Abstract
The ADP/ATP carrier (AAC) proteins play a central role in cellular metabolism as they facilitate the exchange of ADP and ATP across the mitochondrial inner membrane. We present evidence here that in yeast (Saccharomyces cerevisiae) mitochondria the abundant Aac2 isoform exists in physical association with the cytochrome c reductase (cytochrome bc1)-cytochrome c oxidase (COX) supercomplex and its associated TIM23 machinery. Using a His-tagged Aac2 derivative and affinity purification studies, we also demonstrate here that the Aac2 isoform can be affinity-purified with other AAC proteins. Copurification of the Aac2 protein with the TIM23 machinery can occur independently of its association with the fully assembled cytochrome bc1-COX supercomplex. In the absence of the Aac2 protein, the assembly of the cytochrome bc1-COX supercomplex is perturbed, whereby a decrease in the III2-IV2 assembly state relative to the III2-IV form is observed. We propose that the association of the Aac2 protein with the cytochrome bc1-COX supercomplex is important for the function of the OXPHOS complexes and for the assembly of the COX complex. The physiological implications of the association of AAC with the cytochrome bc1-COX-TIM23 supercomplex are also discussed.
INTRODUCTION
ATP synthesized within the mitochondrial matrix by the F1Fo-ATP synthase is transported out across the inner membrane by the ADP/ATP carrier (AAC) proteins, a process that is coupled to the import of ADP into the mitochondrial matrix (Klingenberg, 1989; Gawaz et al., 1990; Drgon et al., 1991; Pebay-Peyroula and Brandolin, 2004; Nury et al., 2006). The AAC proteins span the inner mitochondrial membrane six times with short N- and C-terminal hydrophilic tails in the intermembrane space (Pebay-Peyroula et al., 2003). A number of mitochondrial AAC isoforms have been shown to exist in many organisms. In the yeast Saccharomyces cerevisiae, the model organism of this study, there are three AAC isoforms, encoded by the AAC1, AAC2, and AAC3 genes (Klingenberg, 1989; Gawaz et al., 1990; Drgon et al., 1991). In aerobically grown cells, the Aac2 protein is the most abundant AAC isoform and the Aac1 represents a minor isoform. The AAC3 gene is predominantly expressed under anaerobic growth conditions (Gawaz et al., 1990; Drgon et al., 1991). The Sa1l protein represents another member of the mitochondrial AAC family, which also catalyzes ADP/ATP exchange, but it differs from other AAC family members in that it contains an extended N-terminal hydrophilic region proposed to be involved in Ca2+ binding (Chen, 2004). Deletion of both the AAC2 and AAC3 genes, or of the AAC2 and SAL1 genes, results in a synthetic lethal phenotype under anaerobic and aerobic conditions, respectively (Drgon et al., 1992; Chen, 2004). Thus in addition to their role in ADP/ATP exchange, these AAC family members perform additional overlapping function(s) that are essential for cell viability. It is currently unclear, however, what essential role(s) these AAC family members may have.
The oxidative phosphorylation (OXPHOS) enzymes are large multisubunit complexes embedded in the mitochondrial inner membrane. These enzymes are not randomly organized as individual complexes in the membrane, but rather exist as higher-ordered assembly states termed “supercomplexes,” formed by the stable physical association of proteins between the OXPHOS complexes (Arnold et al., 1998; Boumans et al., 1998; Cruciat et al., 2000; Schägger and Pfeiffer, 2000; Paumard et al., 2002; Schägger, 2002; Pfeiffer et al., 2003; Gavin et al., 2005; Dudkina et al., 2005, 2006; McKenzie et al., 2006; Boekema and Braun, 2007; Heinemeyer et al., 2007; Marques et al., 2007; Zara et al., 2007; Saddar et al., 2008). Formation of supercomplexes has been proposed to concentrate these enzymes into localized areas within the mitochondrial inner membrane, i.e., to form “OXPHOS platforms,” also termed “respirasomes” (Schägger and Pfeiffer, 2000; Dudkina et al., 2006). Two supercomplex assemblies in yeast mitochondria, the dimeric ATP synthase and the cytochrome c reductase (cytochrome bc1)-cytochrome c oxidase (COX) supercomplex have been previously described (Arnold et al., 1998; Cruciat et al., 2000; Schägger and Pfeiffer, 2000; Paumard et al., 2002). In addition, it has recently been established that a subpopulation of the cytochrome bc1-COX complex has the capacity to interact with the TIM23 machinery and also to associate with the Shy1 and Cox14 proteins (van der Laan et al., 2006; Mick et al., 2007; Wiedemann et al., 2007). The TIM23 machinery forms a voltage-sensitive channel in the mitochondrial inner membrane that facilitates the import of nuclearly encoded proteins into the mitochondria (Truscott et al., 2001; Grigoriev et al., 2004; Chacinska et al., 2005; Kutik et al., 2007). The Shy1 and Cox14 proteins are assembly factors specific for the COX biogenesis pathway (Glerum et al., 1995; Mashkevich et al., 1997; Barrientos et al., 2004; Pierrel et al., 2007).
It has not been addressed until now whether the yeast AAC proteins, essential components of the mitochondrial OXPHOS pathway, may also form higher-ordered assemblies or supercomplexes with the other OXPHOS complexes. Although one report has indicated that some AAC protein may be associated with a minor population of the ATP synthase in bovine mitochondria (Ko et al., 2003), it has been generally considered until now that the AAC proteins exist as physically separate entities in the inner membrane, possibly forming homo-dimers, and where the monomeric AAC has been proposed to represent the enzymatically active form of AAC (Klingenberg, 1989; Pebay-Peyroula et al., 2003; Nury et al., 2005, 2006; Bamber et al., 2007a,b).
In this study, we have analyzed the assembly state of the AAC proteins in yeast mitochondria. We demonstrate here that the predominant Aac2 isoform exists together with other AAC proteins and in a higher-ordered complex composed of the cytochrome bc1-COX supercomplex and the TIM23 translocase. Using His-tagged version of Aac2 we demonstrate here that the cytochrome bc1-COX supercomplex and the TIM23 machinery can be specifically copurified with the Aac2 protein.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
S. cerevisiae strains used in this study are wild type (WT; W303–1A, Mat a, leu2, trp1, ura3, his3, ade2), and Δaac2 (W303–1B, AAC2::KAN, Chen, 2004). The yeast strains harboring His-tagged ATP synthase subunits Su e and F1β were previously described (Brunner et al., 2002; Mueller et al., 2004).
Loss of the mitochondrial DNA (mtDNA) from the Δaac2 expressing the HisAac2 protein was achieved by plating the cells onto YPD (glucose) plates containing ethidium bromide (25 μg/ml) and incubating for 2 d. A single cell colony was picked and streaked onto fresh ethidium bromide-containing YPD plates at 30°C. Single cell colonies were picked, and their inability to grow on YPG (glycerol plates) supplemented with 0.1% galactose (to induce HisAac2) was confirmed. The resulting strain is termed here Δaac2+HisAac2, rho0 and its parent strain containing the mtDNA is referred to as Δaac2+HisAac2, rho+.
Yeast strains were maintained and cultured using standard protocols at 30°C on YP-0.5% lactate media supplemented with 3% glycerol and 0.2% galactose or in the case of the Δaac2 and rho0 strains (and the WT when analyzed in parallel) in YP-0.5% lactate media supplemented with 2% galactose.
Expression of the HisAac2 Derivative
For the expression of HisAac2, a DNA fragment encoding HisAac2 (an Aac2 derivative bearing a 12-histidine tag at the N-terminus) was generated through PCR and was cloned as an XbaI/PstI fragment into a Yip351-based vector and downstream from the galactose-inducible GAL10 promoter. The recombinant plasmids were integrated into the yeast genome of the WT and Δaac2 strains at the LEU2 locus following linearization with BstEII. Expression of the HisAac2 protein was induced by the inclusion of galactose to the growth media.
Two-Dimensional Blue-Native SDS-PAGE Electrophoresis
Mitochondria (200 μg protein) were solubilized in lysis buffer (34 mM potassium acetate, 34 mM HEPES-KOH, pH 7.4, 11.4% glycerol, and 1 mM PMSF) with digitonin (0.5–1.5%, as indicated) for 30 min on ice and then were subjected to a clarifying spin of 30,000 × g for 30 min at 4°C. The supernatant from each sample was analyzed on a blue-native (BN) gel, using 3.5–10 or 7–17% gradient gels, as indicated, (Cruciat et al., 2000; Saddar et al., 2008). When indicated, a second-dimension SDS-PAGE separation was performed after the BN-PAGE step. To do so, the excised BN-PAGE gel strip was inserted on top of a SDS-PAGE gel, sealed with agarose solution (0.7% agarose, wt/vol; 0.5% SDS, wt/vol; and 15 mM β-mercaptoethanol) and was electrophoresed, followed by Western blotting, the nitrocellulose membrane was immunodecorated with subunit-specific antibodies, as indicated. The molecular-weight markers used for the BN-PAGE are bovine serum albumin (66 kDa), alcohol dehydrogenase (150 kDa), β-amylase (200 kDa), apoferritin (443 kDa), and thyroglobulin (670 kDa).
Affinity Purification of His-tagged Aac2 Protein
Mitochondria (200 μg protein) harboring the HisAac2 derivative were solubilized with digitonin (0.5%) as described above for the BN-PAGE sample preparation. After the clarifying spin the sample (70 μl) was supplemented with additional lysis buffer (150 μl), digitonin (final concentration 0.4%), imidazole, and bovine serum albumin, to final concentrations of 20 mM and 3 mg/ml, respectively. The sample was applied to Ni-NTA beads (pre-equilibrated in the lysis buffer) and incubated with constant turning at 4°C for 1.5 h. The beads were washed twice with lysis buffer containing 20 mM imidazole and 0.2% digitonin and once with lysis buffer-imidazole without digitonin. The bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE.
Accumulation and Affinity Purification of F1β Translocation Intermediate
Radiolabeled F1β protein was imported into WT and Δaac2+HisAac2 mitochondria for 30 min at 4°C, in the absence of ATP, and in the absence or presence of valinomycin, as previously described (Schleyer and Neupert, 1985; Gallas et al., 2006; Wiedemann et al., 2007). After import, mitochondria were reisolated by centrifugation, washed, and lysed in buffer containing digitonin (1.5%). Ni-NTA chromatography was performed as described above, with the exception that the binding and wash steps contained 1% digitonin. The level of radiolabeled F1β recovered on the Ni-NTA beads was quantified using a Storm 840 Phosphorimager.
Enzyme Measurements
Measurements of NADH-cytochrome bc1 and COX enzyme activities were performed, essentially as previously described (Tzagoloff et al., 1975). Mitochondria (20 μg protein) were solubilized with sodium deoxycholate (0.5%) before the enzyme assay. The NADH-cytochrome bc1 activity was assayed spectrophotometrically by following the reduction exogenously added horse heart cytochrome c at 550 nm in the presence of 0.1 mM KCN; the COX activity was measured by following the oxidation of exogenously added ferro-cytochrome c at 550 nm. In both cases the relative specific activities of the enzymes were calculated for both WT and Δaac2 mitochondria.
Miscellaneous
Mitochondrial isolation, protein determinations, and SDS-PAGE were performed according to published methods (Laemmli, 1970; Bradford, 1976; Herrmann et al., 1994).
RESULTS
AAC Exists in a Higher-ordered Complex That Comigrates with the Cytochrome bc1-COX-TIM23 Supercomplex
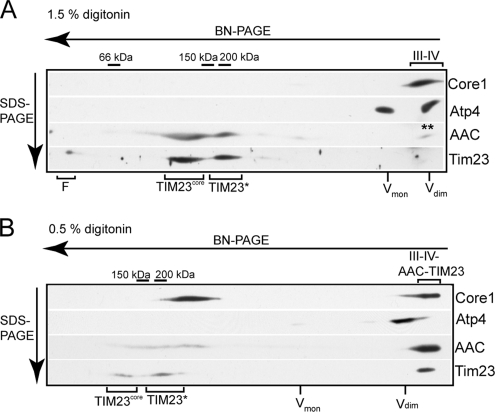
Mitochondrial membrane proteins were solubilized with the mild detergent digitonin, and protein complexes were analyzed by BN-PAGE followed by second-dimension SDS-PAGE resolution (Figure 1A). When solubilized with 1.5% (wt/vol) digitonin, the AAC protein, was found to be predominantly distributed between two closely migrating fractions in the range of the 150- and 200-kDa marker protein complexes, respectively (Figure 1A). No AAC protein was detected in the region of the BN-PAGE gel corresponding to the monomeric (30 kDa) or dimeric (60 kDa) AAC proteins, indicating that all the AAC protein solubilized under these conditions was present in oligomeric complexes larger than the dimeric form of the AAC protein. A small fraction of the AAC protein (observed more easily on longer exposures) was also found migrating in a higher molecular mass fraction where the cytochrome bc1-COX supercomplex (monitored by Core1) and dimeric ATP synthase (monitored through Atp4) also fractionated. Thus a population of AAC may have the capacity to interact with one of these OXPHOS supercomplexes in a supercomplex assembly form, and the relatively high level of digitonin used for the solubilization step may have largely interfered with this association.
Figure 1.
Assembly of the AAC protein and its comigration with the cytochrome bc1-COX-TIM23 supercomplex. (A and B) Mitochondria isolated from WT yeast were solubilized in digitonin (1.5%, A, and 0.5%, B), clarified by centrifugation, and directly analyzed by BN-PAGE (5–10% gradient gel). After the separation of the solubilized complexes by BN-PAGE, proteins were further resolved by a second-dimension SDS-PAGE step. After Western blotting, immunodecoration with specific antisera against Core1, Atp4, Tim23, and AAC was performed as indicated. The position of the cytochrome bc1-COX complex is indicated by III-IV and the TIM23 populations, TIM23core and TIM23*, are also indicated. The position of the AAC protein comigrating with the cytochrome bc1-COX-TIM23 complex is indicated by III-IV-AAC-TIM23. The positions of molecular-weight markers are also indicated. The positions of the dimeric and monomeric ATP synthase complexes are indicated by Vdim and Vmon, respectively, and the front of the BN-PAGE is indicated by F.
In a similar manner, a subpopulation of the TIM23 machinery has been recently described to associate with the cytochrome bc1-COX supercomplex, and this association is sensitive to the level of digitonin used during the lysis step (van der Laan et al., 2006; Saddar et al., 2008). Under the detergent conditions used here (1.5% digitonin), the TIM23 complex does not remain associated with the cytochrome bc1-COX complex, but rather is largely distributed between two populations, which, like AAC, migrate close to the 150- and 200-kDa marker complexes, respectively (Figure 1A). The TIM23 complex has been previously described to exist in multiple forms, which on BN-PAGE gels have collectively been grouped into two populations: the TIM23core (∼100–140 kDa in size) and the TIM23* (∼150–300 kDa in size) forms (Chacinska et al., 2005; van der Laan et al., 2006), as indicated in Figure 1A.
We lowered the concentration of digitonin used during the lysis step to 0.5% (conditions known to maintain the association of TIM23 with the cytochrome bc1-COX complex) to address what effect this had, if any, on the assembly state of the AAC protein (Figure 1B). When the detergent concentrations were reduced in this manner, the vast majority of the solubilized AAC protein and a significant fraction of the TIM23 complex were observed to comigrate with the cytochrome bc1-COX supercomplex. Furthermore, under these conditions, the size of the cytochrome bc1-COX supercomplex exceeded that of the dimeric ATP synthase (monitored through Atp4), indicating that the size of this supercomplex was greater under these mild detergent conditions (Figure 1B). The observed increase in size is presumably due to the presence of additional components, such as AAC and the TIM23 complex, which remained associated with the cytochrome bc1-COX supercomplex under these milder conditions.
On the basis of these data, we conclude that the AAC protein solubilized under the conditions used here does not exist as a physically separate entity in the mitochondrial membrane, but rather assembles into higher-ordered complexes. Furthermore, using mild detergent solubilization conditions a significant fraction of AAC comigrates with the cytochrome bc1-COX-TIM23 supercomplex.
Expression of His-tagged Aac2 Derivative
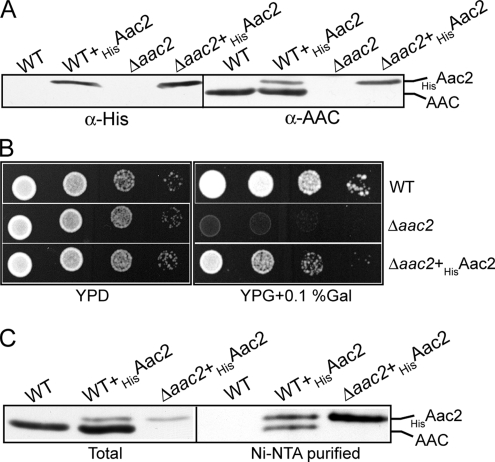
The AAC antibody used in this analysis can recognize all three yeast AAC isoforms (results not shown), but because the Aac2 protein represents the majority of the AAC protein expressed under the aerobic conditions used to cultivate the yeast cells (Aac3 is largely expressed under anaerobic conditions and Aac1 is a minor isoform), we conclude that we are predominantly monitoring the Aac2 isoform in the above BN-PAGE analysis. However, to analyze the assembly states and interacting partners of the Aac2 isoform directly, we cloned the Aac2 isoform as an N-terminally His-tagged derivative, HisAac2, and expressed it in both the WT and Δaac2 null mutant yeast strains (Figure 2). The content of AAC/HisAac2 protein in mitochondria isolated from these strains WT+HisAac2 and Δaac2+HisAac2 was analyzed following SDS-PAGE and Western blotting, respectively (Figure 2A). Expression of the His-tagged Aac2 protein in both strains was confirmed using a α-His antibody. Although the expression of HisAac2 was under control of the strong GAL10 promoter, the steady-state levels of the His-tagged protein remained clearly substoichiometric to the endogenously expressed Aac2 protein, as indicated by parallel decoration with the AAC antibody (Figure 2A). Thus the addition of the His-tag appears to have compromised the stability of the Aac2 protein; however, the HisAac2 protein could largely complement the respiratory-deficient phenotype of the Δaac2 null mutant, indicating that although reduced in levels, the HisAac2 derivative was functional (Figure 2B).
Figure 2.
Expression and characterization of the HisAac2 derivative. (A) Mitochondria were isolated from WT and Δaac2 strains expressing the HisAac2 proteins, WT+HisAac2 and Δaac2+HisAac2, respectively, and also from the WT and Δaac2 control strains. The presence and levels of the HisAac2 derivative and the endogenous AAC proteins were analyzed by SDS-PAGE, Western blotting, and immunodecoration with antibodies specific for the His-tag (left) or for AAC (right), as indicated. (B) The WT, Δaac2 cells and Δaac2+HisAac2 strains were initially grown on YPAD plates and then were resuspended in water at a concentration of 1 OD578 nm/ml. A 10-fold serial dilution series of these strains was then generated, and 2 μl of each of the resulting dilutions was spotted onto YPD (glucose) and YPG (glycerol) supplemented with 0.1% galactose plates (YPG + 0.1% Gal) and incubated at 30°C. (C) Mitochondria isolated from the WT and Δaac2 strains expressing the HisAac2 protein, and the WT control strain, were solubilized with digitonin (0.5%) and subjected to Ni-NTA chromatography, as described in Materials and Methods. An aliquot of the total material applied to the Ni-NTA beads (Total, corresponds to 5% of input) and the Ni-NTA bead-purified material and were analyzed by SDS-PAGE, Western blotting, and immunodecoration with AAC-specific antisera. (A and C) Note under the exposure times used here, the presence of the less abundant AAC isoform(s), Aac1/Aac3, is not observed in Δaac2 samples, but can be seen after prolonged exposure times (not shown).
We initially investigated if the Aac2 protein existed in a complex with another AAC protein. WT mitochondria harboring both the HisAac2 and endogenous Aac2 proteins were solubilized with digitonin and subjected to Ni-NTA affinity chromatography. The nontagged endogenous AAC protein copurified with the HisAac2 protein under these detergent conditions (Figure 2C). Recovery of endogenous AAC protein on the Ni-NTA beads was specific for the presence of the His-tagged Aac2 derivative, because AAC was not recovered on the beads in the parallel control WT sample (i.e., contains endogenous Aac2, but not the His-tagged Aac2 derivative). These data demonstrate the ability of HisAac2 to coassemble with another AAC protein. We consider it most likely that the HisAac2 protein exists together with another Aac2 protein, because the level of endogenous AAC protein recovered with HisAac2 from WT mitochondria was significantly greater than that from the Δaac2 mitochondria (Figure 2C), where a weak endogenous AAC signal (corresponding to Aac1 and/or Aac3 isoforms) was only observed upon prolonged exposure of the Western blot (results not shown).
Aac2 Physically Interacts with the Cytochrome bc1-COX-TIM23 Complex
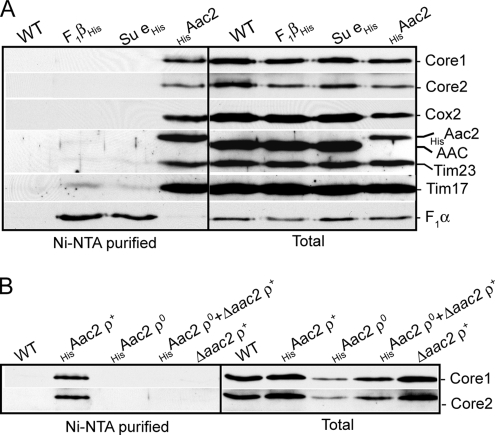
To investigate if the Aac2 protein physically interacts with the cytochrome bc1-COX-TIM23 complexes as its behavior in the BN-PAGE experiments above would suggest (Figure 1B), we analyzed whether components of the cytochrome bc1-COX and TIM23 complexes could be specifically copurified with the detergent solubilized HisAac2 protein. Mitochondria isolated from the Δaac2 strain harboring the His-tagged Aac2 derivative were solubilized with digitonin, and mitochondrial proteins were subjected to Ni-NTA chromatography (Figure 3A). His-tagged Aac2 was recovered on the beads, in contrast to the endogenous AAC protein solubilized from control WT mitochondria, thus indicating recovery of AAC on the beads was specific for the His-tag on the Aac2 protein. The HisAac2 isoform was found to be in association with the cytochrome bc1-COX supercomplex, as evidenced by the corecovery of Core1, Core2, and Cox2 proteins on the Ni-NTA beads with the HisAac2 protein. Furthermore, corecovery of the Tim23 and Tim17 proteins with HisAac2 confirmed the physical association of Aac2 protein in a complex also with the TIM23 machinery. Recovery of the cytochrome bc1-COX-TIM23 complexes on the Ni-NTA beads was specific for the presence of the His-tagged Aac2 protein, as they were not present on the Ni-NTA beads from the WT control mitochondria analyzed in parallel. Furthermore, subunits of the abundant F1Fo-ATP synthase, were not recovered on the beads with the HisAac2 protein (monitored by F1α), further demonstrating the specificity of the association of the Aac2 protein with the cytochrome bc1-COX and TIM23 complexes.
Figure 3.
Affinity purification of the cytochrome bc1-COX-TIM23 complex with the HisAac2 protein. (A) Mitochondria harboring the His-tagged F1β, Su e or Aac2 proteins, or control WT mitochondria were solubilized with digitonin (0.5%) and subjected to Ni-NTA chromatography, as described in Figure 2C. After SDS-PAGE and Western blotting, blots were immunodecorated with antisera specific for the cytochrome bc1 (Core1, Core2), COX (Cox2), AAC, TIM23 (Tim17, Tim23), and ATP synthase (F1α) complexes, as indicated. (B) Mitochondria isolated from control WT cells, Δaac2 expressing the HisAac2 protein with a rho+ or rho0 background (Δaac2+HisAac2, ρ+ and Δaac2+HisAac2, ρ0, respectively), were lysed with digitonin and subjected to Ni-NTA chromatography. In addition a parallel sample of Δaac2+HisAac2, ρ0 mitochondria was premixed with Δaac2 (rho+) mitochondria before lysis and analyzed in parallel. Ni-NTA purification and subsequent analysis was performed as described in Figure 2C.
We also performed in parallel affinity purification of the abundant F1F0-ATP synthase complex using His-tagged F1β or Su e proteins, components of the F1- and F0-ATP synthase sectors, respectively (Brunner et al., 2002; Mueller et al., 2004). Recovery of the F1F0-ATP synthase was successfully achieved using these tagged proteins, as demonstrated by the recovery of F1α on the Ni-NTA beads. In contrast to the HisAac2 purification, the cytochrome bc1-COX and TIM23 complexes were not co-recovered on the Ni-NTA beads with His-tagged F1β or Su e proteins (Figure 3A). Furthermore, the AAC protein was not observed to copurify with the affinity-purified F1F0-ATP synthase complexes. These results further demonstrate the specificity of the co-recovery of the cytochrome bc1-COX-TIM23 complex with the HisAac2 protein.
Next we generated a rho0 derivative of the Δaac2 strain expressing the HisAac2 protein. Rho0 mitochondria lack assembled cytochrome bc1-COX supercomplexes because central components of these complexes (cytochrome b, Cox1, Cox2, and Cox3) are mtDNA encoded. Although present in the Δaac2-HisAac2, rho0 mitochondria (albeit at levels reduced relative to WT), Core1 and Core2 proteins were not recovered on the Ni-NTA beads with the HisAac2 protein (Figure 3B). These data show that copurification of the Core1 and Core2 proteins with the HisAac2 derivative was dependent on their assembly into the fully assembled cytochrome bc1-COX supercomplex.
Finally, we addressed whether the recovery of the Aac2 protein and the cytochrome bc1-COX complex reflects their physical interaction in organello or whether it is due to their association in a postlysis manner. To do so, we performed a prelysis mixing experiment where the Δaac2-HisAac2, rho0 mitochondria (i.e., lacking assembled cytochrome bc1-COX complex) were combined and lysed together with Δaac2, rho+ mitochondria (i.e., containing cytochrome bc1-COX complex, but lacking HisAac2). If the observed copurification of the cytochrome bc1-COX complex with HisAac2 was due to their physical association in a postlysis manner then the cytochrome bc1-COX complex from the Δaac2 mitochondria should be recovered with the HisAac2 protein originating from the rho0 mitochondria. However, this was not the case, because the Core1 and Core2 proteins were not copurified with the HisAac2 protein from the Δaac2-HisAac2, rho0 mitochondria (Figure 3B). Copurification of the cytochrome bc1-COX subunits with HisAac2 was only observed with the HisAac2 protein solubilized from the Δaac2-HisAac2, rho+ mitochondria (Figure 3B). Taking these results together, we conclude that Aac2 physically coassembles with the cytochrome bc1-COX supercomplex in organello and not in a postlysis manner. We conclude therefore that Aac2 assembles with the cytochrome bc1-COX-TIM23 complex in the mitochondrial membrane.
The Interaction between the AAC Protein and the Cytochrome bc1-COX-TIM23 Supercomplex Is Functionally Relevant
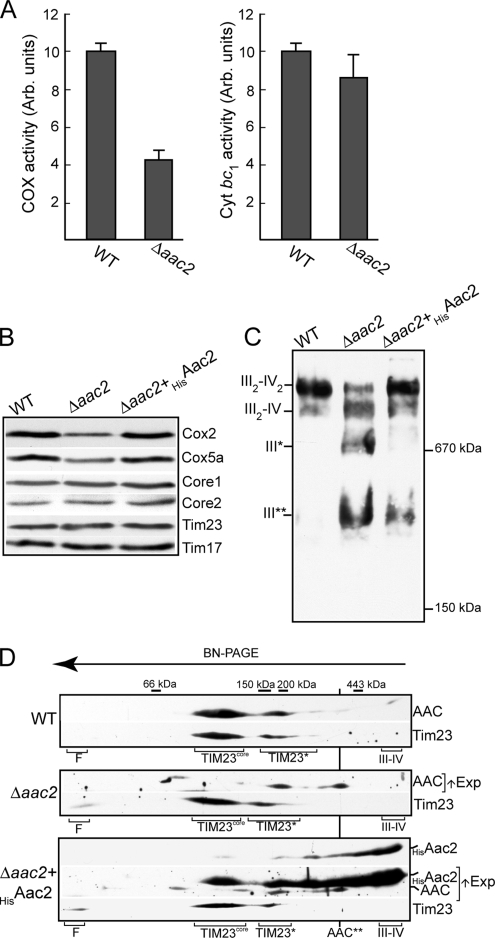
We next addressed the consequence of the physical absence of the Aac2 protein on the cytochrome bc1-COX and TIM23 complexes. We first measured the cytochrome bc1 and COX enzyme activities in mitochondria isolated from the WT and Δaac2 strains. The levels of COX enzyme activity was found to be reduced by ∼55% in the Δaac2 mitochondria, relative to the WT control (Figure 4A). The cytochrome bc1 enzyme activity levels, on the other hand, were not as significantly reduced in the absence of Aac2; a reduction of ∼15% was measured in the aac2 null mitochondria, relative to the WT control (Figure 4A). Consistent with the observed reduction in COX enzyme activity, the levels of the COX subunits tested (Cox2 and Cox5a) were found to be reduced in the Δaac2 mitochondria when compared with WT mitochondria (Figure 4B). Expression of the HisAac2 protein in the Δaac2 cells restored Cox2 and Cox5a levels to those of WT. Thus the presence of the Aac2 protein appears to be important to support normal levels of the COX complex subunits. The steady-state levels of the cytochrome bc1 complex subunits (Core1 and Core2) appeared to be relatively unaffected in the absence of Aac2, indicating that Aac2 was not required for efficient cytochrome bc1 biogenesis and/or stability, results that are consistent with the measured cytochrome bc1 enzyme levels. The levels of the TIM23 complex, as judged by the Tim17 and Tim23 levels, did not appear to be adversely affected in the absence of Aac2 (Figure 4B).
Figure 4.
Assembly of the cytochrome bc1-COX supercomplex is affected in the absence of the Aac2 protein. (A) The levels of cytochrome c oxidase (COX) and cytochrome bc1 enzyme activity were measured using isolated WT and Δaac2 mitochondria from YP-Gal grown cultures. Assays were performed on two independent isolations on each type of mitochondria, and each time triplicate samples were assayed. The mean of the resulting relative specific activities and their SDs were calculated. For both enzymes, the activity measured in the WT control was set to an arbitrary unit of 10, and those of the Δaac2 mutant were then expressed as a fraction of the WT. (B) Mitochondria (50 μg protein) isolated from WT and Δaac2 and Δaac2+HisAac2 strains were analyzed by SDS-PAGE, Western blotting, and the steady-state levels of components of the COX (Cox2, Cox5a), cytochrome bc1 (Core1 and Core2), TIM23 (Tim17, Tim23) complexes were analyzed using subunit specific antisera, as indicated. (C and D) Mitochondria were isolated from the WT and Δaac2 and Δaac2+HisAac2 strains and solubilized with digitonin (1.5%). (C) One-dimensional BN-PAGE analysis (5–10% gradient gel) was performed, followed by Western blotting and immunodecoration with Core1-specific antisera. The positions of the III2-IV2 and III2-IV assembly states of the cytochrome bc1-COX supercomplex and of cytochrome bc1 subpopulations (III*, III**) are indicated. (D) BN-PAGE (7–17% gradient gel) followed by a second-dimension SDS-PAGE analysis, and Western blotting was performed. Immunodecoration with AAC and Tim23 antisera and F1Fo-ATP synthase complexes (decorated with Atp4 antisera, not shown) was performed as described in Figure 1. The Aac1/Aac3 isoforms are visualized in the Δaac2 and Δaac2+HisAac2 mitochondria after a prolonged exposure of the AAC signal, indicated by ↑Exp. The largest AAC complex observed is indicated by AAC**, and other abbreviations are as in Figure 1.
The assembly state of the cytochrome bc1-COX supercomplex in the absence of the Aac2 protein was next addressed. Mitochondria isolated from WT, the Δaac2 null and the Δaac2 strain harboring the HisAac2 derivative were solubilized with digitonin, and the assembly states of the cytochrome bc1-COX were analyzed by a one-dimensional BN-PAGE gel (Figure 4C). In WT mitochondria, as indicated by immunoblotting with Core1 antiserum, the cytochrome bc1-COX supercomplex was predominantly present in a complex of a size that corresponds to the previously described III2-IV2 stoichiometric form (III, cytochrome bc1; IV, COX) (Schägger and Pfeiffer, 2000; Pfeiffer et al., 2003). A minor fraction was present in a slightly smaller form, which has previously been termed the III2-IV complex (Figure 4C). In the absence of Aac2, a perturbation in the assembly state of the cytochrome bc1-COX complex was observed on a couple of levels. First, decreased levels and altered ratios of the III2-IV2 and III2-IV supercomplex forms were observed, whereby an increase in the level of the III2-IV form relative to the III2-IV2 form was seen in the Δaac2 mitochondrial extracts. Second, additional Core1-containing complexes of ∼670 kDa and smaller (indicated by III* and III**, respectively) were observed in the Δaac2 mitochondria and in contrast to the WT control (Figure 4C). Expression of HisAac2 in the Δaac2 strain largely restored the altered cytochrome bc1-COX assembly state back to that of WT mitochondria, because the majority of the cytochrome bc1-COX complex in the Δaac2+HisAac2 mitochondria appeared from its electrophoretic profile to resemble the abundant III2-IV2 form with a minor population being present as the III2-IV form. Some Core1-containing complex (III**) was also observed in the Δaac2+HisAac2 mitochondria, suggesting complementation of the assembly defect of the cytochrome bc1-COX supercomplex was not complete upon expression of the HisAac2 derivative (Figure 4C).
Thus we conclude that the Aac2 protein is important to secure the normal assembly of the III2-IV2 stoichiometric-form of the cytochrome bc1-COX supercomplex. In the absence of Aac2, the significant increase of the III2-IV assembly form (relative to the III2-IV2 form) may correlate with the reduced, and possibly limiting, levels of the COX complex in the Δaac2 mitochondria. The assembly state of the F1Fo-ATP synthase (as monitored through Atp4) was not perturbed in the absence of Aac2 (results not shown).
The assembly state of the AAC proteins and the TIM23 complex was also analyzed in the WT, Δaac2, and Δaac2+HisAac2 mitochondria by BN-PAGE followed by SDS-PAGE analysis (Figure 4D). In WT mitochondria under these detergent conditions, the majority of the TIM23 machinery was released from the cytochrome bc1-COX supercomplex and, as previously described in Figure 1, was observed to partition between the two diverse TIM23core and TIM23* populations. Both the TIM23core and TIM23* populations were observed in the Δaac2 mitochondria, indicating that the assembly states of these TIM23 forms are not grossly altered in the absence of Aac2. However, a slight mobility shift in the TIM23* population was observed in the Δaac2 mitochondria, indicating that the size of at least some of the TIM23* forms may be smaller in the absence of Aac2. The alteration in the mobility of the TIM23* forms was partially restored in the Δaac2 mitochondria harboring the HisAac2 derivative, where the TIM23* forms appeared to have a more uniform size. An increased level of Tim23 running at the front of the gel was observed in the Δaac2 mitochondria, both in the absence and presence of the His-tagged Aac2 derivative, suggesting that the stability of the TIM23 complex may be altered in the absence of WT levels of the Aac2 protein.
As previously described in WT mitochondria, the AAC protein largely partitioned between two major fractions and exhibited an electrophoretic profile similar to that of the TIM23core and TIM23* populations. Because the other AAC isoform(s) are of similar size as that of the endogenous Aac2 protein, they cannot be distinguished in the WT extracts as a result of the abundance of the Aac2 protein, the major AAC isoform. Decoration of the Δaac2 mitochondrial extracts with the AAC antiserum, followed by prolonged exposure, however, allowed the detection of the other minor AAC isoforms(s). These AAC isoform(s) were distributed between a number of complexes, ranging from ∼90 through 400 kDa. These AAC isoform complexes did not largely comigrate with TIM23core, but an AAC complex in the 200-kDa size range, did partially overlap with the TIM23* population (Figure 4D). Because the AAC antiserum used in this analysis recognizes both the Aac1 and Aac3 isoforms, we cannot distinguish which isoform(s) these may represent. Interestingly, the majority of the HisAac2 protein was recovered comigrating with the cytochrome bc1-COX supercomplex, and only a minor form was recovered with the AAC complexes in the 150–200-kDa range. It is possible that the HisAac2 protein has enhanced the affinity for the cytochrome bc1-COX complex because of the presence of the N-terminal His-tag. Longer exposure times (indicated by ↑Exp. in Figure 4D) allowed detection of the endogenous AAC isoforms and of the less abundant populations of HisAac2. As was observed in Δaac2 mitochondria, the other AAC isoforms present in the HisAac2-containing mitochondria partitioned between the 90- and 400-kDa AAC complexes, with an abundant 200-kDa AAC complex partially overlapping with a TIM23* population.
We conclude on the basis of these data that the assembly state of the TIM23 complex is not grossly altered in the absence of Aac2; however, a subtle affect on the TIM23* population may be observed in the null mutant extracts. It is possible that another AAC isoform (possibly Aac1 and/or Aac3) may also have the capacity to interact with TIM23*-containing complexes.
Aac2 Can Be Recovered in Association with a Functional TIM23-PAM Machinery
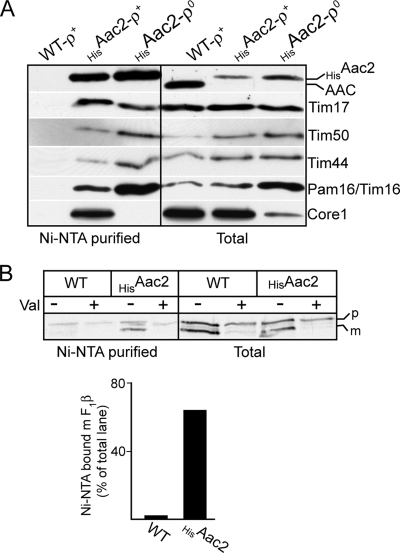
Complete transport of preproteins across the TIM23 complex into the matrix requires the concerted activity of the TIM23 translocase and its associated PAM complex, the “presequence translocase motor.” The PAM complex is composed of mtHsp70 (Ssc1), Tim44, Pam16/Tim16, Pam17, and Pam18/Tim14, which together regulate the mtHsp70 protein and its physical association with the TIM23 complex (Kutik et al., 2007). Evidence for the association of the TIM23 and associated PAM complex with the cytochrome bc1-COX complex has been previously presented (van der Laan et al., 2006; Wiedemann et al., 2007; Saddar et al., 2008). In addition to the TIM23 subunits, e.g., Tim17 and Tim50, components of the PAM motor, e.g., Tim44, and Pam16/Tim16, were recovered with the Ni-NTA affinity-purified HisAAC2 protein from both the rho+ and rho0 mitochondria (Figure 5A). We conclude therefore that the Aac2 protein can be recovered in association with both the TIM23 and PAM complexes. Furthermore, because the rho0 mitochondria are deficient in the mitochondrially encoded cytochrome bc1-COX subunits, we can conclude that the association of TIM23-PAM machinery with HisAac2, unlike Core1, does not depend on the presence of a fully assembled cytochrome bc1-COX supercomplex.
Figure 5.
Aac2 can interact with the TIM23-PAM machinery. (A) Mitochondria isolated from the Δaac2+HisAac2, ρ+ (HisAac2-ρ+) and Δaac2+HisAac2, ρ0 (HisAac2-ρ0) strains and WT control were solubilized with digitonin (0.5%) and subjected to Ni-NTA chromatography, as described in Materials and Methods. The Ni-NTA purified material was analyzed by SDS-PAGE, Western blotting, and immunodecoration with AAC, Tim17, Tim44, Tim50, Pam16/Tim16, and Core1 antisera, as described in Figure 2C. (B) Radiolabeled F1β was imported into mitochondria at 4°C in the presence or absence of added valinomycin, as indicated. After lysis of mitochondria with digitonin (1.5%), samples were subjected to Ni-NTA chromatography, as described in Figure 2C. Samples were subjected to SDS-PAGE, Western blotting, and autoradiography (top panel). The level of radiolabeled F1β in each sample was quantified by phosphorimaging. The level of mature size F1β species recovered on the Ni-NTA beads from the WT and Δaac2+HisAac2 samples was expressed as a percentage of the level of mature F1β present in their corresponding “total” lanes.
Finally, we tested whether the TIM23-PAM complex found in association with the Aac2-cytochrome bc1-COX supercomplex represented a translocation-active complex. The precursor of the β subunit of the F1-ATP synthase can be trapped as a translocation intermediate when the in vitro import reaction is performed at low temperature. Under these conditions, the F1β translocation intermediate spans both the TOM and the TIM23-PAM complexes and exposes its N-terminus to the matrix, where it becomes proteolytically matured by the mitochondrial processing peptidase (Schleyer and Neupert, 1985; Wiedemann et al., 2007). Radiolabeled F1β precursor was imported into WT and Δaac2+HisAac2 mitochondria at 4°C, and we tested the ability of the accumulated translocation intermediate to copurify with the HisAac2 protein. The mature-size radiolabeled F1β protein was recovered on the Ni-NTA beads when it was accumulated in the HisAac2-containing, but not WT, mitochondria (Figure 5B, top and bottom panels). The nonimported F1β precursor protein which accumulates on the outside of the mitochondria appeared to be recovered on the Ni-NTA beads in a largely nonspecific manner, as recovery of the precursor was observed from WT and HisAac2 bearing mitochondria, and even was observed when import was inhibited in the absence of membrane potential due to the presence of valinomycin. It is likely therefore that the nonimported species is prone to aggregation and hence nonspecific recovery on the beads.
Taken together, we demonstrate that the mature-size F1β translocation intermediate can be recovered in association with the HisAac2 protein, an observation that provides support that the TIM23-PAM machinery associated with the Aac2-cytochrome bc1-COX supercomplex is actively involved in the protein import process.
DISCUSSION
In this study we have analyzed the assembly state of the yeast mitochondrial ADP/ATP carrier protein, Aac2, and present evidence for the association of this AAC isoform with another component of the OXPHOS system, the cytochrome bc1-COX supercomplex and its associated TIM23 machinery. The oligomeric state of the mitochondrial AAC proteins has been the focus of much discussion in the literature, where its existence and function as a dimer or monomer has been debated. Evidence to support that the monomeric form of the enzyme may represent the enzymatically functional unit of the AAC protein as an ADP/ATP transporter has been recently presented (Pebay-Peyroula et al., 2003; Nury et al., 2005; Bamber et al., 2007a,b). Using mild detergent solubilization conditions combined with BN-PAGE analysis, we have demonstrated that yeast AAC proteins fractionate in higher molecular weight complexes, thus indicating their ability to interact with other proteins in the mitochondrial membrane, rather than existing as monomeric proteins. Using a His-tagged Aac2 protein, we show here that these higher-ordered Aac2-complexes contain at least one other AAC protein, as an endogenously expressed AAC protein, most likely the Aac2 protein, can be coaffinity-purified with the His-tagged Aac2 derivative. The fact that the Aac2 protein exists together with other AAC protein(s) does not exclude the possibility that the operational unit of the ADP/ATP translocating activity could still be the AAC monomer.
We demonstrate here that the Aac2 protein copurifies with the cytochrome bc1-COX supercomplex. The association of Aac2 with the cytochrome bc1-COX supercomplex was confirmed using a His-tagged Aac2 derivative and Ni-NTA affinity purification studies. It is important to note that the levels of HisAac2 are clearly substoichiometric to the endogenous WT Aac2 levels and thus may be a limiting factor and affects the level of cytochrome bc1-COX recovered on the Ni-NTA beads. The association between the AAC proteins and the cytochrome bc1-COX complex is highly sensitive to detergent concentration and is observed here using low concentrations of the mild detergent digitonin. This apparent sensitivity to detergent may explain why the association of AAC proteins with the solubilized cytochrome bc1-COX supercomplex has not been described before. Under the 0.5% digitonin solubilization conditions, the majority of the solubilized AAC protein was found in association with the cytochrome bc1-COX supercomplex. Approximately 70% of the total AAC protein, and almost all (more than 90%) of the cytochrome bc1 and COX complexes, were solubilized under the digitonin conditions used here (similar solubilization efficiencies of these complexes were observed for both the 0.5 and 1.5% conditions; results not shown). Thus we can conclude that a significant fraction of the total mitochondrial AAC protein associates with the cytochrome bc1-COX supercomplex, the latter, like AAC is known to be an abundant mitochondrial protein complex. It is possible that the fraction of the AAC protein not solubilized under these detergent conditions may form a separate AAC subpopulation within the mitochondria, i.e., one that is not associated with the cytochrome bc1-COX complex and thus potentially differs in its environment and solubility profile.
Why does the Aac2 protein exist in association with the cytochrome bc1-COX supercomplex in mitochondria? Under conditions of oxidative phosphorylation, external ADP3− is transported into the mitochondria coupled to the exchange for ATP4−, which is transported from the matrix across the inner membrane (Duszyński et al., 1981; Klingenberg, 1989; Pebay-Peyroula and Brandolin, 2004; Nury et al., 2006). The exchange of ADP3− for ATP4− is not charge-compensated and is supported energetically through the membrane potential generated by the activity of the OXPHOS complexes. In fact it has been estimated that ∼30% of the energy generated by the respiratory chain complexes is required to support the exchange of ADP for ATP (Duszyński et al., 1981). Both the cytochrome bc1 and COX complexes play an active role in establishing the mitochondrial membrane potential through their H+-pumping activities (Saraste, 1999). It is therefore possible that a direct physical association between the Aac2 protein and these H+-pumping complexes may be favorable in supporting the energetic demands of the ADP/ATP exchange process. However, the association of AAC with the cytochrome bc1-COX supercomplex is clearly not essential for function, as ADP/ATP exchange across the inner membrane can occur also in mitochondria deficient for these OXPHOS complexes.
On the other hand, it is possible that the coassembly of Aac2 is favorable for the cytochrome bc1-COX complex. In support of this, we demonstrate that in the absence of Aac2 a decrease in COX enzyme levels was detected, an observation that is consistent with previously published cytochrome spectral analysis of Δaac2 cells (Fontanesi et al., 2004). In addition, an alteration in the assembly state of the cytochrome bc1-COX supercomplex was observed in the absence of Aac2. In WT mitochondria, under the conditions used here, the cytochrome bc1-COX supercomplex was almost exclusively present in the III2-IV2 or larger forms. In the absence of Aac2 we observed a significant change in the ratio of III2-IV to III2-IV2 complexes and also the presence of some free cytochrome bc1 subforms. BN-PAGE analysis, followed by a second-dimension SDS-PAGE and Cox2 immunodecoration, indicated that the COX complex (although reduced in levels) was exclusively recovered in the III2-IV2 and III2-IV supercomplex forms, i.e., no free COX complex was observed, in the Δaac2 mitochondria (results not shown). Thus the decreased levels of the COX complex observed in the Δaac2 mitochondria may limit the formation of the normal III2-IV2 assembly state of the cytochrome bc1-COX supercomplex. It is possible that the association of Aac2 with the cytochrome bc1-COX supercomplex may support the stability of the assembled COX complex. Alternatively, Aac2 may directly support the assembly of the COX complex through affecting the assembly state and possibly the function of COX assembly factors, Shy1 and Cox14, which have been shown to associate with the cytochrome bc1-COX supercomplex (Mick et al., 2007). Our preliminary data would indicate that the association of AAC with the cytochrome bc1-COX complex may reflect an interaction between the AAC protein and the COX-associated subunits, rather than the cytochrome bc1-aspect of the complex. Analysis of mutants known to exhibit a perturbed assembly of the cytochrome bc1-COX supercomplex (e.g., null mutants of Su e and Su g of the ATP synthase; Saddar et al., 2008) indicated that AAC did not partition with the free cytochrome bc1 complex, but rather remained in association with the remaining cytochrome bc1-COX supercomplex (results not shown). Thus the association of AAC with this supercomplex may involve the COX-related proteins directly or possibly a combination of cytochrome bc1 and COX subunits.
We also describe here that the association of HisAac2 with the cytochrome bc1-COX supercomplex involves the TIM23-PAM complex and in a manner that can occur independently of the mitochondrially encoded cytochrome bc1 and COX subunits. This association could be facilitated through a direct interaction of a HisAac2-containing complex with TIM23 components or indirectly, via some nuclearly encoded subunits of the cytochrome bc1-COX complex. When solubilized under conditions that caused their release from the cytochrome bc1-COX supercomplex, the AAC and TIM23 complexes both partitioned between two predominant fractions in the 100–300-kDa size range. Despite their similar electrophoretic profiles on the BN-PAGE gels, we consider it unlikely that these comigrating complexes simply represent exclusive AAC-TIM23 subassemblies. In the absence of Aac2, the assembly state of the abundant TIM23core complex was not affected. Furthermore, the Aac1/Aac3 isoforms in the Δaac2 null mutant did not appear to largely comigrate with the TIM23core complexes. Thus it is unlikely that the AAC proteins are an integral part of the TIM23core population. The TIM23* complexes form a diverse group of TIM23 forms ranging from ∼150–300 kDa in size. Although the formation of the TIM23* class of complexes does not depend on the presence of Aac2, the electrophoretic profile of the TIM23* complexes was shifted to a slightly smaller size in the absence of Aac2. Thus it is possible that a subcomplex of TIM23*-AAC may exist within the population of TIM23* complexes, possibly because of their corelease from the solubilized cytochrome bc1-COX supercomplex.
The TIM23-PAM complex associated with Aac2-cytochrome bc1-COX supercomplex represents an import active complex, as shown by the copurification of the TIM23-spanning translocation intermediate with HisAac2. The import of a matrix-targeted precursor protein across the TIM23-PAM machinery was found to be partially inhibited in Δaac2 mitochondria, but was comparable to that measured with other respiratory-deficient mitochondria (results not shown). Therefore we cannot conclude if the activity of the TIM23-PAM complex is specifically affected in the absence of the Aac2 isoform. Given that only a minor change in the assembly state of the TIM23* complex was observed in the absence of Aac2 and that other AAC isoform(s) may overlap in their capacity to interact with the TIM23* complex, it is possible that the function of this subpopulation of the TIM23 translocase is not significantly perturbed in the absence of Aac2 alone. In this respect it is important to note that the double deletion of the genes encoding the Aac2 and Aac3 isoforms, or the AAC2 and SAL1 genes, results in a lethal phenotype (note the Δaac2,Δaac3 mutant is inviable under anaerobic conditions; Drgon et al., 1992; Chen, 2004). We propose here that the essential (and overlapping) role played by the Aac2, Aac3, and Sal1 proteins may be related to the functional integrity of the associated TIM23 complex, a complex required for cell viability.
In summary, we demonstrate here that the yeast Aac2 protein associates with the cytochrome bc1-COX-TIM23 supercomplex and this finding represents another example of the supercomplex organizational state of the mitochondrial OXPHOS complexes. The involvement of AAC proteins in human disease (e.g., autosomal-dominant progressive external ophthalmoplegia) and apoptosis has been well documented in the literature (Zoratti and Szabò, 1995; Bauer et al., 1999; Napoli et al., 2001; Zamzami and Kroemer, 2001; Chen, 2002; Komaki et al., 2002; Fontanesi et al., 2004). In these reports it has been indicated that the AAC proteins may play a structural role vital to the integrity of the mitochondrial membrane potential (Chen, 2002; Fontanesi et al., 2004). The described association of the AAC proteins with the cytochrome bc1-COX supercomplex, both H+-pumping complexes, and the TIM23 complex, a voltage-sensitive channel, may be therefore particularly important for our future understanding of the molecular basis of phenotypes associated with AAC dysfunction. We are currently evaluating whether other forms of the AAC proteins, Aac1 and Aac3, and the functionally related Sal1 protein, also display the capacity to assemble into the cytochrome bc1-COX-TIM23 supercomplex.
ACKNOWLEDGMENTS
We thank Dr. Jean Velours (Bordeaux, France) for the kind gift of the Atp4 antiserum and Drs. Walter Neupert and Kai Hell (Munich, Germany) for the Tim23, Tim17, Tim50, and Pam16/Tim16 antisera. We thank Dr. Xin Jie Chen (Syracuse, NY) for the kind gift of the Δaac2 strain and Dr. David Mueller (Chicago, IL) for the generous gift of the strain expressing the His-tagged F1β protein. We are grateful to Dr. Sonika Saddar for helpful discussions. This research was supported by funding from the U.S. Public Health Service Grant RO1GM61573 to R.A.S.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0402) on July 9, 2008.
REFERENCES
- Arnold I., Pfeiffer K., Neupert W., Stuart R. A., Schägger H. Yeast mitochondrial F1FO-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J. 1998;17:7170–7178. doi: 10.1093/emboj/17.24.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber L., Slotboom D. J., Kunji E. R. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents as demonstrated by differential affinity purification. J. Mol. Biol. 2007a;371:388–395. doi: 10.1016/j.jmb.2007.05.072. [DOI] [PubMed] [Google Scholar]
- Bamber L., Harding M., Monné M., Slotboom D. J., Kunji E. R. The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes. Proc. Natl. Acad. Sci. USA. 2007b;104:10830–10834. doi: 10.1073/pnas.0703969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A., Zambrano A., Tzagoloff A. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 2004;23:3472–3482. doi: 10.1038/sj.emboj.7600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.K.A., Schubert A., Rocks O., Grimm S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J. Cell Biol. 1999;147:1493–1501. doi: 10.1083/jcb.147.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekema E. J., Braun H. P. Supramolecular structure of the mitochondrial oxidative phosphorylation system. J. Biol. Chem. 2007;282:1–4. doi: 10.1074/jbc.R600031200. [DOI] [PubMed] [Google Scholar]
- Boumans H., Grivell L. A., Berden J. A. The respiratory chain in yeast behaves as a single functional unit. J. Biol. Chem. 1998;273:4872–4877. doi: 10.1074/jbc.273.9.4872. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner S., Everard-Gigot V., Stuart R. A. Su e of the yeast F1Fo-ATP synthase forms homodimers. J. Biol. Chem. 2002;277:48484–48489. doi: 10.1074/jbc.M209382200. [DOI] [PubMed] [Google Scholar]
- Chacinska A., et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 2005;120:817–829. doi: 10.1016/j.cell.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chen X. J. Induction of an unregulated channel by mutations in adenine nucleotide translocase suggests an explanation for human ophthalmoplegia. Hum. Mol. Genet. 2002;11:1835–1843. doi: 10.1093/hmg/11.16.1835. [DOI] [PubMed] [Google Scholar]
- Chen X. J. Sal1p, a calcium-dependent carrier protein that suppresses an essential cellular function associated With the Aac2 isoform of ADP/ATP translocase in Saccharomyces cerevisiae. Genetics. 2004;167:607–617. doi: 10.1534/genetics.103.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat C., Brunner S., Baumann F., Neupert W., Stuart R. A. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 2000;275:18093–18098. doi: 10.1074/jbc.M001901200. [DOI] [PubMed] [Google Scholar]
- Drgon T., Sabová L., Nelson N., Kolarov J. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett. 1991;289:159–162. doi: 10.1016/0014-5793(91)81059-h. [DOI] [PubMed] [Google Scholar]
- Drgon T., Sabová L., Gavurniková G., Kolarov J. Yeast ADP/ATP carrier (AAC) proteins exhibit similar enzymatic properties but their deletion produces different phenotypes. FEBS Lett. 1992;304:277–280. doi: 10.1016/0014-5793(92)80637-v. [DOI] [PubMed] [Google Scholar]
- Dudkina N. V., Eubel H., Keegstra W., Boekema E. J., Braun H. P. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc Natl Acad Sci USA. 2005;102:3225–3229. doi: 10.1073/pnas.0408870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina N. V., Heinemeyer J., Sunderhaus S., Boekema E. J., Braun H. P. Respiratory chain supercomplexes in the plant mitochondrial membrane. Trends Plant Sci. 2006;11:232–240. doi: 10.1016/j.tplants.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Bogucka K., Letko G., Küster U., Kunz W., Wojtczak L. Relationship between the energy cost of ATP transport and ATP synthesis in mitochondria. Biochim. Biophys. Acta. 1981;637:217–223. doi: 10.1016/0005-2728(81)90160-2. [DOI] [PubMed] [Google Scholar]
- Fontanesi F., Palmieri L., Scarcia P., Lodi T., Donnini C., Limongelli A., Tiranti V., Zeviani M., Ferrero I., Viola A. M. Mutations in AAC2, equivalent to human adPEO-associated ANT1 mutations, lead to defective oxidative phosphorylation in Saccharomyces cerevisiae and affect mitochondrial DNA stability. Hum. Mol. Genet. 2004;13:923–934. doi: 10.1093/hmg/ddh108. [DOI] [PubMed] [Google Scholar]
- Gallas M. R., Dienhart M. K., Stuart R. A., Long R. M. Characterization of Mmp37p, a Saccharomyces cerevisiae mitochondrial matrix protein with a role in mitochondrial protein import. Mol. Biol. Cell. 2006;17:4051–4062. doi: 10.1091/mbc.E06-04-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin P. D., Prescott M., Devenish R. J. F1FO-ATP synthase complex interactions in vivo can occur in the absence of the dimer specific subunit e. J. Bioenerg. Biomembr. 2005;37:55–66. doi: 10.1007/s10863-005-4128-8. [DOI] [PubMed] [Google Scholar]
- Gawaz M., Douglas M. G., Klingenberg M. Structure-function studies of adenine nucleotide transport in mitochondria. II. Biochemical analysis of distinct AAC1 and AAC2 proteins in yeast. J. Biol. Chem. 1990;265:14202–14208. [PubMed] [Google Scholar]
- Glerum D. M., Koerner T. J., Tzagoloff A. Cloning and characterization of COX14, whose product is required for assembly of yeast cytochrome oxidase. J. Biol. Chem. 1995;270:15585–15590. doi: 10.1074/jbc.270.26.15585. [DOI] [PubMed] [Google Scholar]
- Grigoriev S. M., Muro C., Dejean L. M., Campo M. L., Martinez-Caballero S., Kinnally K. W. Electrophysiological approaches to the study of protein translocation in mitochondria. Int. Rev. Cytol. 2004;238:227–274. doi: 10.1016/S0074-7696(04)38005-8. [DOI] [PubMed] [Google Scholar]
- Heinemeyer J., Braun H. P., Boekema E. J., Kouril R. A structural model of the cytochrome c reductase/oxidase supercomplex from yeast mitochondria. J. Biol. Chem. 2007;282:12240–12248. doi: 10.1074/jbc.M610545200. [DOI] [PubMed] [Google Scholar]
- Herrmann J. M., Fölsch H., Neupert W., Stuart R. A. In: Cell Biology: A Laboratory Handbook. Celis J. E., editor. San Diego: Academic Press; 1994. pp. 538–544. [Google Scholar]
- Klingenberg M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch. Biochem. Biophys. 1989;270:1–14. doi: 10.1016/0003-9861(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Ko Y. H., Delannoy M., Hullihen J., Chiu W., Pedersen P. L. Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. Biol. Chem. 2003;278:12305–12309. doi: 10.1074/jbc.C200703200. [DOI] [PubMed] [Google Scholar]
- Komaki H., Fukazawa T., Houzen H., Yoshida K., Nonaka I., Goto Y. A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmoplegia patients with mitochondrial DNA with multiple deletions. Ann. Neurol. 2002;51:645–648. doi: 10.1002/ana.10172. [DOI] [PubMed] [Google Scholar]
- Kutik S., Guiard B., Meyer H. E., Wiedemann N., Pfanner N. Cooperation of translocase complexes in mitochondrial protein import. J. Cell Biol. 2007;179:585–591. doi: 10.1083/jcb.200708199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marques I., Dencher N. A., Videira A., Krause F. Supramolecular organization of the respiratory chain in Neurospora crassa mitochondria. Eukaryot. Cell. 2007;6:391–2405. doi: 10.1128/EC.00149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashkevich G., Repetto B., Glerum D. M., Jin C., Tzagoloff A. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 1997;272:14356–14364. doi: 10.1074/jbc.272.22.14356. [DOI] [PubMed] [Google Scholar]
- McKenzie M., Lazarou M., Thorburn D. R., Ryan M. T. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J. Mol. Biol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Mick D. U., Wagner K., van der Laan M., Frazier A. E., Perschil I., Pawlas M., Meyer H. E., Warscheid B., Rehling P. Shy1 couples Cox1 translational regulation to cytochrome c oxidase assembly. EMBO J. 2007;26:4347–4358. doi: 10.1038/sj.emboj.7601862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. M., Puri N., Kabaleeswaran V., Terry C., Leslie A. G., Walker J. E. Ni-chelate-affinity purification and crystallization of the yeast mitochondrial F1-ATPase. Protein Expr. Purif. 2004;37:479–485. doi: 10.1016/j.pep.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Napoli L., et al. A novel missense adenine nucleotide translocator-1 gene mutation in a Greek adPEO family. Neurology. 2001;57:2295–2298. doi: 10.1212/wnl.57.12.2295. [DOI] [PubMed] [Google Scholar]
- Nury H., Dahout-Gonzalez C., Trézéguet V., Lauquin G., Brandolin G., Pebay-Peyroula E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 2005;579:6031–6036. doi: 10.1016/j.febslet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Nury H., Dahout-Gonzalez C., Trézéguet V., Lauquin G. J., Brandolin G., Pebay-Peyroula E. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annu. Rev. Biochem. 2006;75:713–741. doi: 10.1146/annurev.biochem.75.103004.142747. [DOI] [PubMed] [Google Scholar]
- Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D. M., Brethes D., di Rago J. P., Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay-Peyroula E., Dahout-Gonzalez C., Kahn R., Trézéguet V., Lauquin G. J., Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula E., Brandolin G. Nucleotide exchange in mitochondria: insight at a molecular level Curr. Opin. Struct. Biol. 2004;14:420–425. doi: 10.1016/j.sbi.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K., Gohil V., Stuart R. A., Hunte C., Brandt U., Greenberg M. L., Schägger H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Pierrel F., Bestwick M. L., Cobine P. A., Khalimonchuk O., Cricco J. A., Winge D. R. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 2007;26:4335–4346. doi: 10.1038/sj.emboj.7601861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddar S., Dienhart M. K., Stuart R. A. The F1Fo-ATP synthase complex influences the assembly state of the cytochrome bc1-cytochrome oxidase supercomplex and its association with the TIM23 machinery. J. Biol. Chem. 2008;283:6677–6686. doi: 10.1074/jbc.M708440200. [DOI] [PubMed] [Google Scholar]
- Saraste M. Okidative phosphorylation at the fin de siècle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Schägger H., Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim. Biophys. Acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985;43:339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Truscott K. N., Kovermann P., Geissler A., Merlin A., Meijer M., Driessen A. J., Rassow J., Pfanner N., Wagner R. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 2001;8:1074–1082. doi: 10.1038/nsb726. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system: isolation of nuclear and cytoplasmic mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. J. Bacteriol. 1975;122:826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan M., Wiedemann N., Mick D. U., Guiard B., Rehling P., Pfanner N. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr. Biol. 2006;16:2271–2276. doi: 10.1016/j.cub.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Wiedemann N., van der Laan M., Hutu D. P., Rehling P., Pfanner N. Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J. Cell Biol. 2007;179:1115–1122. doi: 10.1083/jcb.200709087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Zara V., Conte L., Trumpower B. L. Identification and characterization of cytochrome bc(1) subcomplexes in mitochondria from yeast with single and double deletions of genes encoding cytochrome bc(1) subunits. FEBS J. 2007;274:4526–4539. doi: 10.1111/j.1742-4658.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- Zoratti M., Szabò I. The mitochondrial permeability transition Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]