Abstract
The serine/threonine kinase-15 (STK15) acts as a cell cycle regulator being overexpressed in various tumors. One mechanism that could contribute to overexpression of STK15 is tumor hypoxia where hypoxia-inducible factor-1 (HIF-1) is a major regulator of transcription. Therefore, we analyzed whether hypoxia and HIF-1 could contribute to overexpression of STK15. We found that hypoxia increased STK15 expression and STK15 promoter activity in HepG2 tumor cells. Overexpression of HIF-1α induced STK15 gene transcription, whereas HIF-1α siRNA and overexpression of prolyl hydroxylase 2 (PHD-2), a negative regulator of HIF-1α, reversed this effect. In addition, site-directed mutagenesis experiments and chromatin immunoprecipitation revealed that from the three putative hypoxia responsive elements (HRE) within the STK15 promoter only HRE-2 was functional and bound HIF-1. Further, siRNA against STK15 inhibited proliferation of HepG2 cells induced by hypoxia. These results show that STK15 gene transcription can be regulated by hypoxia and HIF-1 via HRE-2 of the STK15 promoter. Thus, tumor hypoxia may trigger overexpression of STK15 observed in various tumors.
INTRODUCTION
The serine/threonine kinase-15 (STK15/Aurora A/BTAK) is involved in the regulation of chromosome segregation during mitosis. Inhibition of STK15 in Drosophila melanogaster and Xenopus laevis led to formation of monopolar spindles due to a lack in centrosome duplication (Giet et al., 2005). Vice versa, overexpression of STK15 induces centrosome amplification (Katayama et al., 2001), which may result in chromosomal instability and aneuploidy (Zhou et al., 1998; Miyoshi et al., 2001). Importantly, amplifications of the STK15 gene are frequently found in various carcinomas, including breast (Sen et al., 1997; Zhou et al., 1998; Tanaka et al., 1999; Miyoshi et al., 2001), bladder (Fraizer et al., 2004), colorectal (Bischoff et al., 1998; Katayama et al., 1999), gastric (Sakakura et al., 2001), and malignant brain tumors (Reichardt et al., 2003; Klein et al., 2004). Although in those tumors the amplification of the STK15 gene causes STK15 overexpression, there are also a number of tumors showing STK15 overexpression without gene amplification (Zhou et al., 1998; Klein et al., 2004, 2005). Importantly, this seems to be the case in hepatocellular carcinoma (HCC), where overexpression of STK15 correlated with high grade, high stage, and poor prognosis (Niketeghad et al., 2001; Collonge-Rame et al., 2001; Kim et al., 2003). This indicates that mechanisms different from gene amplification contribute to increased expression of STK15 in HCC.
One possible reason for overexpression of STK15 may be the reduced partial pressure of oxygen inside tumors. It is well known that these hypoxic conditions influence the expression of various proteins involved in proliferation and cell cycle progression (Giatromanolaki and Harris, 2001; Brown, 2002; Semenza, 2002; Wenger, 2002). Thereby, the transcription factor hypoxia-inducible factor-1 (HIF-1) appears to be of special importance, and it was shown that HIF-1 induces the expression of more than 100 genes (Manalo et al., 2005), among them the angiogenesis mediator vascular endothelial growth factor (VEGF) or plasminogen activator inhibitor-1 (PAI-1; Kietzmann et al., 1999). HIF-1 is a heterodimeric protein, consisting of two subunits, HIF-1α and -1β, also known as arylhydrocarbon receptor nuclear translocator protein (ARNT; Hoffman et al., 1991). While HIF-1β is expressed constitutively, HIF-1α is increased under hypoxia. Under normoxia HIF-1α is hydroxylated (Masson et al., 2001; Jaakkola et al., 2001; Ivan et al., 2002) by the action of a family of prolyl hydroxylases (PHDs; Bruick and McKnight, 2001; Epstein et al., 2001; Ivan et al., 2002; Oehme et al., 2002; Koivunen et al., 2007) and an asparagine hydroxylase (Mahon et al., 2001; Hewitson et al., 2002; Lando et al., 2002a). The proline hydroxylation at P402 and P564 of human HIF-1α promotes binding of the von Hippel-Lindau (VHL) tumor suppressor protein (Maxwell et al., 1999; Ohh et al., 2000; Tanimoto et al., 2000; Hon et al., 2002; Min et al., 2002) and degradation via a proteasomal pathway (Salceda and Caro, 1997; Kamura et al., 1998; Kaelin and Maher, 1998; Kallio et al., 1999; Tanimoto et al., 2000; Ivan et al., 2001). Asparagine hydroxylation at N803 prevents recruitment of the coactivator p300 (Lando et al., 2002b). Under hypoxia, hydroxylase activity is impaired, and hydroxylation of HIF-1α is decreased. This leads to its accumulation, dimerization with HIF-1β, and transcriptional activation of target genes via binding to hypoxia responsive elements (HREs; Safran and Kaelin, 2003;Wenger et al., 2005).
Because tumor hypoxia may contribute to the overexpression of STK15 we aimed to obtain new insights into the regulation of STK15 expression under hypoxia. Therefore, we analyzed STK15 expression in HepG2 hepatoma cells cultured under normoxia and hypoxia and investigated the role of HIF-1α as transcriptional activator of the STK15 promoter.
MATERIALS AND METHODS
All biochemicals and enzymes were of analytical grade and were purchased from commercial suppliers.
Cell Culture
HepG2 hepatoma cells were cultured in MEM supplemented with 10% FCS and 1% nonessential amino acids (PAA Laboratories, Linz, Austria) in a normoxic atmosphere of 16% O2, 79% N2, and 5% CO2 (by volume) for 24 h. Then medium was changed and cells were further cultured under normoxia or hypoxia (3% O2, 87% N2, 5% CO2, by volume).
Plasmid Constructs
The human STK15 promoter 5′-flanking region was amplified by PCR from human genomic DNA by using the oligonucleotides 5′-GTCCTAATAAGGTACCTGGAAG-3′ (−1001/−980) as forward and 5′-CCAAGAGCTCAGCCGTTAGAATTC-3′ (+81/+104) as reverse primers. The resulting PCR product was digested with KpnI and XhoI and ligated into the KpnI and XhoI sites of pGL3basic (Promega, Mannheim, Germany), giving pGL3-STK15-WT with the human STK15 promoter from −986 to +15.
The isolated human STK15 promoter contained three putative HREs (HRE-1: −336/−332; HRE-2: −323/−319; and HRE-3: −240/−236). These HREs were mutated by using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands) according to the instruction manual. In brief, mutation was carried out by PCR from pGL3-STK15-WT DNA by using the oligonucleotides 5′-CTACCGCTCCGAGCGGATCCTCACTGCGCACGCTG-3′ and 5′-CAGCGTGCGCAGTGAGGATCCGCTCGGAGCGGTAG-3′ (−351/−317) as primers for HRE-1; 5′-GCGCACGTTCACTGCTCTAGATGAAAGGGCGCCAAGCCG-3′ and 5′-CGGCTTGGCGCCCTTTCATCTAGAGCAGTGAACGTGCGC-3′ (−339/−301) as primers for HRE-2; and 5′-CGCCATCTTACTTACTGGTACCTTCAAAGGTTAGTTCACC-3′ and 5′-GGTGAACTAACCTTTGAAGGTACCAGTAAGTAAGATGGCG-3′ (−258/−219) as primers for HRE-3. All mutations were verified by sequencing of the mutated plasmids (pGL3-STK15-HRE1m-Luc, pGL3-STK15-HRE2m-Luc, pGL3-STK15-HRE3m-Luc, and pGL3-STK15-HRE1m3m-Luc).
The pGL3-Epo-HRE-Luc construct and the expression vectors pcDNA3.1D/ V5-His-hHIF-1α, pcDNA3.1D/V5-His-hHIF-1αPPN (hHIF-1α P402A P564A N803A), pcDNAV5 PHD2, and siSTRIKE U6-HIF-1α were described previously (Kietzmann et al., 2001; Scharf et al., 2005; Flügel et al., 2007; Bonello et al., 2007).
Cell Transfection and Luciferase Assay
HepG2 cells, 4 × 105 per 60-mm dish, were transfected essentially as described (Immenschuh et al., 1998a; Dimova and Kietzmann, 2006). In brief, 2 μg of the appropriate STK15 promoter Firefly luciferase (Luc) constructs were cotransfected in duplicate together with 500 ng of the respective expression vector for HIF-1αV5, HIF-1αV5-PPN, PHD2, or in the controls empty vector and to control transfection efficiency 0.25 μg of a Renilla luciferase expression vector (pRLSV40; Promega). Transfection with 2 μg pGL3-Epo-HRE-Luc together with the above-mentioned expression vectors served as a positive control. After 5 h the medium was changed and the cells were cultured under normoxic conditions for 18 h. Afterward cells were further cultured for 24 h under normoxia or hypoxia. The detection of luciferase activity was performed with the Luciferase Assay Kit (Berthold, Pforzheim, Germany).
RNA Interference
Short hairpin RNA (shRNA) encoding for 19mer siRNA against HIF-1α (Bonello et al., 2007) and for control (Ctr) small interfering RNA (siRNA; Petry et al., 2006) were described and used with the siSTRIKE U6 Hairpin Cloning System (Promega). Two different STK15 siRNA duplexes with the sequence 5′-AAGCACAAAAGCTTGTCTCCA-3′ (Du and Hannon, 2004) and 5′-AUGCCCUGUCUUACUGUCA-3′ (Kufer et al., 2002) were selected from the open reading frame of STK-15 and purchased form Dharmacon (Lafayette, CO). In brief, 100 nM siRNA was used with transfection reagent essentially as described (Flügel et al., 2007).
RNA Preparation and Northern Blot Analysis
Isolation of total RNA and Northern blot analysis were performed as described (Kietzmann et al., 1999). Digoxigenin (DIG)-labeled antisense RNAs served as hybridization probes; they were generated by in vitro transcription from pBS-STK-15. Blots were quantified with a videodensitometer (Biotech Fischer, Reiskirchen, Germany).
Protein Isolation and Western Blot Analysis
For Western Blot analysis proteins were isolated as described (Immenschuh et al., 1998b). When indicated cells were transfected with 7.5 μg expression plasmids for HIF-1α, HIF-1α-PPN, and HIF-1α-siRNA, respectively. Controls were transfected with an empty vector. After 24 h medium was changed, and cells were cultured under normoxia or hypoxia for another 72 h.
A total of 80 μg protein was separated via SDS gel electrophoresis and blotted onto nitrocellulose membranes. The primary rabbit antibody against human STK15 (no. 402; a gift from Dr. Steffi Urbschat, Neurooncology, Saarland University) was raised against a C-terminal oligopeptide with the sequence CNCQNKESASKQS and described (Du and Hannon, 2002). The STK15 antibody, the primary mouse antibodies against V5-Tag (Invitrogen, Karlsruhe, Germany), β-actin (Sigma, Taufkirchen, Germany) as well as the secondary goat anti-rabbit and goat anti-mouse immunoglobulin G were all used in a 1:5000 dilution. The primary mouse antibody against human HIF-1α (Becton Dickinson, Heidelberg, Germany) was used in a 1:2000 dilution. The enhanced chemiluminescence Western blotting system (Amersham, Freiburg, Germany) was used for detection. Under these conditions STK15 was seen as a 46-kDa band and HIF-1α and -1αV5 as 120-kDa bands.
Chromatin Immunoprecipitation
HepG2 cells were exposed to hypoxia for various times. Cells were then fixed with formaldehyde, lysed, and sonicated in order to obtain DNA fragments in a size from 500 to 1000 base pairs. Chromatin was then precipitated with unspecific preimmune serum or the HIF-1α antibody (Becton Dickinson) overnight at 4°C. DNA from chromatin immunoprecipitation (ChIP) was analyzed by quantitative PCR using Taqman Gene Expression Master Mix (Advanced Biotechnologies, Columbia, MD). PCR was performed with primers for a STK-15 promoter fragment without HRE (fw: 5′-CTGTTCTATCCGGTCTCTTCACTT-3′; rv: 5′-TTGGGGATAATTAGGCTTCTGTT-3′) and for a genomic fragment containing HRE-1and -2 (fw: 5′-AGTCGTTTCTGTGGTTTTCTC-3′, rv 5′-GAGATAAAGTCCAAGGAGGTGAAC-3′) at 55°C for 35 cycles. A β-actin PCR was used for normalization with the oligonucleotides 5′-CCCTAAGGCCAACCGTGAAAAGATG-3′ as forward and 5′-AGGTCCCGGCCAGCCAGGTCCAG-3′ as reverse primer.
Because the distance between HRE-1 and -2 of only nine base pairs does not allow generation of ChIP PCR primers, which can be used to detect HIF-1 binding to the HRE-2 only, we aimed to more specifically detect HIF-1α binding to the HRE-2 by a transfection approach. For this, HepG2 cells grown on 10-cm dishes were transfected with 20 μg pGL3-STK15-HRE1m3m-Luc containing mutations in HRE-1 and -3. After cultivation of cells for 72 h they were exposed to hypoxia for 1 h. Cells were then fixed with formaldehyde, lysed, and sonicated in order to obtain DNA fragments in a size from 500 to 1000 base pairs. Chromatin was then precipitated with the HIF-1α antibody (Becton Dickinson) overnight at 4°C. PCR was performed with primers for the STK-15 promoter (fw: 5′-GCCCAATCTACCGCTCCGA-3′; flanking the HRE-2 and the GL2 reverse primer from the luciferase construct 5′-CTTTATGTTTTTGGCGTCTTCC -3′) at 55°C for 35 cycles.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared as already described (Dimova et al., 2005). The sequence of STK15 oligonucleotides used for electrophoretic mobility shift assay (EMSA) are HRE2 5′-CACTGCGCACGCTGAAAGG-3′ (−330/−319) and HRE2m 5′-CACTGCGCAtGaTGAAAGG-3′. For supershift analysis the nuclear extracts were preincubated on ice for 45 min with preimmune serum or HIF-1α antibody before adding the labeled probes.
Proliferation Assay
Proliferative activity was evaluated by 5-bromo-2′ deoxyuridine (BrdU) labeling (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. The absorbance values obtained in the ELISA with the cells under normoxia were set to 100%; values measured under hypoxia were then normalized to the values obtained under hypoxia.
Statistical Analysis
Densitometry data were plotted as fold induction of relative density units, with the zero value absorbance in each figure set arbitrarily at 1 or 100%. Statistical comparisons of absorbance differences were performed by the Mann-Whitney test (Statview 4.5, Abacus Concepts, Berkeley, CA), and p ≤ 0.05 was considered significant. Luc values presented are means ± SEM. Results were compared by ANOVA for repeated Luc measurements followed by the Newman-Keuls test. p ≤ 0.05 was accepted as significant.
RESULTS
Induction of STK15 mRNA and Protein Expression as well as Promoter Activity by Hypoxia in HepG2 Hepatoma Cells
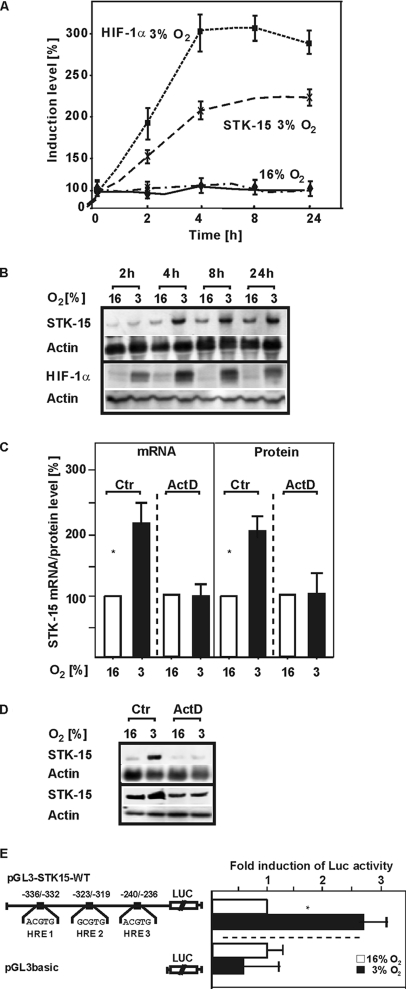
First we investigated whether exposure to hypoxia would increase STK-15 expression in HepG2 hepatoma cells. Exposure of cells to hypoxia increased STK15 mRNA levels in a time-dependent manner and almost maximal levels were reached within 4 h. Then STK-15 mRNA was only slightly increased and remained almost constant at this level up to 24 h (Figure 1, A and B). The STK-15 mRNA induction was followed by an increase in protein levels, which were enhanced by ∼2.3-fold after 4 h (Figure 1, C and D). Further, the increase in STK-15 mRNA was paralleled by an increase in HIF-1α protein levels (Figure 1, A and B). The hypoxia-dependent induction of HIF-1α was clearly visible after 2 h and reached maximal values after 4 h. Then, HIF-1α levels remained stable until 8 h, when they started slightly to decline. Because the HIF-1α protein acts as a transcriptional activator, these data suggested that HIF-1α could activate STK-15 mRNA transcription under hypoxia. Therefore we aimed to investigate whether the enhancement of STK-15 levels was due to transcriptional regulation. To do this, HepG2 cells were pretreated with the transcription inhibitor actinomycin D (ActD) for 30 min before stimulation with hypoxia. Although ActD did not affect the STK-15 mRNA and protein levels under normoxia, it completely abolished the hypoxia-dependent STK-15 mRNA and protein induction (Figure 1, C and D). To further analyze a possible transcriptional regulation of the STK15 promoter by hypoxia, we generated a luciferase reporter gene construct (pGL3-STK15-WT) containing the human STK15 promoter from −986 to +15. After transfection into HepG2 cells, we found that hypoxia induced STK15 promoter activity by ∼2.5-fold (Figure 1E). Thus, a transcriptional regulation within the STK15 promoter appears to mediate the hypoxia-dependent STK15 expression.
Figure 1.
Hypoxia increases STK15 mRNA and protein expression as well as STK15 promoter activity. (A) HepG2 cells were incubated under normoxia (16% O2) or hypoxia (3% O2) and then harvested at different time points. The STK15 mRNA levels were measured by Northern blot and HIF-1α levels were measured by Western blot analysis. The graphs represent STK15 mRNA and HIF-1α protein levels normalized against β-actin and indicate % induction compared with control levels. Values are means ± SEM of five independent culture experiments. (B) Representative Northern and Western blots. Total RNA (15 μg) was subjected to Northern blot analysis as outlined in Materials and Methods. For Western blots, protein samples were immunoblotted and sequentially probed with HIF-1α and β-actin antibodies as described in Materials and Methods. (C) HepG2 cells were pretreated with actinomycin D (Act D; 5 μg/ml) for 30 min, and then they were exposed to hypoxia (3% O2) for 4 h. The STK15 mRNA levels were measured by Northern blot, and STK15 protein levels were measured by Western blot analysis. The graphs represent STK15 mRNA and protein levels normalized against β-actin and indicate % induction compared with control levels. Values are means ± SEM of three independent culture experiments. (D) Representative Northern and Western blots. Total RNA (15 μg) was subjected to Northern blot analysis as outlined in Materials and Methods. For Western blots, protein samples (15 μg) were immunoblotted and sequentially probed with STK15 and β-actin antibodies as described in Materials and Methods. (E) A 1001-bp fragment of the human STK15 promoter 5′-flanking region (−986 to +15) was cloned into the luciferase vector pGL3-basic to give pGL3-STK15-WT. The STK15 promoter contains three potential HRE-sites at positions −336/−332 (HRE-1), −323/−319 (HRE-2), and −240/−236 (HRE-3). HepG2 cells were then transfected with the STK15 promoter construct (pGL3-STK15-WT) or with the pGL3-basic vector as a control as well as Renilla Luc vectors. Normalized Luc activity in the respective controls was set to 1. Values are means ± SEM of three independent experiments. Significant difference, 16% O2 versus 3% O2, *p ≤ 0.05.
Involvement of HIF-1α in the Regulation of STK15 Expression
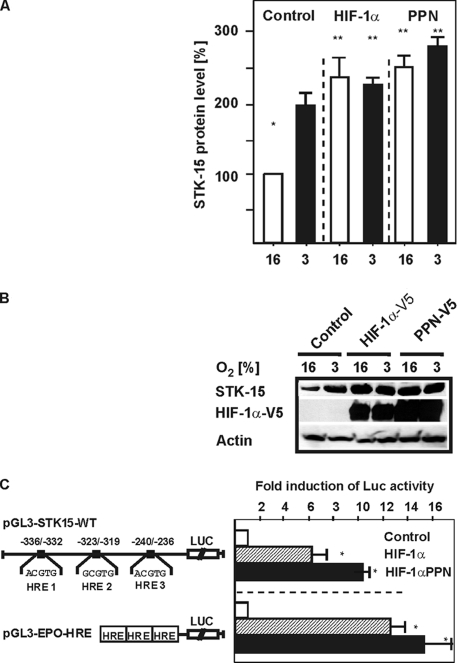
Next, we analyzed whether the transcription factor HIF-1α regulates STK15 expression. First, we measured STK15 protein levels in cells transfected with expression vectors for wild-type HIF-1α (HIF-1αV5) or a HIF-1α mutant (HIF-1αV5-PPN) harboring three mutations (P402A, P564A, and N803A). These mutations led to a very strong HIF-1α transactivity because of a loss of its degradation by the VHL-proteasome system and an increased recruitment of the coactivator p300.
We found that, like hypoxia, overexpression of HIF-1α increased STK15 protein levels by about twofold. Further, the HIF-1αPPN mutant enhanced STK15 protein levels by ∼2.5-fold under both normoxia and hypoxia. Interestingly, this induction by the HIF-1αPPN mutant was not much higher than with HIF-1α alone, although the HIF-1αPPN levels were enhanced compared with the HIF-1αWT levels (Figure 2, A and B).
Figure 2.
STK15 expression is induced by HIF-1α. (A) HepG2 cells were transfected with expression vectors for HIF-1α and the HIF-1α-PPN mutant (hHIF-1α P402A P564A N803A) and cultured under normoxia (16%) and mild hypoxia (8%) for 72h. The STK15 protein levels were measured by Western blot analysis and protein levels under normoxic conditions in the controls were set to 100%. Values are means ± SEM of three independent experiments. *Significant difference, 16% O2 versus 3% O2 and **significant difference, HIF-1α or HIF-1α PPN at 16% O2 and 3% O2 versus control at 16% O2, p ≤ 0.05. (B) Representative Western blot. Protein levels were analyzed by Western blot analysis with the STK15 402-, V5-, and β-actin antibodies. (C) HepG2 cells were cotransfected with the pGL3-STK15-WT construct and in controls with empty expression vector or with vectors for HIF-1α wild-type (HIF-1αV5) and the HIF-1α mutant (HIF-1αV5-PPN) as well as Renilla Luc vectors. The pGL3-Epo-HRE-Luc construct served as an independent control. Normalized Luc activity in the respective controls was set to 1. Values are means ± SEM of three independent experiments. Significant difference, HIF-1α or HIF-1α PPN versus control, *p ≤ 0.05.
In addition, we observed that the STK15 promoter was induced by HIF-1α. After cotransfection of pGL3-STK15-WT with the vector for wild-type HIF-1α, we detected an induction of STK15 promoter activity by about sixfold. Further, HIF-1αV5-PPN cotransfection induced STK15 promoter activity by ∼10-fold (Figure 2C). Similar effects were observed when we used the pGL3-Epo-HRE-Luc construct containing three HRE-sites in front of the SV40-promoter and the Luc-gene as a control (Figure 2C).
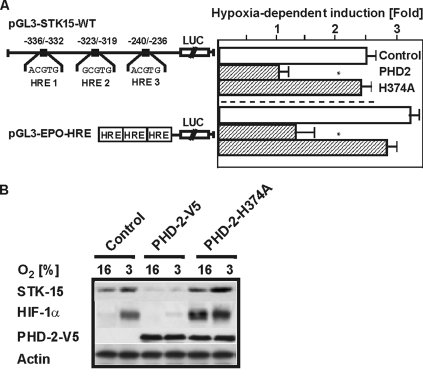
Because HIF-1α is degraded after hydroxylation at the critical proline residues, we thought to investigate whether the most important PHD (prolyl hydroxylase), namely PHD-2, has an effect on STK15 promoter activity. Therefore, we expected a diminished induction of the STK15 promoter under hypoxia after expression of PHD-2. Indeed, we found a 60% reduced induction of the STK15 promoter as well as a 70% reduced induction of the Epo-HRE construct after cotransfection with PHD-2 (Figure 3). By contrast, when we overexpressed the catalytically dead PHD2 mutant H374A in which one of the histidines involved in the coordination of iron was changed into alanine, we found that catalytic-dead PHD2 increased STK15 and HIF-1α levels already under normoxia to about the same levels as under hypoxia (Figure 3B).
Figure 3.
Expression of PHD-2 reduces the induction of the STK15 promoter under hypoxia. (A) HepG2 cells were cotransfected with an expression vector for PHD-2 or catalytically inactive PHD-2-H374A and either the pGL3-STK15-WT or EPO-HRE-Luc construct. Luc values represent the fold induction under hypoxia in comparison to normoxia. Values are means ± SEM of three independent experiments. Significant difference, control versus PHD2, *p ≤ 0.05. (B) Representative Western blot. Proteins were detected by Western blot analysis with the STK15 402-, HIF-1α, V5-, and β-actin antibodies.
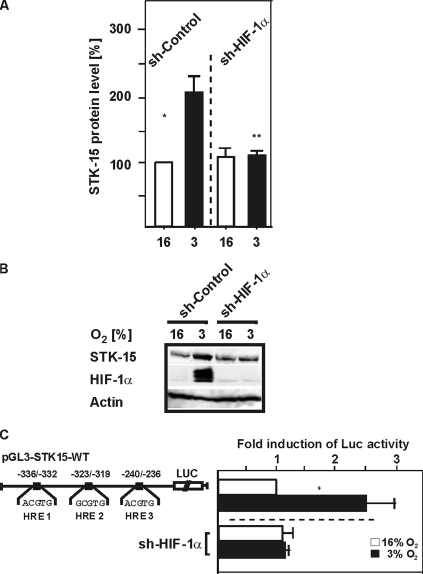
Further, we analyzed a possible reduction of STK15 protein expression by Western analysis after HIF-1α knockdown with shRNA. We found that the hypoxia-dependent STK15 expression was reduced to the expression level found under normoxia (Figure 4, A and B). In addition, depletion of HIF-1α by shRNA abolished the hypoxia-dependent induction of the STK15 promoter (Figure 4C). Together, these data indicate that HIF-1α is involved in the regulation of STK15 expression.
Figure 4.
Expression of HIF-1α siRNA decreases hypoxia-dependent STK15 induction. (A) HepG2 cells were transfected with an expression vector for HIF-1α shRNA (siSTRIKE U6-HIF-1α) and cultured under normoxia and hypoxia. Proteins were detected by Western blot analysis with the STK15 402- and HIF-1α antibodies. The STK15 protein levels in control cells at 16% were set to 100%. Values are means ± SEM of three independent experiments. *Significant difference, 16% O2 versus 3% O2, **significant difference, HIF-1α shRNA at 3% O2 versus control at 3% O2, p ≤ 0.05. (B) Representative Western blot. STK15 levels were analyzed with the STK15 402 antibodies. Analysis with anti-HIF-1α was performed to show transfection efficiency and knockdown of HIF-1α expression via shRNA. (C) HepG2 cells were cotransfected with the pGL3-STK15-WT Luc construct and siSTRIKE U6-HIF-1α as well as Renilla Luc vectors. Normalized Luc activity in the respective controls was set to 1. Values are means ± SEM of three independent experiments. Significant difference, 16% O2 versus 3% O2, *p ≤ 0.05.
Identification of STK15 Promoter Sequences Responsible for the Hypoxia-dependent Induction
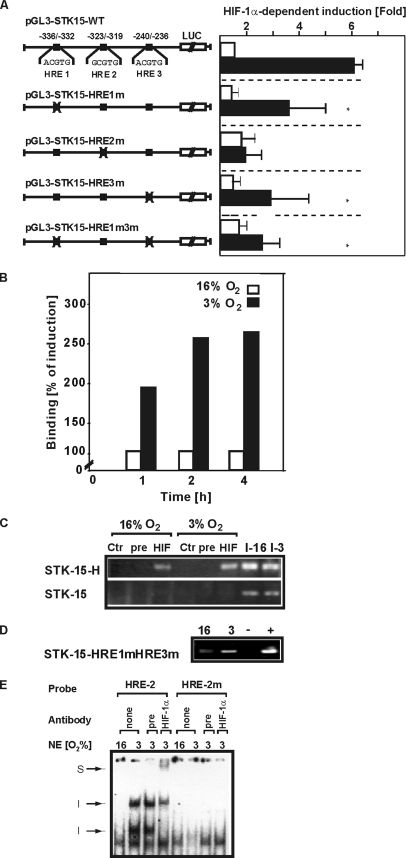
HIF-1α regulates the transcription of target genes usually via binding to HREs. Sequence analysis of the STK15 promoter revealed three potential HRE sites. These HRE sites were mutated, and the mutated STK15 promoter luciferase constructs were cotransfected along with the expression vector for HIF-1αV5 to identify those HREs responsible for HIF-dependent STK15 promoter activation. Although the HIF-1α–dependent promoter activity was reduced after mutation of HRE-1 and -3, we could still observe an induction of promoter activity by HIF-1α. By contrast, the HIF-dependent induction was lost when the HRE-2–mutated construct was used (Figure 5A). These data suggest that the HRE-2 constitutes the major HIF-1 binding site in the STK15 promoter.
Figure 5.
The HRE-2 mediates the HIF-1–dependent induction of the STK15 promoter. (A) HepG2 cells were transfected with either pGL3-STK15-WT or the respective STK15 promoter mutant constructs (pGL3-STK15-HRE1m-Luc, pGL3-STK15-HRE2m-Luc, pGL3-STK15-HRE3m-Luc, and STK15-HRE1m3m-Luc) as well as Renilla Luc vectors and cultured under normoxia and hypoxia. Normalized Luc values in the controls were set to 100%. Values are means ± SEM of three independent experiments. Significant difference, pGL3-STK15-WT versus mutants, *p ≤ 0.05. (B) Cells were incubated for 1 h under normoxia (N) and hypoxia (H) and ChIP was performed by using either no antibody (No), unspecific preimmune serum (pre) or the HIF-1α antibody (HIF). Quantitative PCR was performed with primers for the STK15 promoter fragment containing the three HREs and normalized to β-actin. (C) To detect binding of HIF-1 to the STK-15 promoter, a ChIP assay was performed with the STK15 promoter fragment containing the three HREs (STK-15-H) and a promoter fragment containing no HREs as a control (STK-15). I-16, input 16% O2; I-3, input 3% O2. (D) To detect binding of HIF-1 to HRE2, a ChIP assay was performed with a transfected STK15 promoter construct containing mutations in HRE-1 and HRE-3 (STK-15-HRE1mHRE3m), respectively. (E) EMSA with oligonucleotides spanning the HRE2 of the STK15 promoter. The respective 32P-labeled oligonucleotides with the wild-type HRE2 and mutant HRE2 (HREm) were incubated with 10 μg nuclear extracts. In supershift experiments the nuclear extracts were preincubated on ice for 45 min with preimmune serum or HIF-1α antibody before adding the labeled probes. The DNA–protein complexes were separated by electrophoresis on 5% native polyacrylamide gels and visualized by phosphoimaging.
To confirm the conclusion from the experiments above indicating HIF-1α binding to the STK15 promoter, ChIP assays were performed using an antibody against HIF-1α in cells exposed to hypoxia for different periods of time. Quantitative PCR performed with specific primers for the STK15 constructs revealed that HIF-1α could bind to the STK-15 already under normoxia; however, this binding was enhanced after exposure to hypoxia and maximal binding was observed after 2 h of hypoxia (Figure 5, B and C). These data nicely correlate with the time course of protein accumulation and STK-15 mRNA induction. Further, these data and the transfection data implicated that HRE-2 acts as the major HRE within the STK-15 promoter. However, the distance between HRE-1 and -2 of only nine base pairs did not allow generation of PCR primers enabling detection of HIF-1 binding at HRE-2 with a ChIP assay. Therefore, we aimed to detect HIF-1α binding to the HRE-2 after a transfection approach. For this, we transfected the STK15-HRE1m3m-Luc construct containing mutations in both HRE-1 and -3 and performed a ChIP analysis with a STK-15 promoter and a primer from the Luc gene, respectively. In line with the data from the endogenous promoter we could see HIF-1α binding to this fragment (Figure 5D).
To further confirm that the HRE2 facilitates HIF-1 binding to the STK15 promoter, EMSAs with nuclear proteins and an HRE2 wild-type and HRE2 mutant oligonucleotide were performed. To investigate the presence of HIF-1 in these complexes, an antibody against HIF-1α was included in the binding reaction. When the HRE2 oligonucleotide was incubated with nuclear extracts from normoxic cells, two DNA–protein complexes were found. When nuclear extracts from hypoxic cells were used two additional, DNA–protein complexes with higher molecular weight were present. The formation of these two hypoxia-inducible complexes was affected by the addition of an antibody against HIF-1α. Although the presence of the larger complex was greatly diminished, the formation of the smaller hypoxia-inducible complex was completely abolished after addition of the HIF-1α antibody, and a supershift was formed. Further, an unspecific preimmune serum did not affect the formation of the DNA–protein complexes. By contrast, mutation of the HRE2 eliminated binding of the hypoxia-inducible nuclear proteins (Figure 5). Together, these data show that hypoxia facilitates HIF-1 binding to HRE2 within the STK15 promoter.
Involvement of STK-15 in Hypoxia-dependent Proliferation
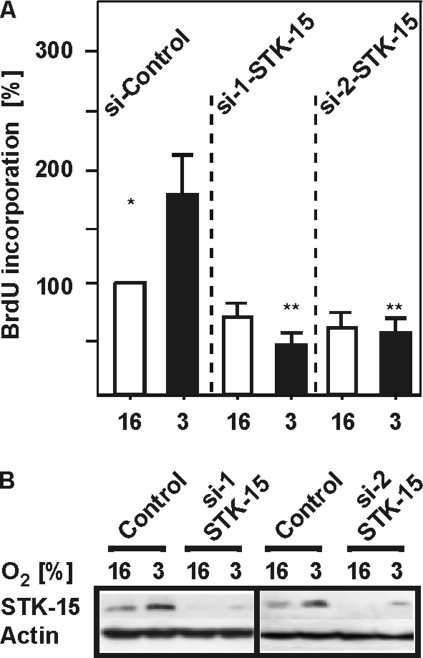
Because STK15 appears to be involved in cell cycle regulation and cellular proliferation, we next tested the role of STK15 in the regulation of HepG2 cell proliferation. To do this, HepG2 cells were transfected with two different siRNAs against STK15 and BrdU incorporation was measured. Evaluation of BrdU incorporation showed that hypoxia increased proliferation by about twofold. This response was completely abrogated by siRNAs depleting STK15 (Figure 6). This indicates that hypoxia-dependent induction of STK15 expression is involved in controlling HepG2 cell proliferation.
Figure 6.
Knockdown of STK15 inhibits the hypoxia-induced proliferation of HepG2 hepatoma cells. (A) HepG2 cells were transfected with control siRNA or siRNA against STK15 (siSTK15a and siSTK15b) and cultured under normoxia and hypoxia for 24 h. Proliferative activity was assessed by BrdU incorporation, and the values under 16% O2 were set to 100%. Values represent means of three different culture experiments. Significant difference, *normoxia versus hypoxia and **hypoxia versus hypoxia+siSTK15; p ≤ 0.05. (B) Representative Western blot. Proteins were detected by Western blot analysis with the STK15 402- and β-actin antibodies.
DISCUSSION
The present study shows that hypoxia can induce STK15 expression in HepG2 hepatoma cells. Transfection experiments involving overexpression of PHD2 and HIF-1α siRNA as well as ChIP assays revealed that the hypoxia-dependent induction involves binding of HIF-1α to the HRE-2 within the STK15 promoter. In addition, the relevance of this induction for cell cycle progression was confirmed by blocking hypoxia-dependent proliferation in HepG2 cells depleted of STK15. To our knowledge the present study shows for the first time that hypoxia directly links HIF-1α with STK15 expression, implicating an important pathophysiological role of this novel pathway in disorders associated with genomic instability.
STK15 belongs to a family of serine/threonine kinases that are involved in the regulation of the chromosome segregation process during mitosis. The importance of STK15 for human tumors becomes apparent by the amplification and overexpression of the gene observed in some tumor entities where it is associated with poor prognosis (Landen et al., 2007). Although overexpression of STK15 mRNA and protein correlates with an amplification of the STK15 gene in some cases, there are other cases where in spite of increased STK15 expression no gene amplification is detectable (Zhou et al., 1998; Klein et al., 2004, 2005). Interestingly, not much is known about STK15 expression in HCC. Although gain of chromosome 20q, where STK15 is located, was observed in HCC (Niketeghad et al., 2001; Collonge-Rame et al., 2001; Kim et al., 2003), this was challenged by another study in which 224 HCC were investigated and STK15 was overexpressed in 137 cases, whereas an amplification of STK15 was detected only in three HCCs (Jeng et al., 2004). Thus, it appears that other mechanisms than amplification could contribute to the progression of HCC. Therefore an understanding of the regulatory pathways mediating an enhanced transcription of STK15 is important to unravel the mechanisms relevant not only for HCC progression but also for other tumors associated with STK15 overexpression.
Our study showed that hypoxia could mediate an increased STK15 expression. Tumor hypoxia is known to be an important regulator for the expression of many genes that are involved in tumorigenesis and cell cycle regulation. Thereby the transcription factor HIF-1α appears to be a key regulatory factor for ∼100 genes from which VEGF, PGI, c-MET, and CXCR4, CDC2, RB1, or PAI-1 are known to play a pivotal role in tumorigenesis and cancer metastasis (for review see Brahimi-Horn and Pouyssegur, 2006). In line, our data now show that HIF-1 is also involved in the regulation of STK15 expression under hypoxia because expression of siRNA against HIF-1α abolished the induction of STK15 under hypoxia. In addition, STK15 expression and promoter activity was induced after expression of HIF-1α and a HIF-1α PPN mutant that cannot be degraded via the proline hydroxylase/VHL-dependent pathway. Interestingly, when compared with wild-type HIF-1α, overexpression of the HIF-1α PPN mutant did not mediate the same additive induction of STK15 protein levels as observed with the STK15 promoter activity (Figure 2, A and B). A possible reason for this is that under these overexpression conditions either PHD-2 or VHL become limiting and that therefore the PPN mutant is not that much more active. Further, the notion that STK15 is directly regulated via the PHD-HIF system is confirmed by the repression of the induction by overexpression of PHD-2. PHD-2 was used as an example PHD because it appeared to be the most important PHD for HIF-1α regulation in Hela cells (Berra et al., 2003) and hepatocytes (Scharf et al., 2005). However, these data do not rule out that the other PHD enzymes would also be involved in HIF-dependent STK15 regulation. In addition, mutagenesis experiments and subsequent reporter gene assays showed that mutation of STK15 HRE-2 abolished promoter induction, and together with EMSA and ChIP assays this indicates that HRE-2 mediates the majority of the HIF-1 induction (Figure 5).
The relevance of the hypoxia-induced STK15 expression for the proliferation of tumor cells was then shown in the experiments where two different siRNAs against STK15 inhibited BrdU incorporation into HepG2 cells. Thus, it may be possible that hypoxia as present in the center of tumors triggers STK15 expression and thus stimulates proliferation and mitosis of cells within this area.
The regulation of STK15 expression by HIF-1 appears not only to be important under conditions of hypoxia but also under conditions leading to the activation of HIF-1α under normoxic conditions. This can be achieved by the action of hormones, growth and coagulation factors, cytokines, and conditions of mechanical-, physical-, or chemical stress (for review see Kietzmann and Görlach, 2005). In liver, these events may be also of importance for situations associated with growth factors and cytokines that stimulate liver regeneration and promote hepatoprotection. Thereby, and to the best of our knowledge, HIF-1 is the first new player linking an environmental signal to the STK15 promoter. So far, the only known STK15 regulators were E4TF1 (Tanaka et al., 2002) and GABP (Udayakumar et al., 2006) of the Ets transcription factor family that act in concert with the coactivator TRAP220/MED1 (Udayakumar et al., 2006). Because HIF-1 acts with the recruitment of the coactivators CBP/p300, it appears that fine tuning of STK15 expression can be achieved by recruitment of coactivators.
In summary, we found that hypoxia-dependent binding of HIF-1α at HRE-2 of the STK15 promoter can up-regulate STK15 expression and HepG2 cell proliferation. Because STK15 is an important regulator of the chromosomal segregation process in mammalian cells these results are of importance for the progression of tumors as well as for the development of anti-tumor therapies.
ACKNOWLEDGMENTS
The authors thank Linda Kunsch for her work on isolating the STK15 promoter fragments.
Abbreviations used:
- ARNT
arylhydrocarbon receptor nuclear translocator protein
- CDC-2
cell division cycle 2
- CIN
chromosomal instability
- c-MET
Met proto-oncogene
- CXCR4
chemokine receptor 4
- E4TF-1
Ets transcription factor 1
- GABP
GA-binding protein transcription factor
- HIF-1
hypoxia-inducible factor 1
- HRE
hypoxia responsive element
- PAI-1
plasminogen activator inhibitor 1
- PGI
phosphoglucose isomerase
- PHD
prolyl hydroxylase
- RB-1
retinoblastoma 1
- STK15
serine/threonine kinase 15
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau tumor suppressor protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0042) on June 18, 2008.
REFERENCES
- Berra E., Benizri E., Ginouves A., Volmat V., Roux D., Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J. R., et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonello S., Zahringer C., BelAiba R. S., Djordjevic T., Hess J., Michiels C., Kietzmann T., Görlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn C., Pouyssegur J. The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull. Cancer. 2006;93:E73–E80. [PubMed] [Google Scholar]
- Brown J. M. Tumor microenvironment and the response to anticancer therapy. Cancer Biol. Ther. 2002;1:453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- Bruick R. K., McKnight S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Collonge-Rame M. A., Bresson-Hadni S., Koch S., Carbillet J. P., Blagosklonova O., Mantion G., Miguet J. P., Heyd B., Bresson J. L. Pattern of chromosomal imbalances in non-B virus related hepatocellular carcinoma detected by comparative genomic hybridization. Cancer Genet. Cytogenet. 2001;127:49–52. doi: 10.1016/s0165-4608(00)00421-0. [DOI] [PubMed] [Google Scholar]
- Dimova E. Y., Kietzmann T. Cell type-dependent regulation of the hypoxia-responsive plasminogen activator inhibitor-1 gene by upstream stimulatory factor-2. J. Biol. Chem. 2006;281:2999–3005. doi: 10.1074/jbc.M512078200. [DOI] [PubMed] [Google Scholar]
- Dimova E. Y., Moller U., Herzig S., Fink T., Zachar V., Ebbesen P., Kietzmann T. Transcriptional regulation of plasminogen activator inhibitor-1 expression by insulin-like growth factor-1 via MAP kinases and hypoxia-inducible factor-1 in HepG2 cells. Thromb. Haemost. 2005;93:1176–1184. doi: 10.1160/TH04-11-0761. [DOI] [PubMed] [Google Scholar]
- Du J., Hannon G. J. The centrosomal kinase Aurora-A/STK15 interacts with a putative tumor suppressor NM23–H1. Nucleic Acids Res. 2002;30:5465–5475. doi: 10.1093/nar/gkf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Hannon G. J. Suppression of p160ROCK bypasses cell cycle arrest after Aurora-A/STK15 depletion. Proc. Natl. Acad. Sci. USA. 2004;101:8975–8980. doi: 10.1073/pnas.0308484101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. C., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Flügel D., Görlach A., Michiels C., Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol. Cell. Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraizer G. C., Diaz M. F., Lee I. L., Grossman H. B., Sen S. Aurora-A/STK15/BTAK enhances chromosomal instability in bladder cancer cells. Int. J. Oncol. 2004;25:1631–1639. [PubMed] [Google Scholar]
- Giatromanolaki A., Harris A. L. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21:4317–4324. [PubMed] [Google Scholar]
- Giet R., Petretti C., Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Hewitson K. S., et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Hon W. C., Wilson M. I., Harlos K., Claridge T. D., Schofield C. J., Pugh C. W., Maxwell P. H., Ratcliffe P. J., Stuart D. I., Jones E. Y. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- Immenschuh S., Hinke V., Ohlmann A., Gifhorn-Katz S., Katz N., Jungermann K., Kietzmann T. Transcriptional activation of the heme oxygenase-1 gene by cGMP via a cAMP response element/activator protein-1 element in primary cultures of rat hepatocytes. Biochem. J. 1998b;334:141–146. doi: 10.1042/bj3340141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M., Haberberger T., Gervasi D. C., Michelson K. S., Gunzler V., Kondo K., Yang H., Sorokina I., Conaway R. C., Conaway J. W., Kaelin W. G., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P., et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O-2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jeng Y. M., Peng S. Y., Lin C. Y., Hsu H. C. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin. Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Maher E. R. The VHL tumour-suppressor gene paradigm. Trends Genet. 1998;14:423–426. doi: 10.1016/s0168-9525(98)01558-3. [DOI] [PubMed] [Google Scholar]
- Kallio P. J., Wilson W. J., O'Brien S., Makino Y., Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- Kamura T., Sato S., Haque D., Liu L., Kaelin W. G., Jr., Conaway R. C., Conaway J. W. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Ota T., Jisaki F., Ueda Y., Tanaka T., Odashima S., Suzuki F., Terada Y., Tatsuka M. Mitotic kinase expression and colorectal cancer progression. J. Natl. Cancer Inst. 1999;91:1160–1162. doi: 10.1093/jnci/91.13.1160. [DOI] [PubMed] [Google Scholar]
- Katayama H., Zhou H., Li Q., Tatsuka M., Sen S. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 2001;276:46219–46224. doi: 10.1074/jbc.M107540200. [DOI] [PubMed] [Google Scholar]
- Kietzmann T., Cornesse Y., Brechtel K., Modaressi S., Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem. J. 2001;354:531–537. doi: 10.1042/0264-6021:3540531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T., Görlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin. Cell Dev. Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Kietzmann T., Roth U., Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia inducible factor-1 in rat hepatocytes. Blood. 1999;94:4177–4185. [PubMed] [Google Scholar]
- Kim G. J., Cho S. J., Won N. H., Sung J. M., Kim H., Chun Y. H., Park S. H. Genomic imbalances in Korean hepatocellular carcinoma. Cancer Genet. Cytogenet. 2003;142:129–133. doi: 10.1016/s0165-4608(02)00834-8. [DOI] [PubMed] [Google Scholar]
- Klein A., Jung V., Zang K. D., Henn W., Montenarh M., Kartarius S., Steudel W. I., Urbschat S. Detailed chromosomal characterization of the breast cancer cell line MCF7 with special focus on the expression of the serine-threonine kinase 15. Oncol. Rep. 2005;14:23–31. [PubMed] [Google Scholar]
- Klein A., Reichardt W., Jung V., Zang K. D., Meese E., Urbschat S. Overexpression and amplification of STK15 in human gliomas. Int. J. Oncol. 2004;25:1789–1794. [PubMed] [Google Scholar]
- Koivunen P., Tiainen P., Hyvarinen J., Williams K. E., Sormunen R., Klaus S. J., Kivirikko K. I., Myllyharju J. An endoplasmic reticulum transmembrane prolyl 4-hydroxylase is induced by hypoxia and acts on hypoxia-inducible factor alpha. J. Biol. Chem. 2007;282:30544–30552. doi: 10.1074/jbc.M704988200. [DOI] [PubMed] [Google Scholar]
- Kufer T. A., Sillje H. H., Korner R., Gruss O. J., Meraldi P., Nigg E. A. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen C. N., Jr, Lin Y. G., Immaneni A., Deavers M. T., Merritt W. M., Spannuth W. A., Bodurka D. C., Gershenson D. M., Brinkley W. R., Sood A. K. Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin. Cancer Res. 2007;13:4098–4104. doi: 10.1158/1078-0432.CCR-07-0431. [DOI] [PubMed] [Google Scholar]
- Lando D., Peet D. J., Gorman J. J., Whelan D. A., Whitelaw M. L., Bruick R. K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002a;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D., Peet D. J., Whelan D. A., Gorman J. J., Whitelaw M. L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002b;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Mahon P. C., Hirota K., Semenza G. L. FIH-1, a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalo D. J., Rowan A., Lavoie T., Natarajan L., Kelly B. D., Ye S. Q., Garcia J. G., Semenza G. L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Min J. H., Yang H., Ivan M., Gertler F., Kaelin W. G., Jr, Pavletich N. P. Structure of an HIF-1alpha-pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Iwao K., Egawa C., Noguchi S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int. J. Cancer. 2001;92:370–373. doi: 10.1002/ijc.1200. [DOI] [PubMed] [Google Scholar]
- Niketeghad F., Decker H. J., Caselmann W. H., Lund P., Geissler F., Dienes H. P., Schirmacher P. Frequent genomic imbalances suggest commonly altered tumour genes in human hepatocarcinogenesis. Br. J. Cancer. 2001;85:697–704. doi: 10.1054/bjoc.2001.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehme F., Ellinghaus P., Kolkhof P., Smith T. J., Ramakrishnan S., Hutter J., Schramm M., Flamme I. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem. Biophys. Res. Commun. 2002;296:343–349. doi: 10.1016/s0006-291x(02)00862-8. [DOI] [PubMed] [Google Scholar]
- Ohh M., Park C. W., Ivan M., Hoffman M. A., Kim T. Y., Huang L. E., Pavletich N., Chau V., Kaelin W. G., Jr Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Petry A., Djordjevic T., Weitnauer M., Kietzmann T., Hess J., Görlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid. Redox. Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- Reichardt W., Jung V., Brunner C., Klein A., Wemmert S., Romeike B. F., Zang K. D., Urbschat S. The putative serine/threonine kinase gene STK15 on chromosome 20q13.2 is amplified in human gliomas. Oncol. Rep. 2003;10:1275–1279. [PubMed] [Google Scholar]
- Safran M., Kaelin W. G., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura C., Hagiwara A., Yasuoka R., Fujita Y., Nakanishi M., Masuda K., Shimomura K., Nakamura Y., Inazawa J., Abe T., Yamagishi H. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br. J. Cancer. 2001;84:824–831. doi: 10.1054/bjoc.2000.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S., Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Scharf J. G., Unterman T. G., Kietzmann T. Oxygen-dependent modulation of IGF binding protein biosynthesis in primary cultures of rat hepatocytes. Endocrinology. 2005;146:5433–5443. doi: 10.1210/en.2005-0948. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- Sen S., Zhou H., White R. A. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ueda A., Kanamori H., Ideguchi H., Yang J., Kitajima S., Ishigatsubo Y. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J. Biol. Chem. 2002;277:10719–10726. doi: 10.1074/jbc.M108252200. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kimura M., Matsunaga K., Fukada D., Mori H., Okano Y. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999;59:2041–2044. [PubMed] [Google Scholar]
- Tanimoto K., Makino Y., Pereira T., Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayakumar T. S., Belakavadi M., Choi K. H., Pandey P. K., Fondell J. D. Regulation of Aurora-A kinase gene expression via GABP recruitment of TRAP220/MED1. J. Biol. Chem. 2006;281:14691–14699. doi: 10.1074/jbc.M600163200. [DOI] [PubMed] [Google Scholar]
- Wenger R. H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Wenger R. H., Stiehl D. P., Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci. STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- Zhou H., Kuang J., Zhong L., Kuo W. L., Gray J. W., Sahin A., Brinkley B. R., Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]