Abstract
The Caenorhabditis elegans teneurin ortholog, ten-1, plays an important role in gonad and pharynx development. We found that lack of TEN-1 does not affect germline proliferation but leads to local basement membrane deficiency and early gonad disruption. Teneurin is expressed in the somatic precursor cells of the gonad that appear to be crucial for gonad epithelialization and basement membrane integrity. Ten-1 null mutants also arrest as L1 larvae with malformed pharynges and disorganized pharyngeal basement membranes. The pleiotropic phenotype of ten-1 mutant worms is similar to defects found in basement membrane receptor mutants ina-1 and dgn-1 as well as in the mutants of the extracellular matrix component laminin, epi-1. We show that the ten-1 mutation is synthetic lethal with mutations of genes encoding basement membrane components and receptors due to pharyngeal or hypodermal defects. This indicates that TEN-1 could act redundantly with integrin INA-1, dystroglycan DGN-1, and laminin EPI-1 in C. elegans development. Moreover, ten-1 deletion sensitizes worms to loss of nidogen nid-1 causing a pharynx unattached phenotype in ten-1;nid-1 double mutants. We conclude that TEN-1 is important for basement membrane maintenance and/or adhesion in particular organs and affects the function of somatic gonad precursor cells.
INTRODUCTION
Teneurins are large transmembrane proteins that play important roles in cell signaling and cell adhesion (Tucker and Chiquet-Ehrismann, 2006; Tucker et al., 2007). Teneurins are phylogenetically conserved among metazoans and they were described in several species, including ten-1 in Caenorhabditis elegans (Drabikowski et al., 2005), ten-m/odz and ten-a in Drosophila (Baumgartner et al., 1994; Levine et al., 1994; Fascetti and Baumgartner, 2002; Rakovitsky et al., 2007), zebrafish (Mieda et al., 1999), and in chicken (Minet et al., 1999; Tucker et al., 2000; Tucker et al., 2001; Rubin et al., 2002) and mouse (Oohashi et al., 1999; Ben-Zur et al., 2000; Zhou et al., 2003). In vertebrates, the four teneurin paralogs were named teneurin-1 to -4, ten-m1 to -m4, or odz-1 to -4.
The extracellular domain of all teneurins is composed of eight tenascin-type EGF-like repeats, a region of conserved cysteines, and YD repeats that are also found in a few bacterial proteins (Minet and Chiquet-Ehrismann, 2000). The intracellular domain contains proline-rich stretches and putative tyrosine phosphorylation sites but is less conserved than the extracellular part and cannot be aligned in a linear way between the phyla. Teneurins are thought to interact in a homophilic manner (Oohashi et al., 1999; Rubin et al., 2002; Bagutti et al., 2003; Leamey et al., 2008) and to date, no other ligand has been identified.
The name “teneurins” refers to their high expression in the developing and adult nervous system (Mieda et al., 1999; Oohashi et al., 1999; Otaki and Firestein, 1999; Ben-Zur et al., 2000; Tucker et al., 2000; Rubin et al., 2002; Zhou et al., 2003). In the developing mouse cortex, all teneurins are expressed in distinctive gradients and may be required for neocortical patterning (Li et al., 2006). Several reports point out their role in the development of visual pathways. Leamey et al. (2008) have found that teneurins are up-regulated in visual versus somatosensory areas of the neocortex. Moreover, expression of different teneurins is largely nonoverlapping and can be found in interconnected regions of the developing visual system (Rubin et al., 1999, 2002; Kenzelmann et al., 2008; Leamey et al., 2008). For instance, teneurin-1 staining is found in the tectofugal pathway, and teneurin-2 is primarily expressed in the thalamofugal pathway. In addition, teneurins were shown to promote neurite outgrowth in vitro (Minet et al., 1999; Rubin et al., 1999) and in vivo (Leamey et al., 2008), suggesting an important function for teneurins in axon guidance and target recognition. Recently, the first vertebrate teneurin knockout was described (Leamey et al., 2007). Teneurin-3 regulates eye-specific patterning in the visual system, and the knockout mice show impaired binocular vision.
Beside prominent expression in the nervous system, teneurins are also found in nonneuronal tissues. They are expressed in alternating parasegments in the fly embryo, as well as in cardiac cells, muscle attachment sites, and the tracheal system in Drosophila (Baumgartner and Chiquet-Ehrismann, 1993; Baumgartner et al., 1994). In the chicken teneurins are found in limb buds, branchial arches, and somites (Tucker et al., 2000, 2001), and in C. elegans ten-1 is expressed in gonadal somatic cells, pharynx, and muscles (Drabikowski et al., 2005). Teneurin expression in each of these tissues is often associated with pattern formation and cell migration.
The in vivo function of teneurins is mainly inferred from studies of C. elegans and Drosophila mutants. Mutation of the fly ten-m gene causes embryonic lethality due to the fusion of adjacent denticle belts (Baumgartner et al., 1994; Levine et al., 1994). Moreover, defects in the ventral nerve cord, cardiac cells and eye patterning are found in late ten-m mutant embryos (Levine et al., 1994; Kinel-Tahan et al., 2007). Similar defects in cuticle and eye development have been observed for the second Drosophila teneurin gene, ten-a (Rakovitsky et al., 2007). In C. elegans, deletion in the ten-1 gene causes a pleiotropic phenotype, including gonad disorganization, nerve cord defasciculation, and defects in distal tip cell migration and axonal pathfinding (Drabikowski et al., 2005).
The single teneurin ortholog in C. elegans, ten-1, is under control of alternative promoters giving rise to two protein variants. The isoforms differ only in their intracellular domains. Their expression patterns are complex but mostly nonoverlapping: TEN-1 long (TEN-1L) is found mainly in the mesoderm, including pharynx, somatic gonad, and various muscles and neurons, and TEN-1 short (TEN-1S) is predominantly expressed in some hypodermal cells and in a subset of neurons (Drabikowski et al., 2005).
We report here the role of TEN-1 in gonadal basement membrane maintenance, as well as in epidermal and pharyngeal development. Mutation of the ten-1 gene leads to gonad rupture and sterility. Germ cell leakage from the gonads has also been reported for basement membrane mutants, e.g., integrin α ina-1, dystroglycan dgn-1, and laminin αB epi-1 (Baum and Garriga, 1997; Huang et al., 2003; Johnson et al., 2006). Furthermore, the genetic interactions between ten-1, ina-1, dgn-1, epi-1, and nid-1 suggest that teneurin, integrin, and dystroglycan have related and partly redundant functions in C. elegans development.
MATERIALS AND METHODS
General Methods and C. elegans Strains
C. elegans strains were maintained at 20°C as described (Brenner, 1974). The following strains were used in this study: wild-type N2, variety Bristol, CH120: cle-1(cg120) I, CB444: unc-52(e444) II, VC518: ten-1(ok641) III; TM0651: ten-1(tm651) III; NG39: ina-1(gm39) III; NG144: ina-1(gm144) III; CB189: unc-32(e189) III; CX2914: nDf16/dpy-17(e164) unc-32(e189) III; CH119: nid-1(cg119) V; CH121: dgn-1(cg121)/dpy-6(e14) unc-115(mn481) X. The tm651 deletion removes nucleotides R13F6: 3661-4550 of the ten-1 coding sequence.
The following GFP marker strains were used: RU7: kdEx7 [ten-1a::gfp]; RU97: ten-1(ok641) kdEx45 [F36A3, III]; JK2049: qIs19 [lag-2::gfp]; SS0747: bnIs1 [pie-1::GFP::PGL-1] (gift of Susan Strome, University of California, Santa Cruz, CA); IM253: urEx131 [lam-1::gfp] (gift of William Wadsworth, Robert Wood Johnson Medical School, Piscataway, NJ), CH1878: dgn-2(ok209) dgn-3(tm1092) dgn-1(cg121); cgEx308 [DGN-1::GFP] (gift of James Kramer, Northwestern University Medical School, Chicago, IL).
Double mutant worms were maintained as [ten-1(ok641);ina-1(gm144); kdEx45], [ten-1(ok641/+);nid-1(cg119)], [ten-1(ok641);dgn-1(cg121/+); kdEx45] or [ten-1(ok641/+);dgn-1(cg121); cgEx308] strains and genotyped by PCR for the phenotypic analysis.
Constructs and Plasmids
The translational Pten-1a::GFP::TEN-1L minigene reporter construct was generated by cloning SpeI-HindIII cDNA fragment and HindIII-XhoI genomic fragment of TEN-1 long variant into p123T vector (Mo Bi Tec, Goettingen, Germany). The following restriction sites were introduced into the primers: SpeI and XhoI flanking the ten-1 coding sequence, SacII at the 5′ end of the ten-1a promoter, and ApaI downstream of the 3′ UTR.
The long intracellular domain, transmembrane domain, and a short fragment of the extracellular part were amplified using 5′-AACAGTCTACCGAATCCCAACC-3′ and 5′-ATAACTAGTATGTTCCAGCACAGGTAAACTACCACG-3′ primers and cDNA from mixed stage N2 worms as a template. For the extracellular domain of ten-1 we used 5′-GCTGAAATACCCACTCGCCAGC-3′ and 5′-ATCTCGAGCTATTCAGATTTTCGGAACTTCC-3′ primers and R06H12 cosmid as a template. The sequence encoding green fluorescent protein (GFP) was amplified from pPD117.01 vector and its NcoI site was mutated to CCTTGG. GFP was fused by PCR to the N-terminus of the ten-1 cDNA fragment, which was cloned into SpeI-NcoI sites of ten-1 minigene. Hemagglutinin (HA) tag was added at the C-terminus of ten-1 coding sequence by PCR and cloned into HpaI-XhoI sites. The Pten-1a::GFP::TEN-1L construct contained 4235 base pairs of the ten-1a promoter and a 512-base pair sequence downstream of the stop codon. PCR fragments were generated with Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA).
Transgenic Animals
Transgenic lines were generated as previously described (Mello et al., 1991). The Pten-1a::GFP::TEN-1L plasmid was injected into ten-1(ok641) mutant worms. Injections of GFP::TEN-1 minigene at low concentration (5 ng/μl) resulted in a very weak GFP fluorescence, mainly in the nervous system. Therefore, we injected the worms with high concentrations of the transgene (40 ng/μl) and obtained several lines giving stronger GFP fluorescence. We used pRF4 [rol-6] as a coinjection marker. This resulted in the line RU152: kdEx121 [Pten-1a::GFP::TEN-1L] used in this study.
RNA Interference
RNA-mediated interference (RNAi) was performed as described (Kamath and Ahringer, 2003). The K08C7.3 RNAi clone was obtained from the Ahringer feeding library. Wild-type and ten-1(ok641) synchronized L4 hermaphrodites were placed on RNAi plates and grown at 15°C for 72 h. Single adult worms were placed on fresh RNAi plates and allowed to lay eggs for 24 h. These plates were examined for 3 d to determine embryonic lethality and postembryonic phenotypes.
Immunostaining of C. elegans Larvae
C. elegans larvae were prepared as previously described (Finney and Ruvkun, 1990). Fixed animals were blocked overnight at 4°C in PBS containing 0.1% Triton X-100 (Triton) and 10% goat serum. Samples were incubated with an antibody against collagen IV LET-2 (NW68, kind gift of James Kramer) overnight at 4°C, washed in PBS containing Triton, and incubated with fluorescein conjugated goat anti-rabbit secondary antibody overnight at room temperature. Finally, fixed larvae were washed in PBS containing Triton and Hoechst, followed by PBS alone.
Electron Microscopy
Worms were washed in M9 and anesthetized in 8% ethanol in M9 for 5 min. They were placed in a fixative (2.5% glutaraldehyde, 1% paraformaldehyde in 0.1M sucrose, and 10 mM PBS, pH 7.4), cut open with a needle at both anterior and posterior ends, and fixed for 2 h. Worms were embedded in 2% agarose, cut into small blocks, and washed three times in PBS. Subsequently, pieces were fixed with a second solution (1% osmium tetroxide, 1.5% potassium ferrocyanide in PBS) for 2 h and washed three times in water. Worms were stained with 1% uranyl acetate for 1 h. Samples were dehydrated in ethanol (10 min in 50% ethanol, 10 min in 70% ethanol, 10 min in 90% ethanol, and 10 min in 100% ethanol) and acetone (10 min). Blocks with worms were embedded in Epon resin (Fluka, Buchs, Switzerland): first in Epon-acetone (1:1) for 1–2 h and then in pure resin for 2–4 h. Samples polymerized for 24–48 h at 60°C and in 60-nm sections were prepared with Ultracut E. Sections were stained in uranyl acetate for 60 min and then 2 min in Millonig's lead acetate stain. Pictures were taken on Philips Morgagni 80 KV microscope (Eindhoven, The Netherlands).
Phenotypic Analysis
Young adult hermaphrodites were placed on separate plates and allowed to lay eggs for 24 h. The progeny were analyzed for embryonic and postembryonic phenotypes: lethality, larval arrest, sterility, and bursting at the vulva.
Time Course of Germline Development and Basement Membrane Breakdown
Synchronized, starved L1 larvae carrying the GFP::PGL-1 marker were placed on bacteria plates. We scored the number of germ cells in 20 worms for each genotype at 0, 8, 12, 16, and 20 h. For the study of basement membrane integrity, we used synchronized worms carrying the LAM-1::GFP marker and analyzed 50–62 worms for each developmental stage.
Microscopy
Animals were mounted on 2% agarose pads in a drop of M9 buffer containing 25 mM sodium azide. Differential interference contrast (DIC) and fluorescence images were acquired with Z1 microscope (Zeiss, Jena, Germany) and AxioCam Mrm camera (Zeiss) using 63×/1.4 NA Plan-Apochromat objective (Zeiss) and AxioVision software.
RESULTS
Both ten-1(ok641) and ten-1(tm651) Are Functional Null Alleles
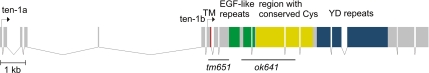
In our previous study we described the ten-1 mutation, ok641, that carries an in-frame 2130-base pair deletion removing four EGF-like repeats and a large part of the conserved cysteines region (Drabikowski et al., 2005). We now obtained another allele, tm651, lacking 890 base pairs and introducing a frameshift into the ten-1 coding sequence (Figure 1). This deletion results in a loss of the transmembrane domain and the entire extracellular part. Therefore, tm651 is most likely a null allele. Because phenotypes of both ten-1 mutants show similar penetrance (Table 1), we assume that ok641 represents a functional null allele as well.
Figure 1.
Genomic organization of ten-1 gene and location of tm651 and ok641 deletions. Exons are depicted as boxes and introns are shown as lines. Expression of ten-1 is regulated by alternative promoters: ten-1a and ten-1b, resulting in two type II transmembrane protein variants differing in the length of their intracellular domain. Fragments of exons encoding different protein domains are labeled as follows: red, single transmembrane domain, green, EGF-like repeats in two groups, yellow, region of conserved cysteines, and blue, stretch of YD repeats. Black horizontal lines show the regions deleted in two ten-1 mutants: tm651 and ok641.
Table 1.
Phenotypes of ten-1 deletion mutants
| Genotype | Embryonic lethality (%) | Larval arrest (%) | Sterile and/or vulva defects (%) | Fertile adults (%) | n |
|---|---|---|---|---|---|
| Wild type | 0.9 | 0 | 0 | 99.1 | 321 |
| ten-1(tm651) | 5.7 | 31.9 | 17.4 | 45.1 | 386 |
| ten-1(ok641) | 6.4 | 32.1 | 16.7 | 44.8 | 346 |
| ten-1(ok641), kdEx121 | 1.2 | 5.5 | 7.2 | 86.1 | 165 |
To confirm this hypothesis, we created heterozygous worms carrying nDf16 deficiency in trans to tm651 or ok641 and investigated whether the mutant phenotypes became aggravated after complete removal of one copy of the ten-1 gene. The ok641/nDf16 and tm651/nDf16 worms displayed a similar range of defects to ok641 and tm651 homozygous animals, and the values observed were very close to those calculated under the assumption of ten-1 mutants being null alleles (Table 2).
Table 2.
Embryonic lethality and larval arrest phenotypes appearing in the progeny of nDf16/ten-1 unc-32 transheterozygotes
| Genotype | Embryonic lethality (%) | Larval arrest (%) | Adults: total (%) | % Unc in adult worms | n |
|---|---|---|---|---|---|
| nDf16/ten-1(tm651) unc-32(e189) | 29.7 | 27.2 | 43.1 | 32.1 | 492 |
| nDf16/ten-1(ok641) unc-32(e189) | 33.5 | 22.6 | 43.9 | 30.1 | 310 |
| Expected value for nDf16/ten-1a | 29.5 | 24.0 | 46.5 | 33.3 |
a The calculated ratio of phenotypes expected if the ten-1 mutants are null mutants.
These data and the fact that ok641 and tm651 deletions affected protein regions that are common to both TEN-1 isoforms, suggested that there was no functional TEN-1 present in any of the ten-1 mutants.
Gonads of ten-1 Mutant Worms Burst Early in Development
Previous studies demonstrated that TEN-1 plays an important role in gonad development and function (Drabikowski et al., 2005). Homozygous ten-1(ok641) worms are viable, but 15–20% are sterile or burst-through-the-vulva due to germ cell leakage in the middle of the gonad. Occasionally, gonads disintegrate completely and germ cells float in the pseudocoelom. We could rescue gonadal and vulval defects by expression of the kdEx121 transgene encoding the long teneurin isoform under its own promoter (Table 1).
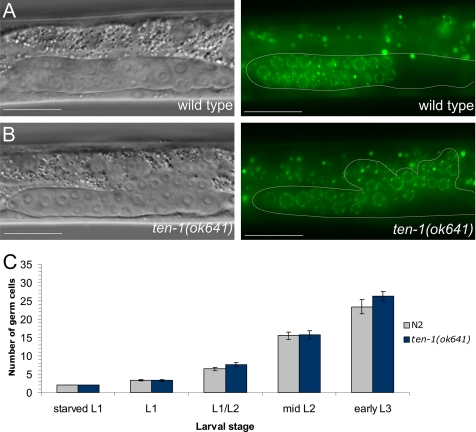
To determine the basis and the developmental stage of gonad bursting, we performed a time course experiment of germ cell proliferation in the early gonads of ten-1(ok641) mutants. We used worms carrying a P-granule GFP marker to distinguish between germ cells and somatic gonad precursor cells. Interestingly, we found that germ cells were released from the gonads of ten-1 mutant already at the early L3 stage (Figure 2B). At the same time point, there were no germ cells present around the developing somatic gonad primordium in the wild-type worms (Figure 2A). A sharp DIC boundary surrounding the gonad was visible in the wild type as well as a large part of ten-1(ok641) gonad (Figure 2, A and B) but absent on the dorsal side of the mutant gonad, where the germ cells leaked out into the pseudocoelom. Gonad bursting was not the result of germline overproliferation causing increased pressure on the gonadal basement membrane (BM), because we did not find any difference in the number of germ cells between wild-type and ten-1 mutants at this stage (Figure 2C).
Figure 2.
Germ cells are released from the early gonad of ten-1(ok641) mutant through the central break. Germ cell number and localization were evaluated using the P-granule marker pie-1::GFP::PGL-1. (A) Wild-type L3 gonad. The somatic gonadal primordium forms in the middle of the gonad, and germ cells fill the two gonad arms (only one arm is shown). (B) Ruptured gonadal primordium of a ten-1(ok641) L3 larva. Germ cells are released into the body cavity and localize in the vicinity of the developing somatic gonad primordium. (C) Time course of germline development in wild-type animals and ten-1(ok641) mutants. There is no germline overproliferation in the early gonads of the ten-1(ok641) mutant. Scale bar, 20 μmm.
Gonadal Basement Membrane Is Not Maintained in the ten-1 Mutant
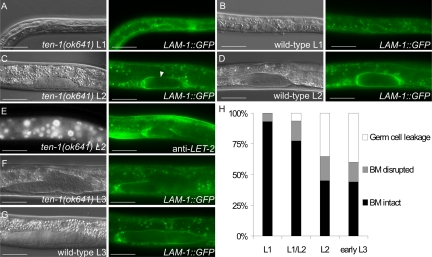
Bursting of the early gonads in the ten-1 mutant suggested that mutant worms have defects in BM formation or maintenance. Therefore, we examined the organization of the BMs in the ten-1(ok641) worms using a laminin-μb LAM-1::GFP marker and an anti-collagen IV antibody that label most BMs in worms.
At hatching, wild-type and the majority of ten-1 mutant gonad primordia were compact and completely surrounded by laminin (Figure 3, A and B). At the L2 stage the laminin layer surrounding the developing gonad of the mutants appeared to get thinner at the dorsal side, but germ cells did not lose contacts and gonads kept their tubular shape, similarly to wild-type (Figure 3, C and D). As the gonadal precursor cells divided, a discontinuity appeared in the ten-1(ok641) gonadal BM that could be seen by a lack of laminin as well as of collagen IV LET-2 (Figure 3, C and E). In L3 larvae germ cells were released in the center of the mutant gonad, where there was no laminin-GFP detectable. Gonad disruption appeared always on the dorsal side, whereas gonad arms were normally covered with BM (Figure 3F). In wild-type animals the gonads remained completely ensheathed by a BM (Figure 3G). A time course of the appearance and the penetrance of BM defects in young ten-1(ok641) larvae is summarized in Figure 3H.
Figure 3.
The basement membrane breaks on the dorsal side of the ten-1(ok641) gonads. Basement membranes were visualized by the LAM-1::GFP marker (A–D and F–G) and the anti-LET-2 immunostaining (E). The ten-1(ok641) L1 gonad (A), wild-type L1 (B), and L2 (D) gonads are uniformly covered by laminin. (C) In the ten-1(ok641) mutant, the gonadal basement membrane becomes thinner or fails to assemble correctly (arrowhead) at the L2 stage. (E) Lack of gonadal BM on the dorsal side of ten-1(ok641) L2 gonad is visualized by collagen IV immunostaining with anti-LET-2. (F) There is no laminin present in the center of the ten-1(ok641) L3 gonad. The basement membrane is absent completely, and germ cells are released. (G) The wild-type L3 gonad is entirely covered by laminin. (H) Time-course analysis of gonadal BM integrity in ten-1(ok641) worms carrying the LAM-1::GFP marker. Scale bar, 20 μmm.
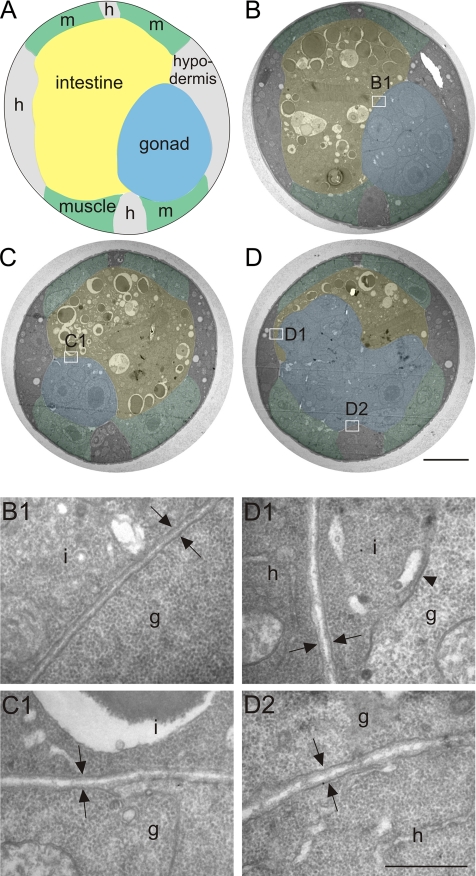
Furthermore, we compared the BM ultrastructure in wild-type and ten-1 mutant worms using transmission electron microscopy of thin sections of L3 larvae. Wild-type gonads were completely ensheathed by BM, which appeared as a thin mesh of extracellular material along the plasma membranes (Figure 4, B and B1). In ten-1(ok641) worms, gonads had a round shape and were entirely covered by BM in sections localized distally from the break (Figure 4C and C1). However, in the midbody region no BM was present on the dorsal side of the broken gonad and germ cells invaded the intestine (Figure 4, D and D1). In contrast, the BMs on the ventral side of the burst gonad as well as the BMs between the intestine and the hypodermis showed a wild-type ultrastructure (Figure 4, D and D2). Moreover, we did not find any whorls or clumps of extracellular material that might suggest a general defect in BM organization. Such a phenotype was described for some BM mutants such as epi-1, lam-1, or dig-1 (Huang et al., 2003; Benard et al., 2006; Kao et al., 2006).
Figure 4.
Basement membrane ultrastructure in ten-1 mutant worms. Schematic cross-section through the midbody of wild-type worm (A). Transmission electron microscopy sections of a wild-type L3 (B) and a ten-1(ok641) mutant (C and D). Tissues are labeled as follows: blue, gonad; yellow, intestine; green, muscles; and gray/unlabeled, hypodermis. Enlargements (B1–D2) are marked on the cross-sections (B–D) with white rectangles. Morphology of wild-type BMs at the boundaries between gonad and intestine (B1, arrows). The gonadal and intestinal BM of the ten-1 mutant appears wild-type in a section 2 μmm distant from the central break (C1, arrows). In the midbody region, the mutant gonad breaks on its dorsal side, and there is no BM present between germ cells and intestine (D1, arrowhead). However, BMs between intestine and hypodermis (D1, arrows) or ventral gonad and hypodermal ridge (D2, arrows) have a normal ultrastructure. Scale bar, 5 μmm (A–D) and 500 nm (B1–D2).
In summary, the gonadal BM in the ten-1(ok641) hermaphrodites was properly assembled at hatching but was not maintained later in development. The localized BM deficiency could result from defects in BM assembly, stability, or protein expression.
Gonadal Defects of ten-1 Mutants Are Similar to Those Found in the Dystroglycan dgn-1, Integrin ina-1, and Laminin epi-1 Mutants
Laminins are secreted proteins that play fundamental roles in BM formation and function (Previtali et al., 2003; Miner and Yurchenco, 2004). EPI-1 is one of two laminin μa chains found in the C. elegans genome. Both C. elegans laminin isoforms are broadly distributed among BMs, but the gonadal BM contains the EPI-1 isoform only (Huang et al., 2003). Dystroglycan and integrins, two cell surface receptors interacting with laminin, are required for BM assembly, adhesion, and signal transduction (Bokel and Brown, 2002; Higginson and Winder, 2005). In C. elegans, gonadal epithelialization defects were reported for the dystroglycan dgn-1(cg121) worms and laminin μa chain epi-1 mutants (Huang et al., 2003; Johnson et al., 2006). Gonads of integrin μa chain ina-1 mutants are oddly sized and show germ cell leakage, but the cause of the defects remains unknown (Baum and Garriga, 1997).
Gonads of dgn-1 mutants and epi-1(RNAi) worms were variably misshapen (Figure 5, C and E), burst during development, and led to worm sterility. Early gonads of ina-1(gm39) worms hardly ever burst (Figure 5F) and rather seemed to be swollen in the center. However, at the L4 stage ina-1 mutant gonads were clearly ruptured, and the germ cells clustered around the developing vulva (Figure 5G), similarly to ten-1(ok641) gonads (Figure 5H). Gonads of adult worms carrying the weaker ina-1 allele, gm144, had enlarged arms but we did not observe any germ cell leakage (unpublished data).
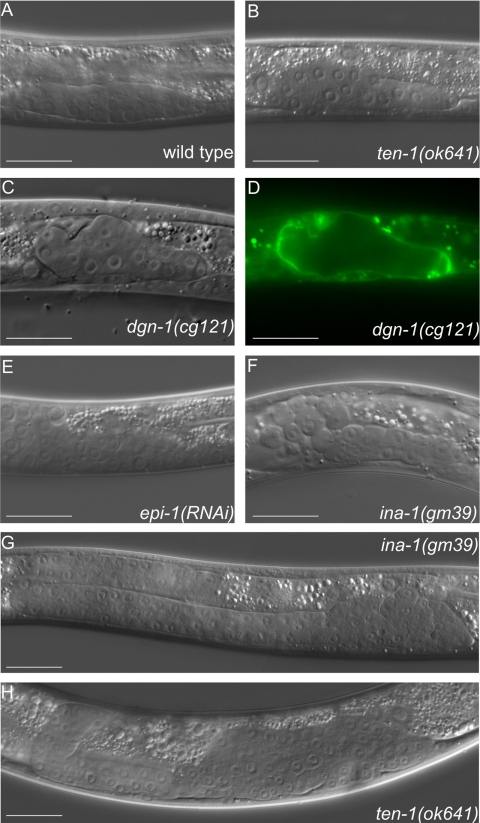
Figure 5.
Misshapen gonadal primordia are found in several basement membrane mutants, i.e., dystroglycan dgn-1, integrin ina-1, and laminin epi-1(RNAi) worms. DIC pictures of early gonads in wild type (A), ten-1(ok641) (B), dgn-1(cg121) (C), and the corresponding LAM-1::GFP pattern (D), epi-1(RNAi) (E), ina-1(gm39) L2 larva (F), ina-1(gm39) L4 larva (G), and ten-1(ok641) L4 larva (H). Mutant gonads do not form a tube-like structure but grow into a disorganized mass. Scale bar, 20 μmm.
We analyzed the organization of the laminin network surrounding the developing gonad in dgn-1 mutants using the LAM-1::GFP marker. Although the DIC pictures of ten-1 and dgn-1 mutants appeared similar, dgn-1(cg121) hermaphrodite gonads did not have any localized breaks as did the ten-1(ok641) gonads. In contrast, the dgn-1 mutant gonads were generally disorganized, and LAM-1::GFP seemed to be more diffuse throughout the gonadal surface in comparison to ten-1 mutant gonads (Figure 5D). Nevertheless, gonadal defects described for dgn-1(cg121), ina-1(gm39), and epi-1(RNAi) worms resembled the defects that we observed in the ten-1 mutants (Figure 5B), suggesting that TEN-1 could be involved in gonadal BM maintenance, together with laminin receptors INA-1 and DGN-1.
In early gonads TEN-1 was found to be expressed in the somatic gonad founder cells Z1 and Z4 (Drabikowski et al., 2005). Also their descendants, the somatic gonad precursor cells (SGPs) during the L2 stage express TEN-1 (Figure 6, A and B). We found that Z1 and Z4 cells were often displaced from the tips of the early L1 gonads in ten-1(ok641) worms (Figure 6C). In almost 20% of the mutants, Z1 and/or Z4 cells interdigitated between germ cell precursors Z2 and Z3, or sometimes one of the SGPs was lost. Because the SGPs are required for the deposition of the gonad BM, the BM defects observed in the ten-1 mutant worms might be due to the inability of the SGPs to form an intact epithelial layer around the gonad primordium. Interestingly, similar gonad epithelialization defects were reported for dgn-1 and epi-1 mutant worms (Johnson et al., 2006).
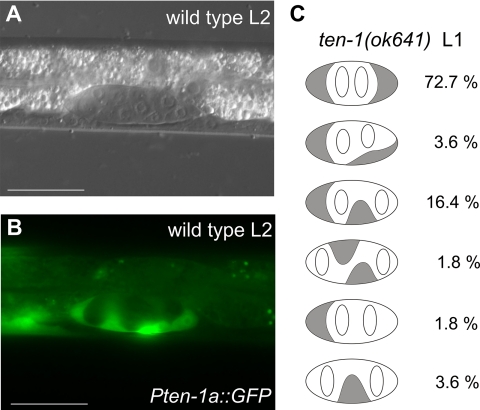
Figure 6.
Teneurin is expressed in somatic cells of the early gonads and SGPs are mislocalized in the L1 gonads of ten-1 mutants. Expression from the upstream promoter of ten-1 is found in the SGPs of L2 gonads in wild-type worms (A and B). In C we present a schematic representation of the position of the Z1 and Z4 (gray shading) and Z2 and Z3 (white) cells in gonads of ten-1(ok641) L1 larvae carrying the lag-2::gfp marker (n = 55). We found that Z1 and Z4 cells are often mispositioned, and the percentage of animals showing the observed patterns is indicated to the right. Scale bar, 20 μmm.
ten-1 Is Synthetic Lethal with dgn-1, ina-1, epi-1, and nid-1
The similar gonadal phenotypes of ten-1, dgn-1, and ina-1 mutants and epi-1(RNAi) worms suggested that TEN-1 could act in a parallel pathway and have a partly redundant function to dystroglycan and/or integrin receptors. To asses the interaction between ten-1 and genes encoding various BM components, we constructed double mutant combinations. In the crosses we used ten-1(ok641) and dgn-1(cg121) null alleles, the weak ina-1 hypomorphic allele gm144, and an RNAi approach in the case of epi-1.
To analyze the genetic interaction network of ten-1 further, we included additional genes encoding BM proteins, namely nidogen nid-1, perlecan unc-52, and collagen XVIII cle-1. C. elegans nidogen deletion does not affect BM assembly (Kang and Kramer, 2000), but nid-1 mutants show defects in neuromuscular junction organization (Ackley et al., 2003) and axonal tract positioning (Kim and Wadsworth, 2000). Interestingly, the nid-1(cg119) null mutant was found to be synthetic lethal with dgn-1 as a result of pharyngeal defects (J. M. Kramer, personal communication). Mutation e444 in the perlecan unc-52 gene causes progressive paralysis in worms as well as gonad disorganization and germ cell release into the body cavity (Gilchrist and Moerman, 1992). Loss-of-function mutation in the collagen cle-1 gene leads to cell migration and axon guidance defects. Some cle-1(cg120) mutant larvae are unable to pump and arrest at the L1 stage with misshapen pharynges (Ackley et al., 2001).
Interestingly, we observed more severe phenotypes in several double mutants than in any single mutant alone (Table 3). Synthetic lethality was found in ten-1(ok641);dgn-1(cg121), ten-1(ok641);ina-1(gm144), ten-1(ok641);nid-1(cg119) double mutants, and ten-1(ok641);epi-1(RNAi) worms. Lack of dystroglycan or nidogen in the ten-1 mutant background led to developmental arrest during late embryogenesis or L1 larval stage in almost 100% of worms. Double-mutant larvae were translucent suggesting a feeding defect. Morphological defects found in epi-1 deficient worms (Figure 7C) were enhanced by ten-1 deletion. More than 90% of ten-1(ok641);epi-1(RNAi) animals arrested during embryogenesis or as early larvae and showed dramatic disorganization of developing tissues (Figure 7E). Moreover, ten-1;ina-1 mutants showed severe morphological defects not found in any single mutant alone (Figure 7, B and D), and nearly 100% of double mutant worms arrested as disorganized embryos or L1 larvae (Figure 7F).
Table 3.
ten-1 is synthetic lethal with dgn-1, ina-1, epi-1, and nid-1
| Genotype | Embryonic lethality | Larval arrest | Sterile and/or vulva defectsa | Fertile adults | n |
|---|---|---|---|---|---|
| Wild type | 0.9 | 0 | 0 | 99.1 | 321 |
| Ten-1(ok641) | 6.4 | 32.1 | 16.7 | 44.8 | 346 |
| dgn-1(cg121) | 5.4 | 2.2 | 92.4 | 0 | 92 |
| Ten-1(ok641);dgn-1(cg121) | 14.0 | 84.2 | 1.8 | 0 | 57 |
| Ina-1(gm144) | 10.0 | 30.6 | 23.5 | 35.9 | 170 |
| ten-1(ok641);ina-1(gm144) | 12.9 | 85.7 | 1.4 | 0 | 70 |
| nid-1(cg119) | 4.2 | 7.3 | 0.3 | 88.2 | 765 |
| ten-1(ok641);nid-1(cg119) | 34.7 | 65.2 | 0 | 0 | 88 |
| epi-1(RNAi) | 17.1 | 29.5 | 53.4 | 0 | 442 |
| ten-1(ok641);epi-1 (RNAi) | 48.5 | 44.2 | 7.3 | 0 | 293 |
| cle-1(cg120) | 0.7 | 0.7 | 1.1 | 97.5 | 283 |
| ten-1(ok641);cle-1(cg120) | 3.4 | 24.5 | 23.1 | 49.0 | 147 |
| unc-52(e444) | 3.5 | 1.5 | 5.6 | 89.4 | 198 |
| ten-1(ok641);unc-52(e444) | 4.8 | 21.5 | 36.5 | 37.2 | 293 |
Percentage of wild-type and mutant worms (single and double mutants) showing the following phenotypes: embryonic lethality, larval arrest, sterility or vulval defects, and wild-type fertile adults.
a The “Vulva defects” category includes protruding vulva and bursting-at-the-vulva phenotypes.
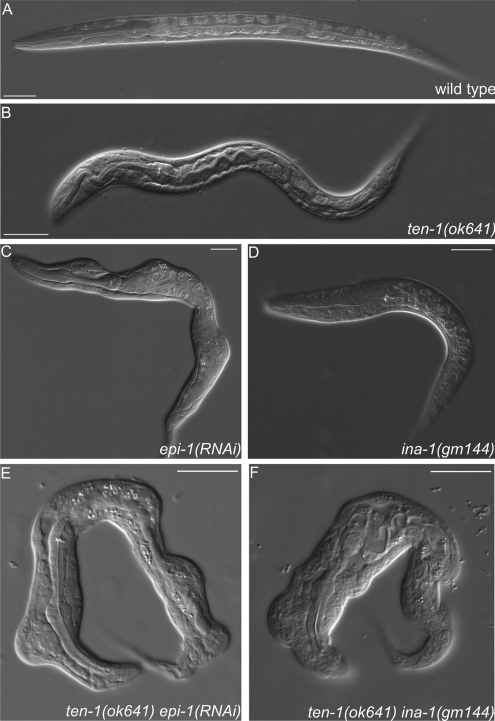
Figure 7.
Morphological defects found in epi-1(RNAi) worms, ten-1(ok641); epi-1(RNAi) animals, and ten-1; ina-1 double mutants. Wild-type (A) and ten-1(ok641) L1 larvae (B). epi-1-depleted worms are often misshapen, but defects are relatively mild (C). Arrested larva of ina-1(gm144) mutant (D). Morphological defects of epi-1(RNAi) worms were enhanced by ten-1(ok641) deletion and caused deformation of the entire body in the arrested larvae (E). Similar defects were found in ten-1(ok641);ina-1(gm144) double mutants (F). Severity and penetrance of the defects were greatly enhanced in the double mutants compared with single mutants. Scale bar, 20 μmm.
In contrast, mutations in unc-52 or cle-1 did not cause synthetic lethality in the ten-1 mutant background. These two mutations did not enhance embryonic lethality, larval arrest, or sterility of the ten-1(ok641) worms. However, we cannot exclude that unc-52 and cle-1 interact genetically with ten-1 in other processes, such as axon guidance or distal tip cell migration.
Teneurin Functions with Nidogen and Dystroglycan in Pharynx Development
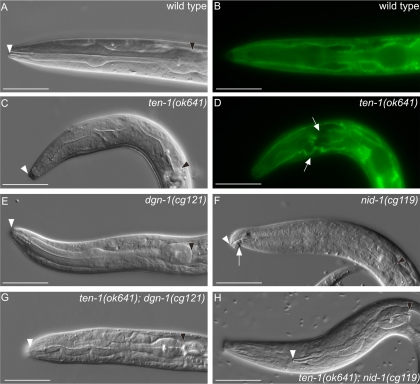
Because larval arrest was significantly increased in several double mutants, we decided to investigate the phenotypes of the starved L1 larvae of ten-1;dgn-1 and ten-1;nid-1 double mutants, suspecting that these three proteins could have an important role in pharyngeal morphogenesis. This hypothesis was supported by the fact that the expression of the long TEN-1 isoform rescued the larval arrest phenotype of ten-1 mutants (Table 1) and GFP::TEN-1L was detectable in the developing pharynx (Figure 8, A and B) until adulthood (Figure 8, C and D). As 30% of ten-1 single mutant worms arrest as L1 translucent larvae, we examined their pharyngeal defects. The wild-type foregut is a short tube, with two bulbs, surrounded by a thick BM (Figure 9, A and B). As viewed by DIC microscopy, ten-1–arrested larvae had variably misshapen pharynges, and the outline of the pharynx was often barely visible (Figure 9C). In addition, we examined the pharyngeal BM organization with the LAM-1::GFP marker and found that it seemed to be disordered and missing in some parts of the pharynx (Figure 9D).
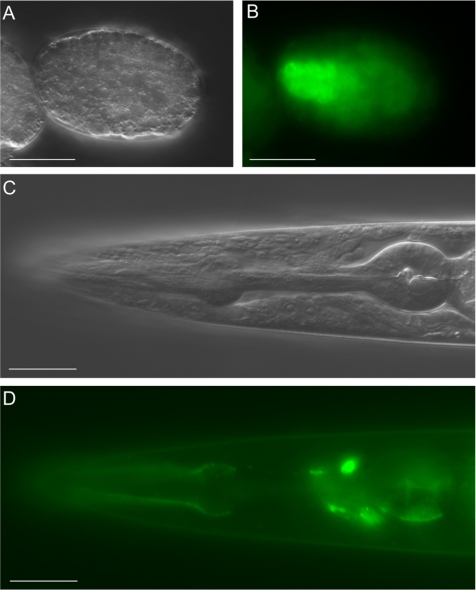
Figure 8.
The long TEN-1 isoform is expressed in the developing and adult pharynx. The GFP::TEN-1 transgene (kdEx121) is expressed in the developing pharynx of the early embryo (A and B) and outlines the adult pharynx (C and D). Expression of the kdEx121 is also found in some head neurons (D). Scale bar, 20 μm.
Figure 9.
Pharyngeal defects in ten-1, nid-1, and dgn-1 single and double mutants. Pharynx morphology of L1 larva is shown. LAM-1::GFP marker labels the pharyngeal basement membrane. Wild-type pharynx is outlined by a sharp DIC boundary visible by DIC microscopy (A). Basement membrane organization in the wild-type larva visualized by LAM-1::GFP (B). Arrested larvae of ten-1(ok641) mutant have misshapen pharynges and the pharyngeal outline is invisible on DIC pictures (C). In the ten-1 mutant, the basement membrane around the pharynx is disorganized or missing in some parts (arrows) (D). The pharynx of the dgn-1 mutant worms shows no obvious defects (E). Arrested larvae of nid-1 mutants have sometimes bent pharynges (arrow) (F) or their pharynges do not attach to the hypoderm (similar to the double mutant shown in H). Variably misshapen pharynges were found in the ten-1;dgn-1 double mutants (G). An unattached pharynx (Pun) phenotype observed in ten-1;nid-1 double mutants (H). White arrowheads mark the anterior and black arrowheads posterior ends of the pharynges. Scale bar, 20 μm.
In contrast to ten-1 mutant worms, only a low percentage of dgn-1 and nid-1 single mutants arrested during larval stages. Pharynges of dgn-1 larvae showed mostly wild-type appearance (Figure 9E), whereas the few nid-1 arrested larvae had a bend in the anterior-most part of their foregut (Figure 9F). Another phenotype found at low penetrance in the nid-1 single mutant was pharynx unattached (Pun), where the pharyngeal epithelium did not connect to the arcade cells of the hypoderm.
Removal of dgn-1 in the ten-1 mutant background enhanced the defects found in the ten-1 single mutant and double mutants of ten-1;dgn-1 arrested as larvae with their pharynges variably misshapen (Figure 9G). Interestingly, ten-1;nid-1 double mutants arrested as larvae that were unable to feed because their pharynges were not attached to the lips (Figure 9H).
In summary, our data suggest that ten-1 and dgn-1 act redundantly in pharyngeal morphogenesis and/or function. Moreover, both ten-1 and dgn-1 caused synthetic lethality in the nid-1 mutant background, implying an important role for these two receptors in the process of pharyngeal attachment.
DISCUSSION
Function of TEN-1 in Somatic Gonad Precursor Cells
We found that TEN-1 is essential for the maintenance of the BM early in development of the gonads in C. elegans. The BM surrounding the gonad was formed properly at hatching but during larval development ruptured at a very specific location on the dorsal side in the middle of the gonad. The ten-1a promoter is active in the SGPs of L1-L2 larvae and RNAi specific for the TEN-1 long variant is known to cause gonadal disorganization (Drabikowski et al., 2005). Therefore, SGPs may play an important role in extracellular matrix production or BM assembly by expression of specific receptors that organize extracellular matrix proteins provided by adjacent tissue. Such a mechanism has been described for several BM proteins. Graham et al. (1997) showed that type IV collagen is assembled on tissues that do not express it themselves, and they postulated that these tissues express receptors that facilitate collagen IV assembly. Another example is fibulin-1, which is secreted by the intestine and deposited on the gonadal surface (Muriel et al., 2005). Also in the case of laminin isoforms it was suggested that their differential distribution is at least partly based on differential assembly mediated by cell surface receptors (Huang et al., 2003). TEN-1 could be a novel receptor promoting BM assembly in the gonad. Another possibility is that teneurin is essential for SGPs polarization, adhesion, or migration. We showed that the position of Z1 and Z4 cells is often altered in the early gonads of ten-1 mutants. This could indicate that TEN-1 plays an important role in SGPs at the early stages of gonad epithelialization and that gonadal BM discontinuity is the consequence of somatic cell mispositioning.
Teneurin Acts Redundantly with BM Receptors Integrin and Dystroglycan
Mutants in the ten-1, ina-1 and dgn-1 genes share several phenotypic features, including gonad disorganization, protruding vulva, defasciculation of the ventral nerve cord, distal tip cell migration, and axonal guidance defects (Baum and Garriga, 1997; Drabikowski et al., 2005; Johnson et al., 2006; Meighan and Schwarzbauer, 2007). Double mutants between ten-1, ina-1, and dgn-1 showed synergistic genetic interaction implying that these three genes act in similar developmental processes and have partly redundant function. However, the mechanism of teneurin signaling remains unclear. Related roles of these receptors in gonad development could not be directly assessed because of functional redundancy in other developmental processes, i.e., pharyngeal or hypodermal morphogenesis.
Although dgn-1 mutants do not show any obvious pharyngeal defects, arrested larvae of ten-1;dgn-1 worms were translucent with misshapen pharynges. This suggests that there is compensation between ten-1 and dgn-1 in pharynx development and function. Interestingly, lack of the ten-1 gene in nid-1 mutant worms had the same effect as the removal of dgn-1 in the nid-1 mutant background (J. M. Kramer, personal communication), and both double mutants show a Pun phenotype. Therefore, loss of teneurin or dystroglycan sensitizes the worms strongly to loss of nidogen, which confirms the functional redundancy between ten-1 and dgn-1.
Furthermore, ten-1;ina-1 mutants were synthetic lethal and arrested as embryos or early larvae, frequently with severe morphological defects. Integrin loss-of-function mutants ina-1(gm39) show malformation of the anterior hypoderm, manifesting as a notched-head phenotype (Baum and Garriga, 1997), whereas ten-1 mutants have low penetrance morphological defects in the posterior body (Drabikowski et al., 2005). Combination of mutations in both genes resulted in worms arrested as L1 larvae with the entire body deformed. Mosaic analysis revealed that INA-1 is important in hypodermis (Baum and Garriga, 1997), and TEN-1S is known to be expressed in hypodermal cells of the developing embryo (Drabikowski et al., 2005). Therefore, mild defects found in single mutants may be due to compensation by activity in a parallel pathway. This strongly suggests that ina-1 and ten-1 could act together in several developmental processes, including hypodermal morphogenesis.
In summary, TEN-1, INA-1, and DGN-1 are not required for BM function in general, but they are crucial in particular tissues and organs such as the gonad, pharynx, or hypodermis. The lack of a phenotype in all BMs could also reflect redundancy between these three receptors, where deletion of a single gene can be compensated for by the presence of other receptors.
ten-1 Is Synthetic Lethal with Genes Encoding Two BM Proteins: Laminin and Nidogen
The ten-1 mutant phenotype resembled in many aspects the phenotypes of epi-1 as well as the laminin binding receptors ina-1 and dgn-1. Laminin epi-1 mutants are generally sick and show cell polarization defects, tissue disorganization, and physical disruption of BMs (Huang et al., 2003). Similar phenotypes have been described for laminin β loss-of-function mutants, lam-1(rh219) (Kao et al., 2006). Thus, mutations in the laminin genes cause more severe defects than dgn-1 or ina-1 single mutants, suggesting that these two receptors might be functionally redundant or that additional laminin receptors exist. Mutation in the ten-1 gene strongly enhanced the effects of epi-1 depletion by RNAi, leading to almost complete lethality of ten-1(ok641);epi-1(RNAi) worms. This result suggests that EPI-1 could be a ligand for TEN-1; however, direct interaction between TEN-1 and EPI-1 needs confirmation by further biochemical studies.
C. elegans nidogen is found in most BMs (Kang and Kramer, 2000), but loss of nid-1 alone causes very mild defects, mainly in the nervous system (Kim and Wadsworth, 2000; Ackley et al., 2003). The defects are, however, dramatically enhanced, if a nid-1 deletion is combined with a mutation in teneurin, BM receptor dgn-1, or axon guidance molecules such as the sax-3 Robo receptor or the unc-40 netrin receptor (J. M. Kramer, personal communication). In such sensitized backgrounds, lack of nid-1 causes a highly penetrant Pun phenotype. It appears that correct attachment of pharyngeal epithelium to arcade cells requires several receptors and guidance molecules as well as nidogen. Currently, NID-1 is considered more as a regulatory molecule (Kim and Wadsworth, 2000; Hobert and Bulow, 2003) rather than being a purely structural component linking laminin and collagen networks (Fox et al., 1991).
Conservation in Higher Organisms
Our data provide the first indication of a link between TEN-1 and BM function. There is little evidence from previous studies in vertebrates suggesting that teneurins could be BM receptors. However, in retrospect, the finding that induction of filopodia formation in neuroblastoma cells by teneurin-2 depends on the substrate and is more prominent on laminin than on poly-l-lysine (Rubin et al., 1999) may reflect a direct interaction between these proteins. Furthermore, chicken teneurin-2 was found to colocalize with laminin in BMs of the optic cup and the heart endocardium (Tucker et al., 2001).
Teneurin-3 knockout mice show defects in the positioning of specific visual circuits, leading to impaired binocular vision (Leamey et al., 2007). Such a mild phenotype in the single mutant might be due to functional redundancy with other teneurins or, in the context of the present study, with BM receptors like integrins and dystroglycan. Our studies in C. elegans could be instructive for further analyses of teneurins, integrins, or dystroglycan function in vertebrates, because they point out redundancy not only between several receptors of the same family but also between structurally distinct receptor families.
ACKNOWLEDGMENTS
We thank Shohei Mitani (Tokyo Women's Medical University School of Medicine, Tokyo, Japan) and the Japanese National BioResource Project for providing the ten-1(tm651) strain and the C. elegans Gene Knockout Consortium for ten-1(ok641) strain; James M. Kramer for the generous gift of antibodies and strains, William Wadsworth and Susan Strome for GFP marker strains; Andrew Fire (Stanford University School of Medicine, Stanford, CA) for GFP expression vectors. We acknowledge Julie Ahringer, Cambridge University Technical Services Limited and Medical Research Council gene service for the RNAi clone. Some strains used in this study were provided by the Caenorhabditis Genetics Center (CGC). We thank Richard P. Tucker for critical reading of the manuscript. This work was supported by the Novartis Research Foundation.
Abbreviations used:
- BM
basement membrane
- DIC
differential interference contrast
- L1
first larval stage
- L2
second larval stage
- L3
third larval stage
- L4
fourth larval stage
- Pun
pharynx unattached
- SGP
somatic gonad precursor cells.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0028) on July 16, 2008.
REFERENCES
- Ackley B. D., Crew J. R., Elamaa H., Pihlajaniemi T., Kuo C. J., Kramer J. M. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 2001;152:1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackley B. D., Kang S. H., Crew J. R., Suh C., Jin Y., Kramer J. M. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 2003;23:3577–3587. doi: 10.1523/JNEUROSCI.23-09-03577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagutti C., Forro G., Ferralli J., Rubin B., Chiquet-Ehrismann R. The intracellular domain of teneurin-2 has a nuclear function and represses zic-1-mediated transcription. J. Cell Sci. 2003;116:2957–2966. doi: 10.1242/jcs.00603. [DOI] [PubMed] [Google Scholar]
- Baum P. D., Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. doi: 10.1016/s0896-6273(00)80347-5. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Chiquet-Ehrismann R. Tena, a Drosophila gene related to tenascin, shows selective transcript localization. Mech. Dev. 1993;40:165–176. doi: 10.1016/0925-4773(93)90074-8. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Martin D., Hagios C., Chiquet-Ehrismann R. Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J. 1994;13:3728–3740. doi: 10.1002/j.1460-2075.1994.tb06682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zur T., Feige E., Motro B., Wides R. The mammalian Odz gene family: homologs of a Drosophila pair-rule gene with expression implying distinct yet overlapping developmental roles. Dev. Biol. 2000;217:107–120. doi: 10.1006/dbio.1999.9532. [DOI] [PubMed] [Google Scholar]
- Benard C. Y., Boyanov A., Hall D. H., Hobert O. DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development. 2006;133:3329–3340. doi: 10.1242/dev.02507. [DOI] [PubMed] [Google Scholar]
- Bokel C., Brown N. H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabikowski K., Trzebiatowska A., Chiquet-Ehrismann R. ten-1, an essential gene for germ cell development, epidermal morphogenesis, gonad migration, and neuronal pathfinding in Caenorhabditis elegans. Dev. Biol. 2005;282:27–38. doi: 10.1016/j.ydbio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Fascetti N., Baumgartner S. Expression of Drosophila Ten-a, a dimeric receptor during embryonic development. Mech. Dev. 2002;114:197–200. doi: 10.1016/s0925-4773(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Fox J. W., et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist E. J., Moerman D. G. Mutations in the sup-38 gene of Caenorhabditis elegans suppress muscle-attachment defects in unc-52 mutants. Genetics. 1992;132:431–442. doi: 10.1093/genetics/132.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham P. L., Johnson J. J., Wang S., Sibley M. H., Gupta M. C., Kramer J. M. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J. Cell Biol. 1997;137:1171–1183. doi: 10.1083/jcb.137.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson J. R., Winder S. J. Dystroglycan: a multifunctional adaptor protein. Biochem. Soc. Trans. 2005;33:1254–1255. doi: 10.1042/BST0331254. [DOI] [PubMed] [Google Scholar]
- Hobert O., Bulow H. Development and maintenance of neuronal architecture at the ventral midline of C. elegans. Curr. Opin. Neurobiol. 2003;13:70–78. doi: 10.1016/s0959-4388(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hall D. H., Hedgecock E. M., Kao G., Karantza V., Vogel B. E., Hutter H., Chisholm A. D., Yurchenco P. D., Wadsworth W. G. Laminin alpha subunits and their role in C. elegans development. Development. 2003;130:3343–3358. doi: 10.1242/dev.00481. [DOI] [PubMed] [Google Scholar]
- Johnson R. P., Kang S. H., Kramer J. M. C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development. 2006;133:1911–1921. doi: 10.1242/dev.02363. [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kang S. H., Kramer J. M. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell. 2000;11:3911–3923. doi: 10.1091/mbc.11.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao G., Huang C. C., Hedgecock E. M., Hall D. H., Wadsworth W. G. The role of the laminin beta subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev. Biol. 2006;290:211–219. doi: 10.1016/j.ydbio.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Kenzelmann D., Chiquet-Ehrismann R., Leachman N. T., Tucker R. P. Teneurin-1 is expressed in interconnected regions of the developing brain and is processed in vivo. BMC Dev. Biol. 2008;8:30. doi: 10.1186/1471-213X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Wadsworth W. G. Positioning of longitudinal nerves in C. elegans by nidogen. Science. 2000;288:150–154. doi: 10.1126/science.288.5463.150. [DOI] [PubMed] [Google Scholar]
- Kinel-Tahan Y., Weiss H., Dgany O., Levine A., Wides R. Drosophila odz gene is required for multiple cell types in the compound retina. Dev. Dyn. 2007;236:2541–2554. doi: 10.1002/dvdy.21284. [DOI] [PubMed] [Google Scholar]
- Leamey C. A., Glendining K. A., Kreiman G., Kang N. D., Wang K. H., Fassler R., Sawatari A., Tonegawa S., Sur M. Differential gene expression between sensory neocortical areas: potential roles for Ten_m3 and Bcl6 in patterning visual and somatosensory pathways. Cereb. Cortex. 2008;18:53–66. doi: 10.1093/cercor/bhm031. [DOI] [PubMed] [Google Scholar]
- Leamey C. A., Merlin S., Lattouf P., Sawatari A., Zhou X., Demel N., Glendining K. A., Oohashi T., Sur M., Fassler R. Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS Biol. 2007;5:e241. doi: 10.1371/journal.pbio.0050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Bashan-Ahrend A., Budai-Hadrian O., Gartenberg D., Menasherow S., Wides R. Odd Oz: a novel Drosophila pair rule gene. Cell. 1994;77:587–598. doi: 10.1016/0092-8674(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Li H., Bishop K. M., O'Leary D. D. Potential target genes of EMX2 include Odz/Ten-M and other gene families with implications for cortical patterning. Mol. Cell. Neurosci. 2006;33:136–149. doi: 10.1016/j.mcn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Meighan C. M., Schwarzbauer J. E. Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 2007;21:1615–1620. doi: 10.1101/gad.1534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M., Kikuchi Y., Hirate Y., Aoki M., Okamoto H. Compartmentalized expression of zebrafish ten-m3 and ten-m4, homologues of the Drosophila ten(m) /odd Oz gene, in the central nervous system. Mech. Dev. 1999;87:223–227. doi: 10.1016/s0925-4773(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Miner J. H., Yurchenco P. D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Minet A. D., Chiquet-Ehrismann R. Phylogenetic analysis of teneurin genes and comparison to the rearrangement hot spot elements of E. coli. Gene. 2000;257:87–97. doi: 10.1016/s0378-1119(00)00388-7. [DOI] [PubMed] [Google Scholar]
- Minet A. D., Rubin B. P., Tucker R. P., Baumgartner S., Chiquet-Ehrismann R. Teneurin-1, a vertebrate homologue of the Drosophila pair-rule gene ten-m, is a neuronal protein with a novel type of heparin-binding domain. J. Cell Sci. 1999;112:2019–2032. doi: 10.1242/jcs.112.12.2019. [DOI] [PubMed] [Google Scholar]
- Muriel J. M., Dong C., Hutter H., Vogel B. E. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development. 2005;132:4223–4234. doi: 10.1242/dev.02007. [DOI] [PubMed] [Google Scholar]
- Oohashi T., Zhou X. H., Feng K., Richter B., Morgelin M., Perez M. T., Su W. D., Chiquet-Ehrismann R., Rauch U., Fassler R. Mouse ten-m/Odz is a new family of dimeric type II transmembrane proteins expressed in many tissues. J. Cell Biol. 1999;145:563–577. doi: 10.1083/jcb.145.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki J. M., Firestein S. Neurestin: putative transmembrane molecule implicated in neuronal development. Dev. Biol. 1999;212:165–181. doi: 10.1006/dbio.1999.9310. [DOI] [PubMed] [Google Scholar]
- Previtali S. C., Dina G., Nodari A., Fasolini M., Wrabetz L., Mayer U., Feltri M. L., Quattrini A. Schwann cells synthesize alpha7beta1 integrin which is dispensable for peripheral nerve development and myelination. Mol. Cell. Neurosci. 2003;23:210–218. doi: 10.1016/s1044-7431(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Rakovitsky N., Buganim Y., Swissa T., Kinel-Tahan Y., Brenner S., Cohen M. A., Levine A., Wides R. Drosophila Ten-a is a maternal pair-rule and patterning gene. Mech. Dev. 2007;124:911–924. doi: 10.1016/j.mod.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Rubin B. P., Tucker R. P., Brown-Luedi M., Martin D., Chiquet-Ehrismann R. Teneurin 2 is expressed by the neurons of the thalamofugal visual system in situ and promotes homophilic cell-cell adhesion in vitro. Development. 2002;129:4697–4705. doi: 10.1242/dev.129.20.4697. [DOI] [PubMed] [Google Scholar]
- Rubin B. P., Tucker R. P., Martin D., Chiquet-Ehrismann R. Teneurins: a novel family of neuronal cell surface proteins in vertebrates, homologous to the Drosophila pair-rule gene product Ten-m. Dev. Biol. 1999;216:195–209. doi: 10.1006/dbio.1999.9503. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Chiquet-Ehrismann R. Teneurins: a conserved family of transmembrane proteins involved in intercellular signaling during development. Dev. Biol. 2006;290:237–245. doi: 10.1016/j.ydbio.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Chiquet-Ehrismann R., Chevron M. P., Martin D., Hall R. J., Rubin B. P. Teneurin-2 is expressed in tissues that regulate limb and somite pattern formation and is induced in vitro and in situ by FGF8. Dev. Dyn. 2001;220:27–39. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1084>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Kenzelmann D., Trzebiatowska A., Chiquet-Ehrismann R. Teneurins: transmembrane proteins with fundamental roles in development. Int. J. Biochem. Cell Biol. 2007;39:292–297. doi: 10.1016/j.biocel.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Martin D., Kos R., Chiquet-Ehrismann R. The expression of teneurin-4 in the avian embryo. Mech. Dev. 2000;98:187–191. doi: 10.1016/s0925-4773(00)00444-5. [DOI] [PubMed] [Google Scholar]
- Zhou X. H., Brandau O., Feng K., Oohashi T., Ninomiya Y., Rauch U., Fassler R. The murine Ten-m/Odz genes show distinct but overlapping expression patterns during development and in adult brain. Gene Expr. Patterns. 2003;3:397–405. doi: 10.1016/s1567-133x(03)00087-5. [DOI] [PubMed] [Google Scholar]