Abstract
It has been proposed previously that latrunculin A, an inhibitor of actin polymerization, delays the onset of anaphase by causing spindle misorientation in fission yeast. However, we show that Δmto1 cells, which are defective in nucleation of cytoplasmic microtubules, have profoundly misoriented spindles but are not delayed in the timing of sister chromatid separation, providing compelling evidence that fission yeast does not possess a spindle orientation checkpoint. Instead, we show that latrunculin A delays anaphase onset by disrupting interpolar microtubule stability. This effect is abolished in a latrunculin A-insensitive actin mutant and exacerbated in cells lacking Ase1, which cross-links antiparallel interpolar microtubules at the spindle midzone both before and after anaphase. These data indicate that both Ase1 and an intact actin cytoskeleton are required for preanaphase spindle stability. Finally, we show that loss of Ase1 activates a checkpoint that requires only the Mad3, Bub1, and Mph1, but not Mad1, Mad2, or Bub3 checkpoint proteins.
INTRODUCTION
In eukaryotes, the interplay between the actin and microtubule (MT) cytoskeletons is essential for a variety of cellular processes, such as growth, polarity, vesicular transport, and cell division. The unicellular yeast Schizosaccharomyces pombe has proven useful in elucidating some of the underlying principles and mechanisms by which these cytoskeletons interact. During interphase, cytoplasmic microtubules are organized in three to four antiparallel bundles arranged along the longitudinal axis of the cell, with their plus ends facing both cell tips and their minus ends near the middle of the cell (Hagan, 1998). The MT bundles are organized from medial microtubule-organizing centers (MTOCs) that function as nuclear attachment sites (Tran et al., 2001). Interphase microtubule bundles maintain cell polarity by depositing factors required for nucleation of actin filaments at cell tips and exert transient forces produced by plus end microtubule polymerization to ensure the nucleus is positioned in the center of the cell (Sawin and Nurse, 1998; Tran et al., 2001). As cells enter mitosis, actin is redistributed from the cell tips to the medial cell cortex to form the cytokinetic actomyosin ring (Marks et al., 1986). Coincidentally, interphase microtubules disappear and are replaced by a mitotic spindle that is composed of 12–16 interpolar microtubules, overlapping in a central zone, that emanates from two spindle pole bodies (SPBs) embedded in opposite sides of a persistent nuclear envelope. An additional 10–12 microtubules originate from each SPB and terminate at the three kinetochores (Ding et al., 1993). Visualization of live S. pombe cells expressing green fluorescent protein (GFP)-tubulin has revealed that mitosis consists of three phases (Nabeshima et al., 1998; Tatebe et al., 2001). Phase 1 is prophase, during which a short, ∼2.0 μm, spindle is formed. In phase 2, the spindle maintains this length and centromeres make frequent, rapid movements between the poles. At the end of phase 2, kinetochores congress to the central spindle region; and shortly afterward, sister chromatids separate (anaphase A) and move back to the SPBs (Funabiki et al., 1993; Tournier et al., 2004). Phase 3 consists entirely of anaphase B, during which the spindle elongates along the longitudinal axis of the cell (∼14 μm). The cytoplasmic face of the two SPBs is associated with astral microtubules, which can exist in two configurations termed convergent and parallel (Hagan and Hyams, 1996). As the spindle elongates, astral microtubules maintain a fixed angle to the spindle axis and are thought to aid spindle alignment by pushing the SPBs away from the cell cortex (Hagan and Hyams, 1996). Finally, the cytokinetic actomyosin ring contracts perpendicularly to the mitotic spindle to ensure that each set of sister chromatids is separated to daughter cells.
At the onset of mitosis, kinetochores are not attached to spindle microtubules. The kinetochore of one sister chromatid then captures microtubule(s) nucleated from one spindle pole. Once its sister kinetochore has captured microtubule(s) from the other pole, the chromosome becomes bioriented. During metaphase, bioriented chromosomes move to the equatorial plane, known in animal cells as the metaphase plate. The fidelity of chromosome biorientation is ensured by a checkpoint that controls the onset of anaphase (Zhou et al., 2002; Cleveland et al., 2003). Components of this checkpoint, which is often referred to as the spindle assembly checkpoint (SAC), were first identified in budding yeast and include the Mad1, Mad2, Mad3, Bub1, Bub3, and Mps1 proteins (Hoyt et al., 1991; Li and Murray, 1991; Weiss and Winey, 1996). Structural and functional homologues of these proteins have been identified in all other eukaryotes so far examined, including fission yeast (He et al., 1997, 1998; Bernard et al., 1998; Millband and Hardwick, 2002). In response to microtubule-disrupting agents, these proteins inhibit the anaphase promoting complex (APC), an E3 ubiquitin ligase that is responsible for the destruction not only of Cyclin B but Securin, an inhibitor of Separase (Zhou et al., 2002). Separase cleaves Scc1/Rad21, a component of the Cohesin complex, to allow sister chromatids to be separated (Uhlmann, 2004). Microtubule-disrupting agents thus block anaphase onset by inhibiting the APC/C, which prevents activation of Separase. The molecular nature of the defect that is sensed at kinetochores, however, remains controversial. Two models have been put forward. In the first model (attachment model), anaphase is initiated when all potential attachment sites on kinetochores are occupied by spindle microtubules (Rieder et al., 1995). In the second model (tension model), the checkpoint is only satisfied when tension is applied across sister kinetochore pairs (Li and Nicklas, 1995; Stern and Murray, 2001). It has been difficult to distinguish between these models because the application of tension aids microtubule attachment and attachment is necessary for the application of tension.
Some years ago, we and others found that addition of latrunculin A, an inhibitor of actin polymerization, delays the onset of anaphase in fission yeast by a mechanism that requires only the Bub1, Mph1, and Mad3 but not Mad1 or Mad2 spindle checkpoint proteins (Gachet et al., 2001; Tournier et al., 2004). It was originally thought that latrunculin A delays the onset of anaphase in fission yeast by causing spindle misorientation by preventing the interaction of astral microtubules with the medial cortical actin cytoskeleton (Gachet et al., 2001, 2004; Oliferenko and Balasubramanian, 2002; Rajagopalan et al., 2004; Tournier et al., 2004). This effect was therefore termed a spindle orientation checkpoint (SOC). More recent studies have shown that cytoplasmic astral microtubules are only nucleated during anaphase B (Zimmerman et al., 2004) and that mitotic spindle orientation is primarily determined by interphase microtubules (Vogel et al., 2007). By tracing spindle microtubule behavior in either wild type cells or cells lacking either the Mal3 (EB-1) or Alp7/Mia1 proteins, Vogel et al. (2007) could find no correlation between spindle angle and spindle length, so the existence of a spindle orientation checkpoint in fission yeast has been questioned. However, the formal relationship between spindle orientation and the timing of anaphase onset could not be formally established in this study because anaphase onset is already delayed in Δmal3 and Δalp7/Δmia1 mutants due to defects in mitotic spindle assembly (Sato et al., 2003; Asakawa et al., 2005). Moreover, mitotic progression was monitored using only a gfp-atb2 (α-tubulin) construct so the precise timing of sister chromatid separation could not be assessed. In this article, we have reanalyzed the influence of spindle angle and latrunculin A on mitotic progression in fission yeast by single-cell analysis of spindle pole and kinetochore behavior in a mutant in which only cytoplasmic, but not spindle, microtubule nucleation is defective.
MATERIALS AND METHODS
Cell Culture
Media, growth, and maintenance of strains were as described previously (Tournier et al., 2004). All experiments were performed at 30°C unless otherwise stated. Latrunculin A was purchased from Invitrogen (Carlsbad, CA) and dissolved in dimethyl sulfoxide at a stock concentration of 1 mg/ml. Cell synchrony was achieved by lactose gradient size selection. Cells were resuspended in fresh medium at 106 cells/ml and released at 30°C unless otherwise stated. The peak synchrony of septation was >40% in each experiment in the absence of drug.
Experimental Procedures
Deletion of the entire open reading frame (ORF) of ase1 was performed by one-step polymerase chain reaction (PCR)-based gene targeting, as described previously (Bahler et al., 1998). To construct an act1-R183A,D184A allele, a 2.62-kb genomic fragment containing act1+ ORF and 760 bp of upstream promoter region was amplified using oligonucleotides (oligos) 1370 and 1371 and cloned into the Sac1 and Sal1 sites of pJK148 to form pJK148-act1. The act1-R183A,D184A mutation was constructed by amplification of act1 from genomic DNA by using a two-step mutagenic PCR process. In the first step, oligos 1346 and 1347 and oligos 1348 and 1349 were used to amplify the 5′ and 3′ arms of the product. The mutagenic arms were then used to prime each other in a secondary PCR that was further amplified with the 1349 and 1346 oligos to generate a 2070-base pair fragment. This was cut with SacII and BamHI, and the product was used to replace the equivalent SacII-BamHI cassette in pJK148-act1+ to form pJK148-act1-R183A,D184A. Both plasmids were verified by sequencing and then linearized with HindIII and transformed into strain JM3315. Transformants were selected on medium lacking leucine at 33.5°C and integration at the leu1 locus verified by PCR. An act1::hygR cassette was generated by PCR amplification of pFA6a-hphMX6 by using the oligos 1374 and 1375 and used to replace the act1-188 allele and confirmed by PCR using oligos 1365 and 1342. The genotypes of the strains used in this study are detailed in Supplemental Table 1. A list of oligonucleotides used is provided in Supplemental Table 2.
Fixation and Live Cell Microscopy
Cells were fixed in 3.7% formaldehyde for 10 min at room temperature. Live analysis of cells was performed in an imaging chamber (CoverWell PCI-2.5; Grace Bio-Labs, Bend, OR) filled with 1 ml of 1% agarose in minimal medium with or without latrunculin A and sealed with a 22- × 22-mm glass coverslip. Fluorescence microscopy was performed on a Deltavision Spectris/RT system containing a CH350L liquid cooled charge-coupled device camera (Photometrics, Tucson, AZ) and an IX70 inverted microscope (Olympus, Tokyo, Japan) with a 100× 1.35 numerical aperture objective equipped with Deltavision data collection system (Applied Precision, Issaquah, WA). Stacks of six Z-sections (0.6 μm apart) were taken at each time point, with exposure times of 1 s for both GFP and cyan fluorescent protein (CFP). Projected images were made for each time point followed by intensity adjustments and conversion to 24-bit TIFF images. The position of the spindle poles and kinetochores were determined using softWoRx software (Applied Precision) and downloaded to Excel (Microsoft, Redmond, WA) for analysis. Actin staining was performed with rhodamine-phalloidin exactly as described previously (Tournier et al., 2004).
RESULTS
Latrunculin A Does Not Delay Anaphase Onset by Disrupting Spindle Orientation
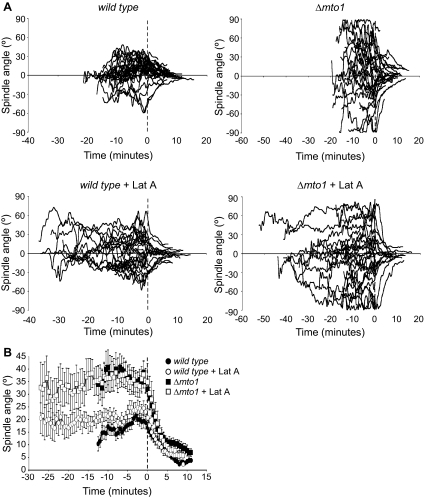
To analyze the relationship between mitotic spindle angle and the timing of anaphase onset in fission yeast, we monitored spindle pole and kinetochore dynamics in wild-type cells and cells lacking Mto1. Mto1 is a centrosomin-like protein that associates with the γ-tubulin complex and is specifically required for microtubule nucleation at interphase MTOCs that surround the nuclear envelope and the outer, but not inner, face of the spindle pole body (Sawin et al., 2004; Venkatram et al., 2004; Zimmerman and Chang, 2005). As a consequence, Δmto1 cells mostly lack cytoplasmic interphase microtubules and completely astral microtubules, but they retain a mitotic spindle. To simultaneously monitor spindle length, spindle angle, and the length of time from mitotic entry to the end of anaphase A, spindle pole body and kinetochore position was monitored in individual movies of ndc80-gfp cdc11-cfp cells. Ndc80 and Cdc11 proteins bind constitutively to the kinetochore and spindle pole body, respectively (Krapp et al., 2001; Wigge and Kilmartin, 2001). The length of the mitotic spindle and its angle from the longitudinal axis of the cell were calculated at each time point. In each movie, the completion of anaphase A was taken as time zero. We find that the average time from entry into mitosis (spindle pole separation) to the end of anaphase A (when individual kinetochores have moved back to spindle poles) was not statistically different in wild-type cells (14.1 ± 1.1 min) to that observed in the absence of Mto1 (14.7 ± 0.7 min) (Figure 1and Table 1). Despite this spindles were markedly more misoriented at mitotic onset in Δmto1 cells than in control cells and, despite oscillations both toward and away from the longitudinal axis of the cell during prometaphase and metaphase, remained markedly more misoriented at anaphase onset in Δmto1 cells (33.6 ± 4.3°) than in the control cells (16.6 ± 2.6°) (Figure 2, A and B, and Table 1). These data provide conclusive evidence that spindle misorientation per se does not influence the timing of anaphase onset in fission yeast.
Figure 1.
Latrunculin A delays anaphase onset independently of spindle orientation. Log phase ndc80-gfp cdc11-cfp (left) or Δmto1 ndc80-gfp cdc11-cfp (right) cells were grown at 30°C either in the absence (top) or presence (bottom) of 1.25 μM latrunculin A for 30 min. Individual cells were then imaged by fluorescence microscopy either in the continued presence or absence of latrunculin A (+Lat A). Spindle length was calculated at 30-s intervals. The completion of anaphase A was taken as T = 0 for each movie. Spindle collapses are those traces in which spindle length reduces to zero.
Table 1.
Effect of latrunculin A on the time spent in prometaphase and metaphase, spindle angle, frequency of preanaphase spindle collapse, and rate of spindle elongation in various strains
| Strain | No. of movies | Phase 1 (min ± SEM) | Phase 2 (min ± SEM) | Phase 1 + phase 2 (min ± SEM) | Angle at start of phase 1 (°±SEM) | Angle at start of phase 2 (°±SEM) | Angle at end of phase 2 (°± SEM) | Rate of spindle elongation (μm/min) | No. of movies with preanaphase spindle collapse (%) |
|---|---|---|---|---|---|---|---|---|---|
| No addition | |||||||||
| Wild type | 29 | 3.1 ± 0.2 | 11.0 ± 0.7 | 14.1 ± 1.1 | 9.7 ± 1.8 | 15.2 ± 2.7 | 16.6 ± 2.6 | 0.82 ± 0.03 | 0/29 (0) |
| Δmto1 | 20 | 2.9 ± 0.3 | 11.8 ± 0.7 | 14.7 ± 0.7 | 40.0 ± 5.9 | 36.9 ± 6.6 | 33.6 ± 4.3 | 0.84 ± 0.05 | 0/20 (0) |
| Δmal3 | 18 | 2.8 ± 0.2 | 18.6 ± 1.9 | 21.3 ± 2.0 | 12.2 ± 2.4 | 16.1 ± 3.3 | 16.0 ± 2.3 | 0.96 ± 0.05 | 0/18 (0) |
| Δase1 | 20 | 2.5 ± 0.2 | 21.9 ± 1.6 | 24.4 ± 1.6 | N.D. | N.D. | N.D. | 0.70 ± 0.03 | 7/20 (35) |
| Δact1 act1+ | 23 | 2.8 ± 0.1 | 12.5 ± 0.8 | 15.3 ± 0.9 | N.D. | N.D. | N.D. | N.D. | 0/23 (0) |
| Δact1 act1(RADA) | 23 | 2.4 ± 0.1 | 12.3 ± 0.8 | 14.6 ± 0.8 | N.D. | N.D. | N.D. | N.D. | 0/23 (0) |
| In the presence of 1.25 μM latrunculin A | |||||||||
| wild type | 28 | 2.5 ± 0.2 | 19.1 ± 1.9 | 21.6 ± 2.0 | 16.1 ± 2.9 | 19.7 ± 2.7 | 22.0 ± 2.0 | 0.79 ± 0.03 | 11/28 (39) |
| Δmto1 | 28 | 2.5 ± 0.1 | 21.0 ± 2.2 | 23.5 ± 2.2 | 27.9 ± 4.6 | 33.4 ± 4.5 | 33.9 ± 5.0 | 0.76 ± 0.05 | 13/28 (46) |
| Δase1 | 20 | 2.4 ± 0.1 | 23.0 ± 2.3 | 25.4 ± 2.2 | N.D. | N.D. | N.D. | 0.61 ± 0.03 | 9/20 (45) |
| In the presence of 2.5 μM latrunculin A | |||||||||
| Δact1 act1+ | 27 | 2.5 ± 0.1 | 21.2 ± 1.9 | 23.7 ± 2.0 | N.D. | N.D. | N.D. | N.D. | 8/27 (30) |
| Δact1 act1(RADA) | 25 | 2.5 ± 0.1 | 13.0 ± 1.0 | 15.5 ± 1.0 | N.D. | N.D. | N.D. | N.D. | 0/25 (0) |
N.D., not determined.
Figure 2.
Effect of latrunculin A on spindle angle. (A) Log phase ndc80-gfp cdc11-cfp cells (left) or Δmto1 ndc80-gfp cdc11-cfp cells (right) were grown at 30°C in the absence (top) or presence (bottom) of 1.25 μM latrunculin A for 30 min. Individual cells were then imaged by fluorescence microscopy and spindle angle relative to the longitudinal axis of the cell (0°) was determined at 30-s intervals. The completion of anaphase A was taken as T = 0 for each movie. Plus and minus values for spindle angle were assigned randomly so that oscillations through 0° could be plotted. (B) The average spindle angle from the longitudinal axis of the cell (0°) was calculated at 30-s intervals for each the movies in A and the average spindle angle plotted as a function of time for ndc80-gfp cdc11-cfp cells (circles) or Δmto1 ndc80-gfp cdc11-cfp cells (squares) either in the absence (closed symbols) or presence (open symbols) of 1.25 μM latrunculin A. The completion of anaphase A was taken as T = 0. Error bars denote SE from the mean.
The lack of relationship between spindle angle and the timing of anaphase onset prompted us to re-examine the effect of latrunculin A on kinetochore and spindle pole body behavior. We found that addition of 1.25 μM latrunculin A to ndc80-gfp cdc11-cfp cells did not effect the rate of spindle formation (phase 1) or the rate of spindle elongation (phase 3), but it caused a pronounced delay in prometaphase and metaphase (phase 2), although this was variable from cell to cell (Figure 1 and Table 1). The length of the delay was not due to the period of incubation because different cell behaviors were observed in a single field of cells. Addition of latrunculin A also caused an increase in the initial angle of the spindle relative to the longitudinal axis of the cell, possibly by inhibiting productive interaction of interphase microtubules with actin at cell tips (Figure 2, A and B, and Table 1). Importantly, addition of latrunculin A also delayed anaphase onset in Δmto1 cells to a similar extent to that observed in wild-type cells (Figure 1), but it did not increase the average angle of the spindle (Figure 2, A and B, and Table 1). These results indicate that latrunculin A does not delay the onset of anaphase by activating a spindle orientation checkpoint, as suggested previously.
Actin Is Required for Preanaphase Spindle Stability in Fission Yeast
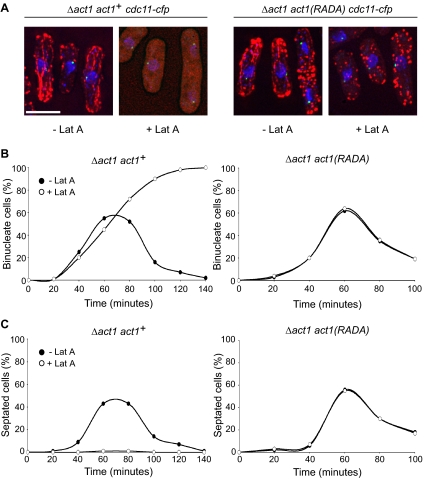
Strikingly, we noted that addition of latrunculin A caused preanaphase mitotic spindles to collapse in a proportion of movies of both wild-type cells and cells lacking Mto1 (Figure 1 and Table 1). This phenotype was not observed in the absence of drug or in cells lacking Mal3 or Dam1, a component of the DASH complex, despite an extended delay in prometaphase and metaphase (Table 1; data not shown). To determine whether anaphase delay and mitotic spindle collapse induced by latrunculin A are due to inhibition of actin polymerization, we constructed an allele of actin that contained two mutations (R183A and D184A) at the leu1 locus. Analogous mutations have been shown previously to confer resistance to latrunculin A in both budding yeast and human cells (Ayscough et al., 1997; Fujita et al., 2003). The endogenous actin gene was deleted so that the act1-R183A,D184A:leu1 allele was the only one expressed. Addition of 20 μM latrunculin A completely disrupted all actin structures in control Δact1 act1+:leu1 cells, as judged by phalloidin staining, but not in Δact1 act1-R183A,D184A:leu1 cells, although actin cables were more difficult to observe (Figure 3A). Addition of 2.5 μM latrunculin A disrupted actin cable formation in Δact1 act1+:leu1 cells, although actin patches were still evident (data not shown), whereas actin staining was unaffected in the Δact1 act1-R183A,D184A:leu1 mutant. Importantly, addition of 1.25 μM latrunculin A to synchronized Δact1 act1+:leu1 cells delayed the appearance of binucleate cells and blocked septum formation but had no effect in Δact1 act1-R183A,D184A:leu1 cells (Figure 3, B and C). These data indicate that the act1-R183A,D184A allele confers latrunculin A resistance in fission yeast. Importantly, whereas addition of 2.5 μM latrunculin A to Δact1 act1+:leu1 ndc80-gfp cdc11-cfp cells caused a delay in anaphase onset and mitotic spindle collapse, the same concentration of latrunculin A had no effect on the timing of prometaphase and metaphase or the stability of the preanaphase spindle in Δact1 act1-R183A,D184A:leu1 ndc80-gfp cdc11-cfp cells (Figure 4and Table 1). These data formally demonstrate that an intact actin cytoskeleton is required for the stability of the preanaphase mitotic spindle (phase 2) but not for spindle assembly (phase 1) or rate of spindle elongation or spindle stability during anaphase B (Figure 4 and Table 1). From this, we argue that latrunculin A delays anaphase onset by influencing mitotic spindle stability rather than by causing spindle misorientation.
Figure 3.
act1(RADA) cells are insensitive to latrunculin A. (A) Log phase act1+:leu1 Δact1 ndc80-gfp cdc11-cfp cells (Δact1 act1+cdc11-cfp) or act1(R183A,D184A):leu1 Δact1 ndc80-gfp cdc11-cfp cells [Δact1 act1(RADA) cdc11-cfp] were incubated in the absence or presence of 20 μM latrunculin A for 30 min at 30°C, and actin structures were identified by phalloidin staining. Actin is shown in red, spindle poles are shown in green, and chromatin is shown in blue. Bar, 5 μm. (B and C) Log phase act1::hygR act1+:leu1 ndc80-GFP:kanR cdc11-CFP:kanR (Δact1 act1+) cells and act1::hygR act1(R183A, D184A):leu1 ndc80-GFP:kanR cdc11-CFP:kanR [Δact1 act1(RADA)] cells grown in YES at 30°C were synchronized by lactose gradient centrifugation. Early G2 cells were then incubated in fresh medium either in the presence (open symbols) or absence (closed symbols) of 1.25 μM latrunculin A. At each time point, cells were fixed and stained with 4,6-diamidino-2-phenylindole (DAPI) and calcofluor to determine the percentage of binucleate cells (B) or septated cells (C) within the culture (n = 500).
Figure 4.
Actin is required for preanaphase spindle stability. Log phase act1+:leu1 Δact1 ndc80-gfp cdc11-cfp cells (left) or act1(R183A,D184A):leu1 Δact1 ndc80-gfp cdc11-cfp cells (right) were incubated on EMM agar pads at 30°C either in the absence (top) or presence (bottom) of 2.5 μM latrunculin A for 30 min. Individual cells were then imaged by fluorescence microscopy either in the continued presence (+Lat) or absence of drug. Spindle length was calculated at 30-s intervals. The completion of anaphase A was taken as T = 0 for each movie.
Ase1 Is Required for Preanaphase Spindle Stability and the Timing of Anaphase Onset
The construction and maintenance of a bipolar spindle requires the concerted action of kinesins and microtubule-associated proteins. Members of the Ase1/PRC1/MAP65 family bundle and stabilize antiparallel microtubules at the spindle midzone during anaphase B (Jiang et al., 1998; Chan et al., 1999; Schuyler et al., 2003). In most species, association of Ase1 (PRC1/MAP65) with the spindle midzone is suppressed by Cdk1-dependent phosphorylation during prometaphase and metaphase (Zhu et al., 2006; Khmelinskii et al., 2007). Consistent with this, metaphase spindles form normally in budding yeast cells lacking Ase1 but collapse shortly after the onset of anaphase. However, recent evidence suggests that when the Cin8 kinesin-5 motor protein is not functional, Ase1 can support preanaphase spindle assembly (Kotwaliwale et al., 2007). In fission yeast, Ase1 binds and is required for the stability of the anaphase B spindle midzone. However, Ase1 also binds weakly to the mitotic spindle before anaphase onset, although there is conflicting evidence as to whether loss of Ase1 effects the timing of anaphase onset, and no role for Ase1 in preanaphase spindle stability has been demonstrated (Loiodice et al., 2005; Yamashita et al., 2005). To readdress this issue, we monitored spindle pole and kinetochore behavior in individual Δase1 ndc80-gfp cdc11-cfp cells. We find that cells lacking Ase1 spent, on average, considerably longer from mitotic entry to the completion of anaphase A (24.4 ± 1.6 min) than control cells (14.1 ± 1.1 min) at the same temperature (Figure 5A and Table 1). This is accompanied by abnormal kinetochore behavior. In control cells kinetochores undergo centromere breathing during prometaphase and metaphase due to microtubule-based tension across bioriented sister chromatids. At the same time, sister chromatid pairs oscillate in a line between separated spindle poles and then congress to the spindle midzone just before the onset of anaphase A (Figure 5B) (Tournier et al., 2004). By contrast, in the absence of Ase1, spindles form but undergo periods of spindle shortening during prometaphase and metaphase (Figure 5B) (Loiodice et al., 2005). During this period, sister kinetochores undergo dynamic separation but chromosome congression is never observed and one or more pairs of sister kinetochores is frequently displaced away from the spindle axis (Figure 5B). Importantly, we find that in 35% of cells lacking Ase1 spindles collapse before anaphase onset (Figure 5A and Table 1). In other cells, anaphase A takes place and either anaphase B does not occur (20%) or spindles collapse during anaphase B (45%) (Figure 5A and Table 1). Similar results were obtained in cen1-gfp cdc11-cfp and Δase1 cen1-gfp cdc11-cfp cells in which only centromere 1 is marked with GFP (Supplemental Figure 1A). To determine whether loss of Ase1 influences the duration of anaphase A, cen1-gfp cdc11-cfp and Δase1 cen1-gfp cdc11-cfp cells were filmed during mitosis at more frequent intervals (10 s). This showed that the duration of anaphase A in Δase1 cells (43 ± 13 s) was not noticeably different from that of control cells (45 ± 12 s) (Supplemental Figure 1, B and C). These results demonstrate that Ase1 is both an important regulator of preanaphase spindle stability and kinetochore dynamics and that it is required for timely anaphase onset in fission yeast.
Figure 5.
Actin and Ase1 are independently required for spindle stability. (A) Log phase Δase1 ndc80-gfp cdc11-cfp cells were incubated on EMM agar pads at 30°C either in the absence (top) or presence (bottom) of 1.25 μm latrunculin A for 30 min. Individual cells were then imaged by fluorescence microscopy in the continued absence (top) or presence (bottom) of latrunculin A (+Lat A). Spindle length was calculated at 30-s intervals relative to the completion of anaphase A (T = 0) for each movie. (B) Images from a representative movie of an ndc80-gfp cdc11-cfp cell (wild type) and a Δase1 ndc80-gfp cdc11-cfp cell (Δase1) during mitosis. Spindle poles are shown in green, and kinetochores are shown in red. Enlarged images of the 10-min time point in the wild-type cell and the 14-min time point in the Δase1 cell are shown at the bottom. Arrows indicates a pair of sister kinetochores that are displaced from the spindle axis. Bars, 1 μm. Spindle pole separation was taken as T = 0 for each movie. (C) Log phase cdc25-22 cells (circles) or Δase1 cdc25-22 (squares) were arrested in late G2 by incubation at 35.5°C for 4 h and then released to the permissive temperature of 25°C either in the absence (closed symbols) or presence (open symbols) of 10 μM latrunculin A. Cells were fixed and stained with DAPI at the times indicated, and the percentage of binucleate cells calculated (n = 500).
Latrunculin A Disrupts an Ase1-independent Spindle Stability Pathway
The similarity in the effect of addition of latrunculin A and deletion of Ase1 on spindle stability persuaded us to examine whether addition of latrunculin A disrupts Ase1 localization or function. Addition of latrunculin A has little effect on the overall length of prometaphase and metaphase in Δase1 cells (25.4 ± 2.2 min) compared with the same cells in the absence of drug (24.4 ± 1.6 min), but preanaphase spindles collapsed somewhat more frequently in the presence of latrunculin A (45%) than in the absence of drug (35%) (Figure 5A and Table 1). Particularly, fewer Δase1 cells initiated anaphase B in the presence of latrunculin A (20%) than in its absence (45%). Because actin is required for cell growth, complete disruption of the actin cytoskeleton prevents cells from attaining a critical cell size necessary for the initiation of mitosis (Rupes et al., 2001). To circumvent this problem, we examined the effect of higher concentrations of latrunculin A in cdc25-22 cells that arrest in late G2 at a size that exceeds that required for mitotic entry. These cells synchronously enter mitosis on release to the permissive temperature even when the actin cytoskeleton is completely destroyed (Tournier et al., 2004). In control cells, the appearance of binucleate (i.e., telophase) cells was delayed by the addition of 10 μM latrunculin A, as observed previously, but was almost completely blocked in cells lacking Ase1 (Figure 5C). Consistent with an additive effect, latrunculin A did not prevent binding of Ase1 to the spindle midzone (data not shown). Together, these data suggest that Act1 and Ase1 are independently required for preanaphase spindle stability in fission yeast.
Loss of Ase1 Activates a Mad1 and Mad2-independent Spindle Checkpoint
We demonstrated previously that in fission yeast latrunculin A delays anaphase by a mechanism that requires the Atf1 transcription factor and Mad3, Mph1, and Bub1, but not Mad1 or Mad2, checkpoint proteins (Gachet et al., 2001; Tournier et al., 2004). The similarities in the effects of latrunculin A and loss of Ase1 on preanaphase spindle stability persuaded us to examine how loss of Ase1 influences the onset of anaphase. As cells enter mitosis, Cdc13 (cyclin B) binds spindle poles and the mitotic spindle before being ubiquitinated by APC/C and subsequently degraded at anaphase onset (Tatebe et al., 2001). In the absence of Ase1, the percentage of cells with spindle pole and spindle-associated Cdc13 is markedly greater in log phase Δase1 cells than in control cells (Figure 6A). Consistently, Cdc13 remained associated with spindles and spindle poles for longer in movies of Δase1 cells than in the control (Figure 6B). These data strongly suggest that loss of Ase1 delays activation of the APC/C. To determine whether components of the spindle assembly checkpoint are required for this delay, we measured the percentage of cells in prometaphase and metaphase in log phase populations of Δase1 ndc80-gfp cdc11-cfp cells or the same cells lacking various spindle checkpoint proteins. This revealed that the delay in anaphase onset in Δase1 cells is dependent on Atf1, Bub1, Mad3, and Mph1, but not on the Mad1 or Mad2 checkpoint proteins (Figure 6C). The differential requirement for Mad2 and Bub1 prompted us to examine their cellular location. In control cells, Mad2 is observed throughout the nucleus in G2 and relocates to a region underlying the unseparated spindle pole as cells enter mitosis and remains close to one of the two spindle poles until after anaphase. Foci of Mad2 that are not associated with the spindle pole during prometaphase and metaphase are only rarely observed in control cells (Asakawa et al., 2005). By contrast, Bub1 is broadly nuclear during interphase and rapidly associates with all kinetochores in prometaphase and metaphase and remains there until a few minutes before anaphase onset (Asakawa et al., 2005). We found that the percentage of cells showing non–SPB-associated Mad2 foci in cells lacking Ase1 (2.5 ± 1.0%; n = 500) was not different from control cells (2.7 ± 0.5%; n = 500) (Figure 6D). By contrast, a higher percentage of log phase Δase1 cells displayed Bub1 foci (9.8 ± 1.3%; n = 500) than in control cells (4.8 ± 1.0%; n = 500) (Figure 6D). In agreement with this, we found that nuclear Bub1 foci were observed for longer in movies of cells lacking Ase1 than in the control (Figure 6E). This provides further genetic evidence that two distinct mitotic checkpoints influence the timing of sister chromatid separation in fission yeast, one checkpoint that requires Mad1 and Mad2, and one checkpoint that does not. Furthermore, these data suggest that addition of latrunculin A and loss of Ase1 delay the onset of anaphase by a similar mechanism, namely, by disrupting preanaphase spindle stability.
Figure 6.
Loss of Ase1 activates a Mad2-independent spindle checkpoint. (A) Log phase cdc13-gfp cells (open bar) and Δase1 cdc13-gfp cells (closed bar) were fixed, and the percentage of spindle pole and spindle pole body associated Cdc13-GFP calculated (n = 500 ± SD). (B) Images from a representative movie of a cdc13-gfp cell (top) and Δase1 cdc13-gfp cell (bottom) in mitosis. Spindle pole separation was taken as T = 0. Bar, 1 μm. (C) Log phase cultures of ndc80-gfp cdc11-cfp cells (+) or Δase1 ndc80-gfp cdc11-cfp cells (Δ) in an otherwise wild-type background or lacking the atf1 (Δatf1), bub1 (Δbub1), mad3 (Δmad3), mph1 (Δmph1), bub3 (Δbub3), mad1 (Δmad1), or mad2 (Δmad2) genes were fixed and imaged by fluorescence microscopy, and the percentage of cells in prometaphase and metaphase (PM + M) was calculated (n = 500 ± SD). (D) Log phase cultures of mad2-gfp cdc11-cfp, mad2-gfp cdc11-cfp Δase1, bub1-gfp cdc11-cfp, or bub1-gfp cdc11-cfp Δase1 cells were fixed and imaged by fluorescence microscopy. The percentage of cells with non–SPB-associated Mad2 foci (Mad2-GFP) or kinetochore associated Bub1 foci (Bub1-GFP) was calculated (n = 500 ± SD). (E) Images from a representative movie of bub1-gfp cdc11-cfp and bub1-gfp cdc11-cfp Δase1 cells. Only the GFP channel is shown. Bar, 1 μm.
Bub3 Is Not Required for the Anaphase Delay Imposed by Latrunculin A or Loss of Ase1
During this analysis, we found that deletion of the Bub3 checkpoint protein did not abrogate the delay imposed by loss of Ase1 (Figure 6C). This was a surprise because Bub3 is required for association of Bub1 to kinetochores (Vanoosthuyse et al., 2004). There have been conflicting reports as to whether Bub3 is required for the anaphase delay imposed by latrunculin A (Rajagopalan et al., 2004; Tournier et al., 2004). To readdress this issue, the percentage of cells in prometaphase and metaphase was monitored after synchronous release of cdc25-22, Δmad3 cdc25-22, Δbub3 cdc25-22, or Δmph1 cdc25-22 cells into mitosis in the presence or absence of 12.5 μM latrunculin A. Although latrunculin A was unable to delay anaphase onset in cells lacking Mad3 or Mph1, anaphase onset was delayed in both control cells and cells lacking Bub3 (Supplemental Figure 2). These data indicate that Bub3 and, by inference, kinetochore association of Bub1 is not required to delay anaphase when interpolar microtubule stability is disrupted.
DISCUSSION
In recent years, the influence of spindle angle and latrunculin A on mitotic progression in fission yeast has been the subject of considerable debate. In this report, we present conclusive evidence that spindle orientation does not influence the timing of anaphase onset in fission yeast. In addition, our data agree with a previous report that spindle orientation is primarily determined by interphase microtubules and that there is no directed spindle repositioning during prometaphase and metaphase (Vogel et al., 2007). This led us to reexamine the effect of latrunculin A on spindle angle and the timing of sister chromatid separation. Although addition of latrunculin A causes some spindle misorientation during prometaphase and metaphase, this is not due to inhibition of astral microtubule contact with the medial actin cytoskeleton, because astral microtubules are only nucleated after anaphase onset (Zimmerman et al., 2004). Instead, we suggest that latrunculin A inhibits productive interaction of interphase microtubules with actin at cell tips, which results in spindle misorientation. Importantly, we show that latrunculin A does not delay anaphase onset by causing spindle misorientation, as previously thought, but rather by disrupting preanaphase spindle stability. This effect is abolished in an actin mutant that cannot bind latrunculin A, revealing a surprising new role for actin in preanaphase spindle stability.
So, why was the effect of latrunculin A on mitotic progression in fission yeast originally misinterpreted? One contributory factor is that in most previous studies we and others have used a variety of gfp-atb2 (α-tubulin) constructs to monitor mitotic progression. This has led to significant discrepancies in the literature. For example, one group has found that cells lacking Mto1 are delayed in anaphase onset (Venkatram et al., 2004), whereas others do not (Sawin et al., 2004). Similarly, different roles for the CLASP homologue (Cls1/Peg1) have been suggested based on the use of different gfp-atb2 constructs (Grallert et al., 2006; Bratman and Chang, 2007). Indeed, we find that latrunculin A does not cause mitotic spindle collapse in nmt1-gfp-atb2 cells at the same concentrations used in this study (data not shown). We reasoned that the choice of gfp-atb2 construct may also explain conflicting reports as to whether Ase1 influences the timing of anaphase onset in fission yeast (Loiodice et al., 2005; Yamashita et al., 2005). By simultaneously monitoring kinetochore and spindle pole movement, we provide compelling evidence that Ase1 is required for the timing of anaphase onset and also is an important regulator of preanaphase spindle stability. Because Ase1 can support spindle assembly in the absence of Cin8 motor protein in budding yeast, a preanaphase role for the Ase1/MAP65/PRC1 family may be conserved (Kotwaliwale et al., 2007).
Importantly, we find that addition of latrunculin A substantially blocks anaphase B in the absence of Ase1. At present, a molecular explanation for the role of actin in mitotic spindle stability remains elusive. One possibility is that actin is a component of a spindle matrix that stabilizes the preanaphase mitotic spindle. An actin meshwork aids chromosome congression during the first meiotic division of starfish oocytes, but this is thought to be necessary only because microtubule-based kinetochore capture is inefficient in large cells (Lenart et al., 2005). Evidence for an actin-based spindle matrix has also been reported in crane fly spermatocytes, and this is thought to act in conjunction with other spindle matrix proteins, such as Chromator, Skeletor, and Megator (Silverman-Gavrila and Forer, 2000; Fabian et al., 2007). In budding yeast, Fin1p forms filaments between the separated spindle poles during mitosis, and it is essential for viability in the absence of Ase1p (van Hemert et al., 2002; Woodbury and Morgan, 2007). Although fission yeast do not possess a close structural homologue of Fin1p, it is possible that actin plays an analogous role. However, budding yeast Fin1p only binds spindle microtubules after anaphase onset (Woodbury and Morgan, 2007), whereas in fission yeast latrunculin A only effects the stability of the preanaphase spindle, suggesting that their roles are not functionally equivalent. Moreover, there is no direct evidence for actin filaments in the nucleus in fission yeast. Regardless, identification of factors required for spindle microtubule stability in the absence of Ase1 may help to illuminate the role of actin in maintaining preanaphase spindle stability.
Previous studies indicate that latrunculin A delays anaphase onset by a mechanism that requires the Atf1 transcription factor and the Mad3, Bub1, and Mph1, but not Mad1 or Mad2 spindle assembly checkpoint proteins (Gachet et al., 2001; Oliferenko et al., 2004; Tournier et al., 2004). A Bub1-dependent, but Mad2-independent, anaphase delay has since been observed in fission yeast cells lacking the Mal3 (EB-1) plus end microtubule binding protein, in cells lacking Msd1, which is required for efficient tethering of spindle microtubules to the spindle pole body, and in atb2-V260I cells, which contain a point mutation in α-tubulin (Asakawa et al., 2005, 2006; Toya et al., 2007). We now demonstrate that the same subset of spindle assembly checkpoint proteins is required to delay anaphase in the absence of Ase1. This provides further evidence that at least two distinct, but overlapping, spindle checkpoint pathways control the onset of anaphase in fission yeast, one pathway that requires Mad1 and Mad2, and one pathway that does not. The role of the Atf1 transcription factor in mitotic checkpoint control remains unclear. Notably, a Mad2-independent checkpoint has also been described in fruit flies (Orr et al., 2007). Several lines of investigation suggest Mad1 and Mad2 are required for monitoring loss of microtubule attachment, but not loss of spindle tension. For example, in budding yeast, Bub1 and Bub3 are bound to kinetochores early in mitosis as part of the normal cell cycle, whereas Mad1 and Mad2 are kinetochore bound only in the presence of spindle damage or kinetochore lesions that interfere with chromosome–microtubule attachment (Gillett et al., 2004). Furthermore, Mad1 and Mad2 are recruited to kinetochores in mammalian cells treated with drugs that cause microtubule–kinetochore detachment, such as high doses of vinblastine or nocodazole, but not in response to low doses of vinblastine or taxol, which cause a loss of spindle tension, whereas both Bub1 and BubR1(Mad3) are recruited to kinetochores under both conditions (Skoufias et al., 2001; Zhou et al., 2002). Our genetic data support the notion that selective disruption of interpolar microtubule stability, either by addition of latrunculin A or inactivation of Ase1, leads to activation of only the spindle tension, and not the kinetochore attachment, checkpoint.
Although Mad2 and BubR1(Mad3) can independently bind to Cdc20 and can inhibit APCCdc20 ubiquitination activity in vitro, this can occur at only very high (superphysiological) concentrations of Mad2, whereas a combination of Mad2 and BubR1(Mad3) yields a far more potent inhibitor of APCCdc20 than Mad2 alone (Hwang et al., 1998; Kim et al., 1998; Sudakin et al., 2001; Tang et al., 2001; Fang, 2002). Indeed, genetic studies in fission yeast suggest that overexpression of mad2 can only induce a spindle checkpoint delay in strains expressing mad3, indicating that Mad2 requires Mad3 for inhibition of APC/C in vivo (Millband and Hardwick, 2002). Conversely, this and previous genetic studies indicate that Mad3 can inhibit APC/C in fission yeast in the absence of Mad2. Notably, Ipl1 (Aurora B) kinase-dependent phosphorylation of Mad3 is required for the spindle checkpoint response to a loss of tension in budding yeast (King et al., 2007). It will be of interest to examine whether Ark1 (Aurora B) and/or Mph1 kinase-dependent phosphorylation of Mad3 is required to inhibit the APC/C in fission yeast cells in which interpolar microtubule stability is disturbed.
So, why might eukaryotic cells possess both spindle tension and kinetochore attachment checkpoints? We showed previously that, as in mammalian cells, sister chromatids congress to the spindle midzone just before the onset of anaphase in fission yeast. When preanaphase spindle stability is disturbed, chromosome congression does not occur. In mammalian cells, Mad1 and Mad2 bind to unoccupied kinetochores in prometaphase and substantially disappear from kinetochores as chromosome congression occurs, whereas other checkpoint proteins, such as Bub1 and BubR1(Mad3), remain bound to kinetochores at the metaphase plate until the onset of anaphase (Chen et al., 1996; Waters et al., 1998). This raises the possibility that a Mad2-independent spindle (tension) checkpoint delays anaphase onset until all chromosomes congress to the spindle midzone (metaphase plate). Because formation of a metaphase plate requires balanced tension across all sister kinetochore pairs, such a checkpoint would provide an additional quality control mechanism to ensure accurate sister chromatid segregation. This might be particularly important in cells in which merotelic kinetochore attachment prevents chromosome congression. If microtubules become detached from kinetochores, this checkpoint signal may be amplified by recruitment of Mad1 and Mad2 to kinetochores to amplify the checkpoint signal blocking the onset of anaphase.
Finally, although we observe prolonged association of Bub1 to kinetochores, we find that the delay over sister chromatid separation imposed by loss of Ase1 or addition of latrunculin A does not require Bub3. This is surprising, since Bub3 is required for association of Bub1 and Mad3 to kinetochores (Millband and Hardwick, 2002; Vanoosthuyse et al., 2004). Moreover, recent evidence suggests that Bub3 is also not required for the metaphase arrest when spindle formation is completely disrupted (Tange and Niwa, 2008; Meadows and Millar, unpublished data). This suggests that, at least in fission yeast, kinetochore localization of checkpoint proteins may not be necessary to delay anaphase when spindle tension is lost or kinetochores become detached.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Takashi Toda, Ken Sawin, and Kathy Gould for supplying strains and to Kate Sullivan (Imaging Facility, National Institute for Medical Research, London, United Kingdom) and Vicky Buck for technical support. J. M. is supported by a project grant from the Association of International Cancer Research and a program grant from the Medical Research Council, United Kingdom.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0164) on June 18, 2008.
REFERENCES
- Asakawa K., Kume K., Kanai M., Goshima T., Miyahara K., Dhut S., Tee W. W., Hirata D., Toda T. The V260I mutation in fission yeast alpha-tubulin Atb2 affects microtubule dynamics and EB1-Mal3 localization and activates the Bub1 branch of the spindle checkpoint. Mol. Biol. Cell. 2006;17:1421–1435. doi: 10.1091/mbc.E05-08-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa K., Toya M., Sato M., Kanai M., Kume K., Goshima T., Garcia M. A., Hirata D., Toda T. Mal3, the fission yeast EB1 homologue, cooperates with Bub1 spindle checkpoint to prevent monopolar attachment. EMBO Rep. 2005;6:1194–1200. doi: 10.1038/sj.embor.7400540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D. G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bernard P., Hardwick K., Javerzat J. P. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 1998;143:1775–1787. doi: 10.1083/jcb.143.7.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratman S. V., Chang F. Stabilization of overlapping microtubules by fission yeast CLASP. Dev. Cell. 2007;13:812–827. doi: 10.1016/j.devcel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Jensen C. G., Jensen L. C., Bush M., Lloyd C. W. The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc. Natl. Acad. Sci. USA. 1999;96:14931–14936. doi: 10.1073/pnas.96.26.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Waters J. C., Salmon E. D., Murray A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Mao Y., Sullivan K. F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Ding R., McDonald K. L., McIntosh J. R. Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J. Cell Biol. 1993;120:141–151. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L., Xia X., Venkitaramani D. V., Johansen K. M., Johansen J., Andrew D. J., Forer A. Titin in insect spermatocyte spindle fibers associates with microtubules, actin, myosin and the matrix proteins skeletor, megator and chromator. J. Cell Sci. 2007;120:2190–2204. doi: 10.1242/jcs.03465. [DOI] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Ichinose S., Kiyono T., Tsurumi T., Omori A. Establishment of latrunculin-A resistance in HeLa cells by expression of R183A D184A mutant beta-actin. Oncogene. 2003;22:627–631. doi: 10.1038/sj.onc.1206173. [DOI] [PubMed] [Google Scholar]
- Funabiki H., Hagan I., Uzawa S., Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y., Tournier S., Millar J. B., Hyams J. S. A MAP kinase-dependent actin checkpoint ensures proper spindle orientation in fission yeast. Nature. 2001;412:352–355. doi: 10.1038/35085604. [DOI] [PubMed] [Google Scholar]
- Gachet Y., Tournier S., Millar J. B., Hyams J. S. Mechanism controlling perpendicular alignment of the spindle to the axis of cell division in fission yeast. EMBO J. 2004;23:1289–1300. doi: 10.1038/sj.emboj.7600156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett E. S., Espelin C. W., Sorger P. K. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 2004;164:535–546. doi: 10.1083/jcb.200308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert A., Beuter C., Craven R. A., Bagley S., Wilks D., Fleig U., Hagan I. M. S. pombe CLASP needs dynein, not EB1 or CLIP170, to induce microtubule instability and slows polymerization rates at cell tips in a dynein-dependent manner. Genes Dev. 2006;20:2421–2436. doi: 10.1101/gad.381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. M. The fission yeast microtubule cytoskeleton. J. Cell Sci. 1998;111:1603–1612. doi: 10.1242/jcs.111.12.1603. [DOI] [PubMed] [Google Scholar]
- Hagan I. M., Hyams J. S. Forces acting on the fission yeast anaphase spindle. Cell Motil. Cytoskeleton. 1996;34:69–75. doi: 10.1002/(SICI)1097-0169(1996)34:1<69::AID-CM7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- He X., Jones M. H., Winey M., Sazer S. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 1998;111:1635–1647. doi: 10.1242/jcs.111.12.1635. [DOI] [PubMed] [Google Scholar]
- He X., Patterson T. E., Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Totis L., Roberts B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hwang L. H., Lau L. F., Smith D. L., Mistrot C. A., Hardwick K. G., Hwang E. S., Amon A., Murray A. W. Budding yeast Cdc 20, a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Jiang W., Jimenez G., Wells N. J., Hope T. J., Wahl G. M., Hunter T., Fukunaga R. PRC 1, a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- Khmelinskii A., Lawrence C., Roostalu J., Schiebel E. Cdc14-regulated midzone assembly controls anaphase B. J. Cell Biol. 2007;177:981–993. doi: 10.1083/jcb.200702145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Lin D. P., Matsumoto S., Kitazono A., Matsumoto T. Fission yeast Slp 1, an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- King E. M., Rachidi N., Morrice N., Hardwick K. G., Stark M. J. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 2007;21:1163–1168. doi: 10.1101/gad.431507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwaliwale C. V., Frei S. B., Stern B. M., Biggins S. A pathway containing the Ipl1/aurora protein kinase and the spindle midzone protein Ase1 regulates yeast spindle assembly. Dev. Cell. 2007;13:433–445. doi: 10.1016/j.devcel.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Schmidt S., Cano E., Simanis V. S. pombe cdc11p, together with sid4p, provides an anchor for septation initiation network proteins on the spindle pole body. Curr. Biol. 2001;11:1559–1568. doi: 10.1016/s0960-9822(01)00478-x. [DOI] [PubMed] [Google Scholar]
- Lenart P., Bacher C. P., Daigle N., Hand A. R., Eils R., Terasaki M., Ellenberg J. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. doi: 10.1038/nature03810. [DOI] [PubMed] [Google Scholar]
- Li R., Murray A. W. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li X., Nicklas R. B. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Loiodice I., Staub J., Setty T. G., Nguyen N. P., Paoletti A., Tran P. T. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol. Biol. Cell. 2005;16:1756–1768. doi: 10.1091/mbc.E04-10-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Hagan I. M., Hyams J. S. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J. Cell Sci. Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- Millband D. N., Hardwick K. G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell Biol. 2002;22:2728–2742. doi: 10.1128/MCB.22.8.2728-2742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K., Nakagawa T., Straight A. F., Murray A., Chikashige Y., Yamashita Y. M., Hiraoka Y., Yanagida M. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol. Biol. Cell. 1998;9:3211–3225. doi: 10.1091/mbc.9.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S., Balasubramanian M. K. Astral microtubules monitor metaphase spindle alignment in fission yeast. Nat. Cell Biol. 2002;4:816–820. doi: 10.1038/ncb861. [DOI] [PubMed] [Google Scholar]
- Orr B., Bousbaa H., Sunkel C. E. Mad2-independent spindle assembly checkpoint activation and controlled metaphase-anaphase transition in Drosophila S2 cells. Mol. Biol. Cell. 2007;18:850–863. doi: 10.1091/mbc.E06-07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Bimbo A., Balasubramanian M. K., Oliferenko S. A potential tension-sensing mechanism that ensures timely anaphase onset upon metaphase spindle orientation. Curr. Biol. 2004;14:69–74. doi: 10.1016/j.cub.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Cole R. W., Khodjakov A., Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes I., Webb B. A., Mak A., Young P. G. G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol. Biol. Cell. 2001;12:3892–3903. doi: 10.1091/mbc.12.12.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Koonrugsa N., Toda T., Vardy L., Tournier S., Millar J. B. Deletion of Mia1/Alp7 activates Mad2-dependent spindle assembly checkpoint in fission yeast. Nat. Cell Biol. 2003;5:764–766. doi: 10.1038/ncb0903-764. author reply 766. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Lourenco P. C., Snaith H. A. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 2004;14:763–775. doi: 10.1016/j.cub.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Nurse P. Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler S. C., Liu J. Y., Pellman D. The molecular function of Ase1p: evidence for a MAP-dependent midzone-specific spindle matrix. Microtubule-associated proteins. J. Cell Biol. 2003;160:517–528. doi: 10.1083/jcb.200210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman-Gavrila R. V., Forer A. Evidence that actin and myosin are involved in the poleward flux of tubulin in metaphase kinetochore microtubules of crane-fly spermatocytes. J. Cell Sci. 2000;113:597–609. doi: 10.1242/jcs.113.4.597. [DOI] [PubMed] [Google Scholar]
- Skoufias D. A., Andreassen P. R., Lacroix F. B., Wilson L., Margolis R. L. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA. 2001;98:4492–4497. doi: 10.1073/pnas.081076898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B. M., Murray A. W. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 2001;11:1462–1467. doi: 10.1016/s0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Sudakin V., Chan G. K., Yen T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Bharadwaj R., Li B., Yu H. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Tange Y., Niwa O. Schizosaccharomyces pombe Bub3 is dispensable for mitotic arrest following perturbed spindle formation. Genetics. 2008;179:785–792. doi: 10.1534/genetics.107.081695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H., Goshima G., Takeda K., Nakagawa T., Kinoshita K., Yanagida M. Fission yeast living mitosis visualized by GFP-tagged gene products. Micron. 2001;32:67–74. doi: 10.1016/s0968-4328(00)00023-8. [DOI] [PubMed] [Google Scholar]
- Tournier S., Gachet Y., Buck V., Hyams J. S., Millar J. B. Disruption of astral microtubule contact with the cell cortex activates a Bub1, Bub3, and Mad3-dependent checkpoint in fission yeast. Mol. Biol. Cell. 2004;15:3345–3356. doi: 10.1091/mbc.E04-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya M., Sato M., Haselmann U., Asakawa K., Brunner D., Antony C., Toda T. Gamma-tubulin complex-mediated anchoring of spindle microtubules to spindle-pole bodies requires Msd1 in fission yeast. Nat. Cell Biol. 2007;9:646–653. doi: 10.1038/ncb1593. [DOI] [PubMed] [Google Scholar]
- Tran P. T., Marsh L., Doye V., Inoue S., Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F. The mechanism of sister chromatid cohesion. Exp. Cell Res. 2004;296:80–85. doi: 10.1016/j.yexcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- van Hemert M. J., Lamers G. E., Klein D. C., Oosterkamp T. H., Steensma H. Y., van Heusden G. P. The Saccharomyces cerevisiae Fin1 protein forms cell cycle-specific filaments between spindle pole bodies. Proc. Natl. Acad. Sci. USA. 2002;99:5390–5393. doi: 10.1073/pnas.072556099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V., Valsdottir R., Javerzat J. P., Hardwick K. G. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell Biol. 2004;24:9786–9801. doi: 10.1128/MCB.24.22.9786-9801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatram S., Tasto J. J., Feoktistova A., Jennings J. L., Link A. J., Gould K. L. Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol. Biol. Cell. 2004;15:2287–2301. doi: 10.1091/mbc.E03-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. K., Raabe I., Dereli A., Maghelli N., Tolic-Norrelykke I. Interphase microtubules determine the initial alignment of the mitotic spindle. Curr. Biol. 2007;17:438–444. doi: 10.1016/j.cub.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Waters J. C., Chen R. H., Murray A. W., Salmon E. D. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P. A., Kilmartin J. V. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 2001;152:349–360. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury E. L., Morgan D. O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat. Cell Biol. 2007;9:106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Sato M., Fujita A., Yamamoto M., Toda T. The roles of fission yeast ase1 in mitotic cell division, meiotic nuclear oscillation, and cytokinesis checkpoint signaling. Mol. Biol. Cell. 2005;16:1378–1395. doi: 10.1091/mbc.E04-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yao J., Joshi H. C. Attachment and tension in the spindle assembly checkpoint. J. Cell Sci. 2002;115:3547–3555. doi: 10.1242/jcs.00029. [DOI] [PubMed] [Google Scholar]
- Zhu C., Lau E., Schwarzenbacher R., Bossy-Wetzel E., Jiang W. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc. Natl. Acad. Sci. USA. 2006;103:6196–6201. doi: 10.1073/pnas.0506926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S., Chang F. Effects of γ-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol. Biol. Cell. 2005;16:2719–2733. doi: 10.1091/mbc.E04-08-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S., Daga R. R., Chang F. Intra-nuclear microtubules and a mitotic spindle orientation checkpoint. Nat. Cell Biol. 2004;6:1245–1246. doi: 10.1038/ncb1200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.