are tightly packed, with little extracellular space. Tissue resistance may be substantial and may establish larger lateral fields with the wound negative. Several epithelial cell types maintain a normal transepithelial resistance, with functional tight junctions up to a wound border (Hudspeth, 1982), whereas for newly apposed cells, tight junctions are formed within 15 min. In healing stratified corneal epithelium, maintenance of functional tight junctions is indicated by the presence of tight junction–specific protein occludin and ZO-1 just one cell back from the leading edge (Danjo and Gipson, 1998). Thus the upper aspect of a migrating sheet of epithelium may “see” the wound as an anode, whereas the lower surface would see a cathode at the wound. In support of this, several reports have demonstrated suprabasal cells rolling over the basal cells to assume a position at the leading edge of healing tongue in reepithelialization (Krawczyk, 1971; Garlick and Taichman, 1994). This is consistent with a role for EF in directing corneal cell movement into a wound but may involve a complex push–pull type of EF-induced behavior on the upper and lower surfaces, respectively, of a migrating corneal epithelial sheet (Figure 10).
Figure 10.
Model illustrating potential role of lateral EF in directing corneal epithelial migration at a wound edge. Beyond ∼1 mm away from a wound, the transcorneal PD indicated at the left (+25 mV inside positive) is supported by the high resistance of the tight junctions in the upper sheet of epithelial cells. At the wound the PD collapses to zero, FN has replaced the normal basement membrane, and the upper sheet of epithelial cells rapidly slides down to make contact with the transient basement membrane within ∼1 h (Kuwabara et al., 1976). Direct measurements indicate that the lateral fields in the tear fluid are higher than those in the stroma. However (see DISCUSSION), the relevant subepithelial voltage drop immediately below the top sheet of corneal epithelium has not been measured and is likely to be substantial, given the tight packing of these differentiated and flattened cells. The lower surface of the cells of a sliding sheet therefore would be migrating toward a cathode. In corneal epithelial wounds, the tight junction–specific protein occludin is present just one cell back from the leading edge (Danjo and Gipson, 1998).
ACKNOWLEDGMENTS
We thank The Wellcome Trust and Dr. James Alexander Mearns’ Trust for financial support, G. Flett for preliminary experiments, Dr. A.M. Rajnicek for helpful discussion, and Dr. J. Zhang (Lawrence Berkeley National Laboratory, University of California, Berkeley, CA) for help with initial staining methods. We thank our referees for their valuable comments and suggestions. This work was supported by The Wellcome Trust and Dr. James Alexander Mearns’ Trust, United Kingdom.
REFERENCES
- Bergethon PR, Trinkaus-Randall V, Franzblau C. Modified hydroxyethylmethacrylate hydrogels as a modelling tool for the study of cell-substratum interactions. J Cell Sci. 1989;92:111–121. doi: 10.1242/jcs.92.1.111. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Loew LM. Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol. 1994;127:117–128. doi: 10.1083/jcb.127.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Loew LM. Graded fibronectin receptor aggregation in migrating cells. Cell Motil Cytoskeleton. 1996;34:185–193. doi: 10.1002/(SICI)1097-0169(1996)34:3<185::AID-CM2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cai X, Foster CS, Liu JJ, Kupferman AE, Filipec M, Colvin RB, Lee SJ. Alternatively spliced fibronectin molecules in the wounded cornea: analysis by PCR. Invest Ophthalmol Vis Sci. 1993;34:3585–3592. [PubMed] [Google Scholar]
- Chiang MJ, Robinson KR, Vanable JW., Jr Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- Cho MR, Thatte HS, Lee RC, Golan DE. Induced redistribution of cell surface receptors by AC electric fields. FASEB J. 1994;8:771–776. doi: 10.1096/fasebj.8.10.8050677. [DOI] [PubMed] [Google Scholar]
- Cho MR, Thatte HS, Lee RC, Golan DE. Reorganisation of microfilament structure induced by ac electric fields. FASEB J. 1996;10:1552–1558. doi: 10.1096/fasebj.10.13.8940302. [DOI] [PubMed] [Google Scholar]
- Cho, M.R., Thatte, H.S., Lee, R.C., and Golan, D.E. (1997). β2 integrins mediate directed migration of human macrophage induced by ac electric fields. Mol. Biol. Cell 8(suppl), 1530 (abstract).
- Danjo Y, Gipson IK. Actin “purse string” filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111:3323–3331. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- den Hartigh JC, van Bergen en Henegouwen PM, Verkleij AJ, Boonstra J. The EGF receptor is an actin-binding protein. J Cell Biol. 1992;119:349–355. doi: 10.1083/jcb.119.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Forrester JV. Clinical patterns of corneal epithelial wound healing. Am J Ophthalmol. 1989;104:481–489. doi: 10.1016/s0002-9394(14)74105-4. [DOI] [PubMed] [Google Scholar]
- Earp HS, Austin KS, Blaisdell J, Rubin RA, Nelson KG, Lee LW, Grisham JW. Epidermal growth factor (EGF) stimulates EGF receptor synthesis. J Biol Chem. 1986;261:4777–4780. [PubMed] [Google Scholar]
- Elner SG, Elner VM. The integrin superfamily and the eye. Invest Ophthalmol Vis Sci. 1996;37:696–701. [PubMed] [Google Scholar]
- Espaillat A, Lee SJ, Arrunategui-Correa V, Foster CS, Vitale A, DiMeo S, Colvin RB. Expression of fibronectin isoforms in rat cornea after an epithelial-scrape wound. Curr Eye Res. 1994;13:325–330. doi: 10.3109/02713689409167295. [DOI] [PubMed] [Google Scholar]
- Fujikawa LS, Foster CS, Gipson IK, Colvin RB. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Biol. 1984;98:128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest. 1994;70:916–924. [PubMed] [Google Scholar]
- Gipson IK, Inatomi T. Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol. 1995;6:3–10. doi: 10.1097/00055735-199508000-00002. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Sugrue SP. Cell biology of the corneal epithelium. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Philadelphia: W.B. Saunders; 1994. pp. 3–16. [Google Scholar]
- Giugni TD, Brastan DL, Haigler HT. Electric field-induced redistribution and postfield relaxation of epidermal growth factor receptors on A431 cells. J Cell Biol. 1987;104:1291–1297. doi: 10.1083/jcb.104.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruler H, Nuccitelli R. Neural crest cell galvanotaxis: new data and a novel approach to the analysis of both galvanotaxis and chemotaxis. Cell Motil Cytoskeleton. 1991;19:121–133. doi: 10.1002/cm.970190207. [DOI] [PubMed] [Google Scholar]
- Grushkin-Lerner LS, Kewalramani R, Trinkaus-Randall V. Expression of integrin receptors on plasma membranes of primary corneal epithelial cells is matrix specific. Exp Eye Res. 1997;64:323–334. doi: 10.1006/exer.1996.0207. [DOI] [PubMed] [Google Scholar]
- Gundorova RA, Brikman IV, Ibadova SI, Issaeva RT. Stimulation of penetrating corneal wound healing by exogeneous fibronectin. Eur J Ophthalmol. 1994;4:202–209. doi: 10.1177/112067219400400403. [DOI] [PubMed] [Google Scholar]
- Holley RW, Armour R, Baldwin JH, Brown KD, Yeb YC. Density-dependent regulation of growth of BSC-1 cells in cell culture: control of growth by serum factors. Proc Natl Acad Sci USA. 1977;74:5046–5050. doi: 10.1073/pnas.74.11.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. The recovery of local transepithelial resistance following single-cell lesions. Exp Cell Res. 1982;138:331–342. doi: 10.1016/0014-4827(82)90182-3. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34–44. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Khoory W, Wu E, Svoboda KK. Intracellular relationship between actin and α-actinin in a whole corneal epithelial tissue. J Cell Sci. 1993;106:703–717. doi: 10.1242/jcs.106.3.703. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyce SD. Electrical profiles in the corneal epithelium. J Physiol. 1972;226:407–429. doi: 10.1113/jphysiol.1972.sp009991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk WS. A pattern of epidermal cell migration during wound healing. J Cell Biol. 1971;49:247–263. doi: 10.1083/jcb.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus MA, Quaranta V, Jones RCR. Surface relocation of α 6 β 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991;115:1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus MA, Stock EL, Jones JC. Analysis of wound healing in an in vitro model: early appearance of laminin and a 125 × 10(3) Mr polypeptide during adhesion complex formation. J Cell Sci. 1990;96:651–660. doi: 10.1242/jcs.96.4.651. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Perkins DG, Cogan DG. Sliding of the epithelium in experimental corneal wounds. Invest Ophthalmol. 1976;15:4–14. [PubMed] [Google Scholar]
- Lin H, Goodman R, Shirley-Henderson A. Specific region of the c-myc promoter is responsive to electric and magnetic fields. J Cell Biochem. 1994;54:281–288. doi: 10.1002/jcb.240540304. [DOI] [PubMed] [Google Scholar]
- Luther PW, Peng HB, Lin JJ. Changes in cell shape and actin distribution induced by constant electric fields. Nature. 1983;303:61–64. doi: 10.1038/303061a0. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Dover CD. Factors influencing perpendicular elongation of embryonic frog muscle cells in a small applied electric field. J Cell Sci. 1991;98:497–506. doi: 10.1242/jcs.98.4.497. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Zhao M. Physiological electrical fields modify cell behavior. Bioessays. 1997;19:819–826. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, O’Brien P, Hudson LG. Overexpression of the epidermal growth factor receptor contributes to enhanced ligand-mediated motility in keratinocyte cell lines. Endocrinology. 1997;138:121–127. doi: 10.1210/endo.138.1.4844. [DOI] [PubMed] [Google Scholar]
- McIntosh LC, Muckersie L, Forrester JV. Retinal capillary endothelial cells prefer different substrates for growth and migration. Tissue & Cell. 1988;20:193–209. doi: 10.1016/0040-8166(88)90041-9. [DOI] [PubMed] [Google Scholar]
- McCloskey MA, Liu ZY, Poo MM. Lateral electromigration and diffusion of Fc ε receptors on rat basophilic leukemia cells: effects of IgE binding. J Cell Biol. 1984;99:778–787. doi: 10.1083/jcb.99.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado BA, Furcht LT. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Invest Ophthalmol Vis Sci. 1995;36:2120–2126. [PubMed] [Google Scholar]
- Messerli, M., and Robinson, K.R. (1997). Endogenous electrical fields affect the distribution of extracellular protein in Xenopus embryos. Mol. Biol. Cell 8(suppl), 1296 (abstract).
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian DL, McCarthy JB, Skubitz AP, Cameron JD, Furcht LT. Characterization of FN-C/H-V, a novel synthetic peptide from fibronectin that promotes rabbit corneal epithelial cell adhesion, spreading, and motility. Invest Ophthalmol Vis Sci. 1993;34:153–164. [PubMed] [Google Scholar]
- Murakami J, Nishida T, Otori T. Coordinated appearance of β 1 integrins and fibronectin during corneal wound healing. J Lab Clin Med. 1992;120:86–93. [PubMed] [Google Scholar]
- Murata T, Ishibashi T, Hajime I. Localizations of epidermal growth factor and proliferating cell nuclear antigen during corneal wound healing. Graefe’s Arch Clin Exp Ophthalmol. 1993;231:104–108. doi: 10.1007/BF00920222. [DOI] [PubMed] [Google Scholar]
- Newton C, Hatchell DL, Klintworth GK, Brown CF. Topical fibronectin and corneal epithelial wound healing in the rabbit. Arch Ophthalmol. 1988;106:1277–1279. doi: 10.1001/archopht.1988.01060140437048. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakagawa S, Awata T, Ohashi Y, Watanabe K, Manabe R. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J Cell Biol. 1983;97:1653–1657. doi: 10.1083/jcb.97.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Nakagawa S, Nishibayashi C, Ranaka H, Manabe R. Fibronectin enhancement of corneal epithelial wound healing of rabbits in vivo. Arch Ophthalmol. 1984;102:455–456. doi: 10.1001/archopht.1984.01040030369040. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakamura M, Mishima H, Otori T. Differential modes of action of fibronectin and epidermal growth factor on rabbit corneal epithelial migration. J Cell Physiol. 1990;145:549–554. doi: 10.1002/jcp.1041450323. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Physiologic electric fields can influence cell motility, growth, and polarity. Adv Cell Biol. 1988;2:213–233. [Google Scholar]
- Ohji M, Mandarino L, SundarRaj N, Thoft RA. Corneal epithelial cell attachment with endogenous laminin and fibronectin. Invest Ophthalmol Vis Sci. 1993;34:2487–2492. [PubMed] [Google Scholar]
- Onuma EK, Hui SW. Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol. 1988;106:2067–2075. doi: 10.1083/jcb.106.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Yamashiro DJ, Maxfield FR, Decker SJ, Vilcek J. Tumor necrosis factor increases the number of epidermal growth factor receptors on human fibroblasts. J Biol Chem. 1987;262:1950–1954. [PubMed] [Google Scholar]
- Pazmany T, Murphy SP, Golinick SO, Brooks SP, Tomasi TB. Activation of multiple transcription factors and fos and jun gene family expression in cells exposed to a single electric pulse. Exp Cell Res. 1995;221:103–110. doi: 10.1006/excr.1995.1357. [DOI] [PubMed] [Google Scholar]
- Poo M, Lam JW, Orida N, Chao AW. Electrophoresis and diffusion in the plane of the cell membrane. Biophys J. 1979;26:1–21. doi: 10.1016/S0006-3495(79)85231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M, Robison KR. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977;265:602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Rijken PJ, Post SM, Hage WJ, van Bergen en Henegouwen PMP, Verkleij AJ, Boonstra J. Actin polymerization localizes to the activated epidermal growth factor receptor in the plasma membrane, independent of the cytosolic free calcium transient. Exp Cell Res. 1995;218:223–232. doi: 10.1006/excr.1995.1150. [DOI] [PubMed] [Google Scholar]
- Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101:2023–2027. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Electric embryos: the embryonic epithelium as a generator of developmental information. In: McCaig CD, editor. Nerve Growth and Nerve Guidance. London: Portland Press; 1996. pp. 131–150. [Google Scholar]
- Ryseck RP, MacDonald-Bravo H, Zerial M, Bravo R. Coordinate induction of fibronectin, fibronectin receptor, tropomyosin, and actin genes in serum-stimulated fibroblasts. Exp Cell Res. 1989;180:537–545. doi: 10.1016/0014-4827(89)90080-3. [DOI] [PubMed] [Google Scholar]
- Sauer H, Hescheler J, Reis D, Diedershagen H, Niedermeier W, Wartenberg M. DC electrical field-induced c-fos expression and growth stimulation in multicellular prostate cancer spheroids. Br J Cancer. 1997;75:1481–1488. doi: 10.1038/bjc.1997.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Sheardown H, Cheng YI. Tear EGF concentration following corneal epithelial wound creation. J Ocul Pharmacol Ther. 1996;12:239–243. doi: 10.1089/jop.1996.12.239. [DOI] [PubMed] [Google Scholar]
- Sheridan DM, Isseroff RR, Nuccitelli R. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J Invest Dermatol. 1996;106:642–646. doi: 10.1111/1523-1747.ep12345456. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- Short SM, Talbott GA, Juliano RL. Integrin-mediated signaling events in human endothelial cells. Mol Biol Cell. 1998;9:1969–1980. doi: 10.1091/mbc.9.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, de Melker AA, Martine Z, de Velasco AM, Janssen H, Calafat J, Jiessen CM. Formation of hemidesmosomes in cells of a transformed murine mammary tumor cell line and mechanisms involved in adherence of these cells to laminin and kalinin. J Cell Sci. 1993;106:1083–1102. doi: 10.1242/jcs.106.4.1083. [DOI] [PubMed] [Google Scholar]
- Sorkin A. Receptor-mediated endocytosis of growth factor. In: Heldin C-H, Purton M, editors. Signal Transduction. London: Chapman & Hall; 1996. pp. 109–123. [Google Scholar]
- Sugrue SP, Hay ED. Interaction of embryonic corneal epithelium with exogenous collagen, laminin, and fibronectin: role of endogenous protein synthesis. Dev Biol. 1982;92:97–106. doi: 10.1016/0012-1606(82)90154-3. [DOI] [PubMed] [Google Scholar]
- Svoboda KK. Embryonic corneal epithelial actin alters distribution in response to laminin. Invest Ophthalmol Vis Sci. 1992;33:324–333. [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissel MJ, Derynck R. Extracellular matrix regulates expression of the TGF-β 1 gene. J Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Liou GI, Wu X, Abney TO, Reinach PS. ETB and epidermal growth factor receptor stimulation of wound closure in bovine corneal epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:2614–2622. [PubMed] [Google Scholar]
- Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, Wilson SE. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp Eye Res. 1997;64:501–504. doi: 10.1006/exer.1996.0226. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Assoian R, Rosner MR. Transforming growth factor-β increases transcription of the genes encoding the epidermal growth factor receptor and fibronectin in normal rat kidney fibroblasts. J Biol Chem. 1988;263:19519–19524. [PubMed] [Google Scholar]
- Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T. Release of TGF-β 1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res. 1997a;16:19–25. doi: 10.1076/ceyr.16.1.19.5119. [DOI] [PubMed] [Google Scholar]
- Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: a potential modulator of corneal wound healing following photorefractive keratectomy. Curr Eye Res. 1997b;16:825–831. doi: 10.1076/ceyr.16.8.825.8984. [DOI] [PubMed] [Google Scholar]
- Virtanen T, Ylatupa S, Mertaniemi P, Partanen P, Tuunanen T, Tervo T. Tear fluid cellular fibronectin levels after photorefractive keratectomy. J Refract Surg. 1995;11:106–112. doi: 10.3928/1081-597X-19950301-10. [DOI] [PubMed] [Google Scholar]
- Vitale AT, Pedroza-Seres M, Arrunategui-Correa V, Lee SL, DiMeo S, Foster CS, Colvin RB. Differential expression of alternatively spliced fibronectin in normal and wounded rat corneal stroma versus epithelium. Invest Ophthalmol Vis Sci. 1994;35:3664–3672. [PubMed] [Google Scholar]
- Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci. 1998;111:2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J Clin Invest. 1992;90:2392–2401. doi: 10.1172/JCI116130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin JM. Regeneration of resistance and ion transport in rabbit corneal epithelium after induced surface cell exfoliation. J Membr Biol. 1988;104:45–55. doi: 10.1007/BF01871901. [DOI] [PubMed] [Google Scholar]
- Wolpert SI, Wong MD, Wang JY, Bass BL. Epithelial-matrix interactions: laminin downregulates enterocyte EGF receptor and IGF-I receptor expression. J Surg Res. 1996;63:345–348. doi: 10.1006/jsre.1996.0273. [DOI] [PubMed] [Google Scholar]
- Wu XY, Trinkaus-Randall V. The expression of integrin subunits α 6 and β 4 by corneal epithelial cells on modified hydrogel surfaces. J Biomed Mater Res. 1997;37:166–175. doi: 10.1002/(sici)1097-4636(199711)37:2<166::aid-jbm4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, Kwiatkowski DJ, Lauffenburger DA, Murphy-Ullrich J, Wells A. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility associated PLC(gamma) signaling pathway. J Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]
- Zhao M, Aguis-Fernandez A, Forrester JV, McCaig CD. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996a;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- Zhao M, Aguis-Fernandez A, Forrester JV, McCaig CD. Directed migration of corneal epithelial sheets in physiological electric fields. Invest Ophthalmol Vis Sci. 1996b;37:2548–2558. [PubMed] [Google Scholar]
- Zhao M, McCaig CD, Aguis-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]