Abstract
For the zonula adherens (ZA) to be established by linear arrangement of adherens junctions (AJs) in epithelial sheet cells, critical for the epithelial cell sheet formation and intercellular barrier function, myosin-2 is supposedly integrated into the ZA with the result of overlapping localization of E-cadherin/actin/myosin-2. Here, we immunofluorescently showed that myosin-2 failed to be integrated into the ZA in cultured epithelial-type ZO1(ko)/2(kd) Eph4 cells lacking ZO-1 and -2 (zonula occludens-1 and -2) by knockout and knockdown, respectively. Instead, a linearized but fragmented arrangement of AJs was formed in the way that it was positive for E-cadherin/actin, but negative for myosin-2 (designated prezonula-AJ). Transfection of full-length ZO-1 or ZO-2, or ZO-1 lacking its PDZ (PSD-95/discs large/zonula occludens-1)-1/2 domains (but not one lacking PDZ-1/2/3) into ZO1(ko)/2(kd) Eph4 cells restored the junctional integration of myosin-2 with prezonula-AJ to establish the ZA. Transfection of dominant-active RhoA or Rho-kinase (ROCK), as well as administration of lysophosphatidic acid or Y27632, which activates RhoA or inhibits ROCK, respectively, suggested that RhoA regulated the junctional integration of myosin-2 into ZA in a manner such that ROCK played a necessary but not-sufficient role. Fluorescence resonance energy transfer analyses revealed that spatiotemporal Rho-activation occurred in a ZO-1/2–dependent way to establish ZA from primordial forms in epithelial cells.
INTRODUCTION
ZO-1/2/3 (zonula occludens-1/2/3) are membrane-associated guanylate kinase-family proteins present in tight junctions (Woods and Bryant, 1993; Anderson, 1995; Kim, 1995). In epithelial cells, ZO-1/2 are exclusively located at the zonula occludens (ZO) composed of tight junctions (TJs); in the absence of the ZO, they are enriched at the cell–cell adherens junctions (AJs) (Stevenson et al., 1986; Itoh et al., 1993). In epithelial cells lacking ZO-1/2, evidence has accumulated suggesting that ZO-1/2 play critical roles in the formation and function of epithelial-typed fully linearized AJs and TJs, i.e., the zonula adherens (ZA) and ZO, respectively (Umeda et al., 2006; McNeil et al., 2006: Fanning et al., 2007; Hernandez et al., 2007; Ikenouchi et al., 2007). In this respect ZO-1/2 reportedly bind to AJ- and TJ-constitutive proteins (Itoh et al., 1997, 1999a,b; Yamamoto et al., 1997; Schmidt et al., 2004). The functional roles of ZO-1/2 in the formation of ZA and ZO are believed to be coordinated by apposite switching mechanisms. ZO-1-knockout mice as well as ZO-2-knockout mice were recently revealed as embryonic lethal, suggesting a nonredundant relationship between them (Katsuno et al., 2008; Xu et al., 2008). ZO-3 has only been detected in epithelia, being localized in ZO in a ZO-1/2–dependent manner (Inoko et al., 2003). However, because its knockout shows no phenotypes in cell- and mouse-level analyses (Adachi et al., 2006), the functional relevance of ZO-3 remains unclear.
In cultured epithelial-type ZO1(ko)/2(kd) Eph4 cells, in which the expression of ZO-1/2 was suppressed by knockout and knockdown, respectively, we found that they were required for claudin polymerization necessary to form the ZO with paracellular barrier function (Umeda et al., 2006). The linearization of E-cadherin-based cell-cell AJs was also significantly delayed by the deficiency of ZO-1/2 (Ikenouchi et al., 2007), although the detailed analyses of the delayed process remained to be elucidated. In the present study, our analysis of ZO1(ko)/2(kd) Eph4 cells suggests that the E-cadherin–based AJ, associated with actin but not with myosin-2, represents a previously undescribed type of junctional state, the “prezonula-AJ,” which we have observed for the first time in cells lacking both ZO-1/2 function. In contrast, the ZA in ZO1(wt)/2(wt) Eph4 cells is positive for E-cadherin, actin, and myosin-2. The junctional integration of myosin-2 with the prezonula-AJ is likely to be a critical step in finally establishing the ZA, and here it has been suggested that this process is dependent on ZO-1/2 and RhoA.
MATERIALS AND METHODS
Antibodies
Rat anti-ZO-1 monoclonal antibody (mAb) was used as described previously (Kitajiri et al., 2004) and rabbit anti-ZO-2 polyclonal antibody was generated using the C-terminal region (1582–1737 amino acids [a.a.]) of mouse ZO-2 by H. Nojima in our laboratory. Rat anti-mouse E-cadherin mAb (ECCD2) was generously provided by M. Takeichi (Riken, CDB, Kobe, Japan) (Shirayoshi et al., 1986). The hybridoma cell line of mouse anti-myosin-2 mAb (CM2-23) was purchased from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Rat anti-green fluorescent protein (GFP) mAb and Alexa 350-phalloidin were purchased from Nacalai Tesque (Kyoto, Japan), and Invitrogen, (Carlsbad, CA), respectively.
Cell Culture
ZO1(ko)/2(kd) Eph4 cells and ZO1(ko)/2(ko) F9 cells were established by Umeda et al. (2006). Eph4 and F9 cells with or without ZO-1/2 were cultured in DMEM including 10% fetal calf serum. Visceral endodermal differentiation of F9 cells was performed as described previously (Adachi et al., 2006). Briefly, under monolayer culture conditions, cells were plated at a density of 6 × 103 cells/cm3. The next day, 10−6 M of retinoic acid was added to the medium and cultured in the presence of retinoic acid for an additional 7 d. For the calcium depletion assay, 12 h after incubation in a calcium-free medium, the medium was changed to DMEM containing calcium and fixed at time points of 0, 2, 4, 8, and 12 h after calcium repletion, followed by immunofluorescence microscopy.
Assay to Evaluate the Susceptibility of Cell–Cell Adhesion of ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 Cells to Extracellular Trypsin Treatment
The confluent ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells were treated with 0.125% trypsin in 1 mM EDTA/PBS at 37°C for 10 min. After the digestion was stopped by the addition of 10% fetal calf serum/DMEM, the reaction mixture was applied to the slide glass, and the particle numbers were counted in a Neubauer cell counter (Erma, Tokyo, Japan).
Construction and Chemicals
Rac1-DA, RhoA-DA, and Cdc-42-DA were subcloned into CAGGS-N-Venus from pEF-Rac1-DA, pEF-RhoA-DA, and pEF-RhoA-DA. Y-27632, latrunculin A and blebbistatin were purchased from Sigma-Aldrich (St. Louis, MO). ZO-1 deletion mutants 5A (181-1745 a.a.), 5B (411-1745 a.a.), and 5C (505-1745 a.a.) were generated by polymerase chain reaction (PCR) amplification and subcloned into CAG-N-Venus vector. The Expression vector of pEGFP-myosin light chain (MLC) was kindly gifted by Dr. H Hosoya (University of Hiroshima, Hiroshima, Japan)(Uchimura et al., 2002).
Immunofluorescence Microscopy
Cells grown on coverslips were fixed in 2% formaldehyde and processed for immunofluorescence microscopy as described previously (Itoh et al., 1999a).
Fluorescence Resonance Energy Transfer (FRET) Analyses
Raichu FRET probes for Rac1 and RhoA were generous gifts from M. Matsuda (Kyoto University, Kyoto, Japan). Raichu probes were transiently transfected into ZO-1(wt)/2(wt) or ZO-1(ko)/2(kd) Eph4 cells by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's recommended procedure. Twenty-four h later, cells were incubated in a calcium-free medium for 12 h, and the medium was changed to a medium with normal calcium levels. After changing the medium, cells were imaged every 5 min with an Olympus IX-71 microscope equipped with a 75-W xenon arc lamp, two filter changers, a temperature-controlled chamber, and a cooled charge-coupled device camera, CoolSNAP HQ, controlled by MetaMorph software (Molecular Devices, Sunnyvale, CA). The ratio image of yellow fluorescent protein (YFP)/cyan fluorescent protein (CFP) was created with MetaMorph software and used to represent FRET efficiency.
In the IMD mode images (Ratio), eight colors from red to blue are used to represent the YFP/CFP ratio, with the intensity of each color indicating the mean intensity of YFP and CFP. The upper and lower limits of the ratio range are shown on the right.
Live Imaging Analyses on GFP-MLC–transfected Cells
The expression vectors of pEGFP-MLC were transiently transfected into ZO1-(ko)/2(kd) Eph4 cells by using Lipofectamine 2000 (Invitrogen) following the manufacturer's recommended procedure. Twenty-four h later, cells were imaged every minute with the same equipment as used for FRET analyses. Seven minutes after imaging, the same volume of medium containing lysophosphatidic acid (LPA) as that in the dishes was added at the final concentration of 50 μM LPA.
RESULTS
Perturbation of Formation of ZA by Deficiency of ZO-1/2
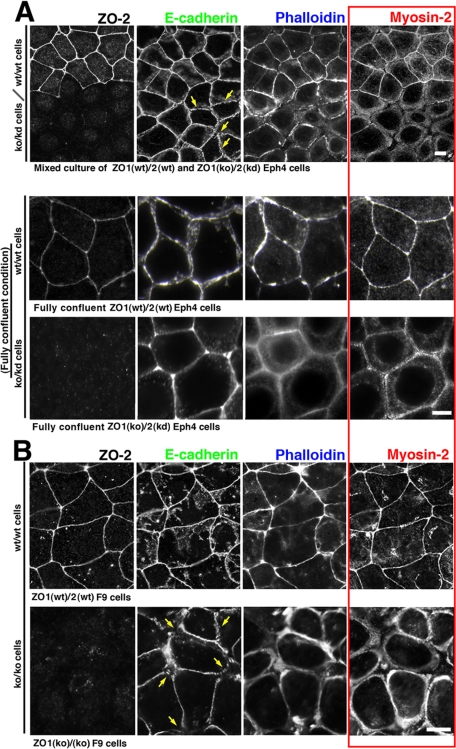
To examine the ZO-1/2–dependent role of the actomyosin-based circumferential ring in establishing the fully developed ZA in epithelial cells, we analyzed the junctional localization of E-cadherin, actin and myosin-2 in cultured ZO1(ko)/2(kd) Eph4 cells in comparison with ZO1(wt)/2(wt) Eph4 cells by immunofluorescence. A mixture of ZO1(ko)/2(kd) and ZO1(wt)/2(wt) Eph4 cells was cultured and examined by immunofluorescence microscopy (Figure 1). In fully confluent ZO1(wt)/2(wt) Eph4 cells with linear ZO-1/2 signals at TJs, the linearly arranged signals for E-cadherin/actin/myosin-2 overlapped at the ZA, as shown previously (Bertet et al., 2004; Zallen and Wieschaus, 2004; Hildebrand, 2005). In contrast in confluent ZO1(ko)/2(kd) Eph4 cells, AJs were associated with partially linearized patterns of E-cadherin and actin, but not with myosin-2, a previously unreported AJ state, which we have designated the “prezonula-AJ.” It was noteworthy that myosin-2 was distributed in the cortical layer of the cytoplasm and, in the fully confluent condition, took a precircumferential ring-like arrangement with actin, separated from but along with the prezonula-AJ in ZO1(ko)/2(kd) Eph4 cells. It was contrasted to ZO1(wt)/2(wt) Eph4 cells in which myosin-2 was integrated into ZA together with actin.
Figure 1.
ZO-1/2–dependent integration of myosin-2 in ZA. (A) Immuno- fluorescence micrographs for E-cadherin/ myosin-2/ZO-2 and fluorescence micrographs for actin due to phalloidin-staining in a mixed or non-mixed culture of ZO1(wt)/2(wt) and ZO1(ko)/ 2(kd) epithelial-type Eph4 cells. (B) Immunofluorescence micrographs for E-cadherin/myosin-2/ZO-2 and fluorescence micrographs for actin due to phalloidin- staining in ZO1(wt)/2(wt) and ZO1(ko)/2(ko) F9 cells differentiated into epithelial type. In contrast to the linear colocalization of E-cadherin/myosin-2/actin at ZA in ZO1(wt)/2(wt) Eph4 and F9 cells, myosin-2 was not detected at prezonula-AJs in ZO1(ko)/2(kd) Eph4 or ZO1(ko)/2(ko) F9 cells, but rather it was distributed in the cortical layer with actin, distinctly from but along with the prezonula-AJ. Note the fragmentation of E-cadherin-based linear staining patterns (arrows). Bars, 5 μm.
Similar results, namely the integration and dissociation of myosin-2 with the ZA, were observed, respectively, in ZO1(wt)/2(wt) and ZO1(ko)/2(ko) F9 cells after differentiation to epithelium. However, association of actin with prezonula-AJ was not detectable in epithelial-typed ZO1(ko)/2(ko) F9 cells, in contrast to the partial association of actin with prezonula-AJ in ZO1(ko)/2(kd) Eph4 cells. In epithelially differentiated ZO1(ko)/2(ko) F9 cells, the immunofluorescence signal for actin was associated with the myosin-2 signal. The differences between Eph4 and F9 cells were possibly due to differences in the methodology of ZO-2 suppression, i.e., by knockdown or knockout, or possibly due to differences in cell type. Together, it seemed likely that the junctional integration of myosin-2 with prezonula-AJ was a key step in the ZO-1/2–dependent establishment of the ZA apparatus in wild-type epithelial sheet cells.
Susceptibility of Cell–Cell Adhesion of ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 Cells to Extracellular Trypsin Treatment
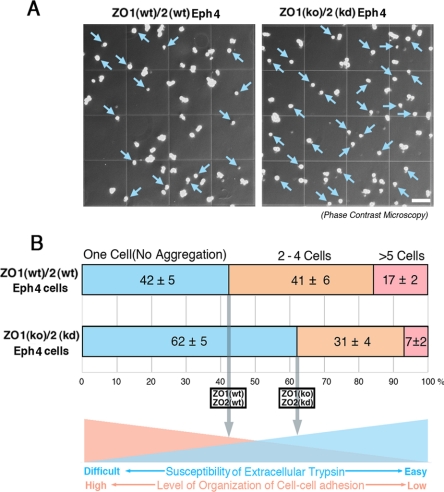
To access the possibility that the ZO-1/2–dependent integration of myosin-2 into zonula adherens was important for highly organized cell–cell adhesion to finally establish the linear arrangement of zonula adherens, the susceptibility of cell–cell adhesion of the confluent ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells to the extracellular trypsin treatment was examined. For this purpose, we treated the confluent ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells by 0.125% trypsin in 1 mM EDTA/phosphate-buffered saline (PBS) at 37°C for 10 min, dispersing the confluent epithelial sheet cells to single cells and aggregates of two to four cells and/or more than five cells. Then, we counted the single cells and aggregates of two to four cells and/or more than five cells (Figure 2A). As a result, the ratios in number of single cells against cell aggregates were significantly higher in ZO1(ko)/2(kd) Eph4 cells than those in ZO1(wt)/2(wt) Eph4 cells, suggesting that the cell–cell adhesion of ZO1(ko)/2(kd) Eph4 cells was more susceptible to the extracellular trypsin treatment compared with that of ZO1(wt)/2(wt) Eph4 cells (Figure 2B). Hence, it was likely that the cell–cell adhesion in ZO1(wt)/2(wt) Eph4 cells was more highly organized than that in ZO1(ko)/2(kd) Eph4 cells in such a manner that the cell–cell adhesion of ZO1(wt)/2(wt) Eph4 was more insusceptible to extracellular trypsin treatment than that of ZO1(kd)/2(ko) Eph4 cells.
Figure 2.
Susceptibility of cell–cell adhesion of ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells to extracellular trypsin treatment. (A) Phase-contrast micrographs of ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells after extracellular trypsin treatment. To examine the level of organization of cell-cell adhesion, we treated the confluent ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells by 0.125% trypsin in 1 mM EDTA/PBS at 37°C for 10 min. The numbers of single cells are likely to be large in ZO1(ko)/2(kd) cells lacking ZO-1/2, compared with those in ZO1(wt)/2(wt) Eph4 cells (blue arrows). Bar, 0.125 mm. (B) The ratios of single cells after extracellular trypsin treatment in ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells. To estimate the susceptibility of ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells to extracellular trypsin treatment, single cells as well as two- to four cell- and more than five-cell aggregates were counted. Six independent experiments were statistically analyzed. The ratios of single cells are significantly high in ZO1(ko)/2(kd) Eph4 cells, compared with those in ZO1(wt)/2(wt) Eph4 cells. These results suggest that in ZO1(wt)/2(wt) Eph4 cells, cell–cell adhesion is more highly organized in such a way that cell–cell adhesion is more insusceptible to extracellular trypsin.
Time Course of Organization of AJs in ZO1(wt)/ZO2(wt) and ZO1(ko)/2(kd) Eph4 Cells
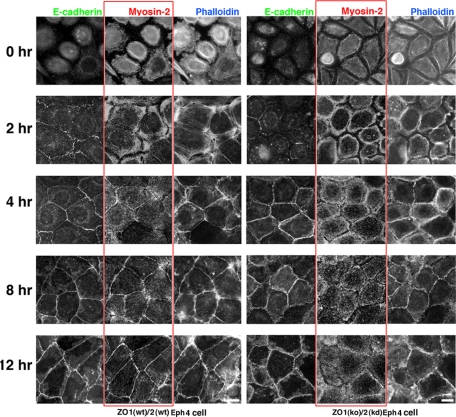
To follow the time course of the organized arrangement of AJs, ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells were subjected to 12-h incubation under calcium-free conditions, which effectively abolished E-cadherin–based cell–cell adhesion. The calcium-free medium was then replaced by a physiological medium, raising the calcium concentration from the depleted level to 2 mM, and the cells were immunofluorescence labeled for E-cadherin/myosin-2/ZO-2 (to confirm the knockdown of ZO-2), and fluorescently labeled for actin by Alexa-350-phalloidin, at 0, 2, 4, 8, and 12 h after calcium repletion (Figure 3). At 2 h after the calcium switching in ZO1(wt)/2(wt) epithelial Eph4 cells, E-cadherin accumulated in punctate AJs, with no accumulation of actin or myosin-2. Myosin-2 and actin were distributed around cell periphery in the cortical layer at 2 h, followed by junctional integration at 4 h after calcium repletion. A mature ZA positive for E-cadherin, actin, and myosin-2 developed by 8–12 h and was maintained thereafter.
Figure 3.
Time course of the establishment of ZA or prezonula-AJ in ZO1(wt)/2(wt) or ZO1(ko)/2(kd) Eph4 cells after repletion of calcium concentration to physiological molarity. Cells were stained for E-cadherin/myosin-2/actin/ZO-2 at time points 0, 2, 4, 8, and 12 h after calcium switch. In ZO1(wt)/2(wt) Eph4 cells, spot-AJs are formed at 2 h followed by subsequent formation of ZAs, positive for E-cadherin/myosin-2/actin/ZO-2. In contrast in ZO1(ko)/2(kd) Eph4 cells, prezonula-AJs, positive for E-cadherin/actin, but negative for myosin-2/ZO-2, are formed at 4 h. In ZO1(ko)/2(kd) Eph4 cells, myosin-2 is not integrated into prezonula-AJs but distributed in the cortical layer of cytoplasm thereafter. Bar, 5 μm.
Contrastingly in ZO1(ko)/2(kd) Eph4 cells, at 2 h after calcium repletion, E-cadherin accumulated in punctate-AJs, but no association of actin and myosin-2 with punctate-AJs was detected. At 4 h, actin had accumulated in prezonula-AJ, but myosin-2 was absent from prezonula-AJ and was instead detected around cell periphery along with actin. This combination of prezonula-AJ, positive for E-cadherin/actin but not for myosin-2, and cytoplasmic myosin-2 and actin continued to 8–12 h and beyond.
Restoration of Junctional Integration of Myosin-2 in ZO1(ko)/2(kd) Eph4 Cells by the ZO-1 Deletion Mutants
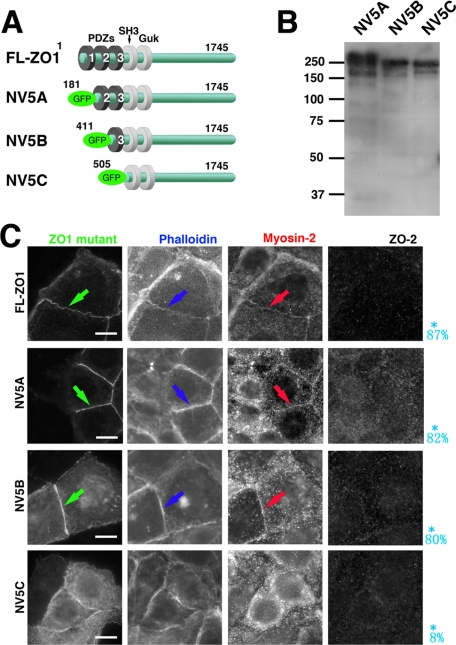
Next, to examine the specific roles of ZO-1 and -2 in the junctional integration of myosin-2 into ZA, we transfected full-length ZO-1 and/or ZO-2 into ZO1(ko)/2(kd) Eph4 cells and found that myosin-2 was junctionally integrated into the ZA, as it was in ZO1(wt)/2(wt) Eph4 cells (Figure 4). The deletion mutants of ZO-1 lacking the PDZ-1 domain or PDZ-1/2 were localized in ZA, positive for myosin-2, and thus amenable to the junctional integration of myosin-2 into ZA (Figure 4). In contrast, the mutant of ZO-1 lacking PDZ-1/2/3, although it did localize in the prezonula-AJ, did not restore junctional integration of myosin-2, resulting in a combination of myosin-2-negative prezonula-AJ and the cortical distribution of cytoplasmic myosin-2. These results suggested that PDZ-3 might play a role for the junctional integration of myosin-2 into ZA.
Figure 4.
Role of PDZ-3 domain of ZO-1 in restoration of formation of ZA. (A) Schematic drawing which shows constructs of ZO-1 deletion mutants. ZO-1 deletion mutants 5A (181-1745 a.a.), 5B (411-1745 a.a.), and 5C (505-1745 a.a.) were generated by PCR amplification and subcloned into CAG-N-Venus vector, producing GFP-NV5A(NV5A), GFP-NV5B(NV5B), and GFP-NV5C(NV5C) constructs, respectively. (B) Immunoblotting of total cell lysates of ZO1(ko)/2(kd) Eph4 cells transiently expressing each mutant of NV5A, NV5B, and NV5C. Detection by anti-GFP mAb. (C) Immunofluorescence of ZO1(ko)/2(kd) Eph4 cells transiently expressing each mutant of NV5A, NV5B, and NV5C for myosin-2, ZO-2, and fluorescence for GFP-ZO-1 mutant and actin due to phalloidin-staining. The mutants of ZO-1 lacking the PDZ-1 or PDZ-1/2 domains (NV5A and NV5B) are localized in ZA positive for myosin-2, similarly effective for junctional integration of myosin-2 into ZA (arrows). In contrast, the mutant lacking the PDZ1/2/3 domain of (NV5C), though localized in prezonula-ZA, does not restore junctional integration of myosin-2, resulting in combination of myosin-2–negative prezonula-AJ and cytoplasmic actomyosin-positive ring like structures. The probability of integration of myosin-2 into cell–cell AJ to form ZA, reduced from 20 transfectants in the immunofluorescence micrographs, are shown in blue letters. Bars, 5 μm.
In these experiments, when we could not distinguish two lines of myosin-2 staining on both sides of cell–cell adhesion at the magnification of 40× (without use of oil) by using the objective lens, we stated that myosin-2 was integrated into zonula adherens. We estimated that, in this condition, the critical distance between myosin-2 staining should be within 0.5 μm for myosin-2 staining to be judged to be integrated into zonula adherens. It was noteworthy that ZO-1–related constructs needed to be expressed on both sides of the cells for integration of myosin-2 to ZA, suggesting the cell–cell adhesion-dependent manner of organization of integration of myosin-2 to establish ZA.
Dissociation of Myosin-2 from ZA by Latrunculin A, Blebbistatin and Y27632 in Wild-Type ZO1(wt)/2(wt) Eph4 Cells
We determined that certain inhibitors of cytoskeleton-related events and signaling, namely, latrunculin A, blebbistatin, and Y27632, dissociated myosin-2 from the zonula adherens without causing extensive changes in cell morphology. When wild-type ZO1(wt)/2(wt) Eph4 cells were treated with latrunculin A or blebbistatin, myosin-2 and actin became dissociated from the ZA, with faint traces of E-cadherin persisting (Supplemental Figure 1S). On treatment with either latrunculin A or blebbistatin, myosin-2 signals were separated from the ZA that was further disrupted in a manner as evidenced by the disappearance of immunofluorescence signals for E-cadherin (Ivanov et al., 2005).
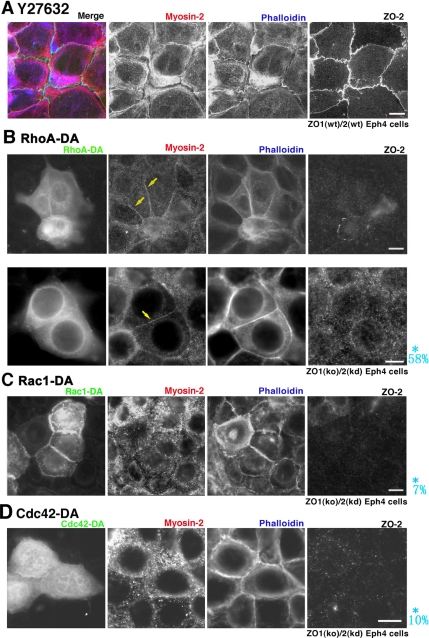
We next examined the effects of Y27632, an inhibitor of ROCK (Ishizaki et al., 2000), on the ZA in wild-type ZO1(wt)/2(wt) Eph4 cells(Figure 5). As a result, we found that Y27632 dissociated myosin-2 from the ZA, consistent with a previous report on MCF7 cells (Shewan et al., 2005). Phosphorylation of myosin-2 is reportedly a downstream event in the ROCK pathway. However, transfection of phosphomimic forms of myosin-2 (Uchimura et al., 2002) did not restore the junctional integration of myosin-2 in ZO1(ko)/2(kd) Eph4 cells (data not shown). Taken with the finding that the transfection of ROCK-I into ZO1(ko)/2(kd) Eph4 cells did not restore the junctional integration of myosin-2 (data not shown), it seemed possible that RhoA, a factor upstream of ROCK, regulated the junctional integration of myosin-2 into ZA in a manner such that ROCK played a necessary but not a sufficient role.
Figure 5.
Role of RhoA in formation of ZA. (A) Effects of Y27632 in formation of ZA. When ZO1(wt)/2(wt) Eph4 cells become confluent and are incubated with Y27632, an inhibitor of Rho-kinase, signals for myosin-2/actin (due to phalloidin-staining) suggest that ZA is disintegrated so that the circumferential ring is separated from prezonula-AJ. (B) Transfection of dominant active RhoA (RhoA-DA). In RhoA-DA–expressing ZO1(ko)/2(kd) Eph4 cells, signals for myosin-2/actin are overlapped and linearly arranged at ZA (arrows). (C and D) Transfection of dominant-active Rac1 (Rac1-DA) or dominant-active Cdc42 (Cdc42-DA). Actin is associated with the linearized but fragmented AJ, prezonula-AJ, with which myosin-2 is not associated. A myosin-2 signal exists with weak actin-signal in the cell periphery. Asterisk (*), probability of integration of myosin-2 into cell-cell AJ to form ZA, deduced from 20 transfectants the immunofluorescence micrographs, are shown in blue letters. Bars, 5 μm.
Effects of RhoA in Myosin-2 Integration to ZA in ZO1(ko)/2(kd) Eph4 Cells
Next, to examine the role of RhoA on myosin-2 junctional integration, we transfected a dominant-active form of GFP-RhoA (RhoA-DA), and, for the sake of comparison, dominant-active forms of GFP-Rac1 (Rac1-DA) and GFP-Cdc42(Cdc42-DA), into ZO1(ko)/2(kd) Eph4 cells (Figure 5). The transfectants were immunofluorescently stained for myosin-2/ZO-2 as well as fluorescently stained for actin due to phalloidin-staining. In RhoA-DA–transfected ZO1(ko)/2(kd) Eph4 cells, the linearly arranged actin signals co-localized with myosin-2-signals in the ZA, although myosin-2-signals were not detected in the prezonula-AJ in nontransfected ZO1(ko)/2(kd) Eph4 cells. Thus, it was suggested that RhoA-DA effectively induced integration of myosin-2 into the ZA in ZO1(ko)/2(kd) Eph4 cells. It was noteworthy that RhoA-DA needed to be expressed on both sides of the adjacent cells for integration of myosin-2 to ZA, suggesting the cell–cell adhesion-dependent way of RhoA-induced integration of myosin-2 to establish ZA.
In contrast, when Rac1-DA or Cdc42-DA was transfected into ZO1(ko)/2(kd) Eph4 cells, the myosin-2-signals did not integrate into AJs, but rather they remained in the cortical layer of cytoplasm. The signals for actin and E-cadherin were, however, detected in AJs, and as a result such junctions were judged as arrested in the prezonula-AJ state. It was noted that Rac1-DA, but not Cdc42-DA, promoted actin linearity, compared with nontransfectants.
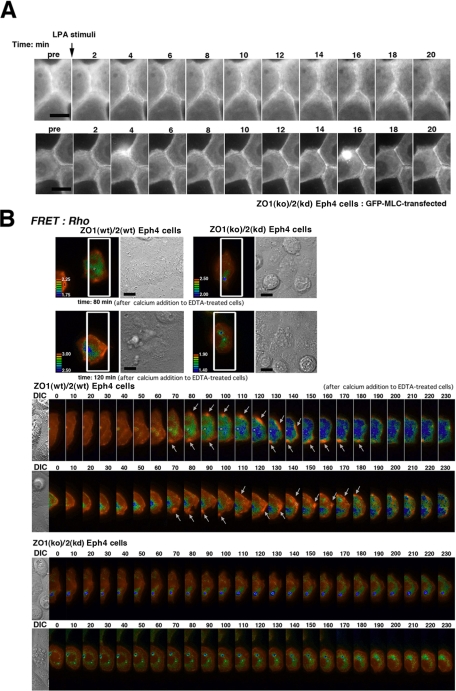
Next, to further confirm the role of RhoA in myosin-2 integration to zonula adherens, we examined the effects of the administration of LPA (lysophosphatidic acid), activator of RhoA, to ZO1(ko)/2(kd) Eph4 cells. As followed by the real-time recording of GFP-MLC-2–transfected ZO1(ko)/2(kd) Eph4 cells, addition of LPA likely induced the integration of myosin-2 to zonula adherens at time point of 6 ∼20 min (Figure 6A and Supplemental Movies 1 and 2). Collectively, it was suggested that RhoA effected the integration of myosin-2 to ZA.
Figure 6.
Activation of RhoA during ZO-1/2–dependent establishment of ZA. (A) LPA stimulation on GFP-MLC–transfected ZO1(ko)/2(kd) Eph4 cells. It is recorded in live imaging that myosin-2 is broadly distributed in a ring-like arrangements at the cell periphery before LPA administration, but after LPA administration myosin-2 is integrated into cell–cell AJ to form ZA in a sharply linearized way. (B) FRET analyses. Top images show ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells at the time point of 80 or 120 min, respectively, for which the regions to be lined up below are marked by white boxes. The Raichu-RhoA–expressing ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells are lined up below, at 10-min intervals from the point of calcium switching. Note the spatiotemporally specific activation of RhoA in Raichu-RhoA–expressing ZO1(wt)/2(wt) Eph4 cells during the establishment of the ZA, contrasted with ZO1(ko)/2(kd) Eph4 cells (arrows).
FRET Analysis on Activation of RhoA in Establishing ZA
To spatiotemporally elucidate the role of activation of RhoA in the junctional integration of myosin-2, we applied FRET analyses in a time-course–dependent spatiotemporal survey of RhoA and Rac1 activation, by using Raichu FRET-based biosensors (Nakamura et al.; 2005, Itoh et al., 2002). For this purpose, Raichu probes were transiently introduced into ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells. After incubation of the Raichu-probed ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells in a calcium-free medium for 12 h, the calcium-free medium was replaced with a normal medium to switch the calcium concentration to a calcium level of 2 mM. We then immediately (within 5 min) selected a single cell from the Raichu transfectants, as determined by its suitability for live imaging in terms of the expression level of the Raichu probe and the state and position of the cell in the epithelial cell sheets (Figure 6B); two representative examples of each FRET pair from three to five independent experiments are shown. As revealed in the survey for Rho activation, there was a general tendency for the level of active RhoA to be enhanced in the cell boundary in ZO1(wt)/2(wt) and ZO1(ko)/2(kd) Eph4 cells at all time points after calcium repletion. Notably, however, distinct patterns of RhoA activation were identified in a time course-dependent way in ZO1(wt)/2(wt) but not in ZO1(ko)/2(kd) Eph4 cells. In the ZO1(wt)/2(wt) Eph4 cells, specific zones showing extremely high levels of activated RhoA occurred around cell–cell contact points at 70 min after calcium repletion, and they moved along the cell border until 180 min (Figure 6B and Supplemental Movies 3–6).
The absence of extremely highly activated zones of RhoA around the cell–cell contacts in ZO1(ko)/2(kd) Eph4 cells (Figure 6B, Supplemental Figure 2, and Supplemental Movies 7–10) suggested that the extreme level of activation of RhoA around cell–cell contacts was dependent on ZO-1/2. In ZO1(wt)/2(wt) Eph4 cells the time course after calcium repletion for the generation of highly activated zones was consistent with the time course of the integration of myosin-2 into the ZA and also consistent with the finding that RhoA-DA was able to induce myosin-2 junctional integration in ZO1(ko)/2(kd) Eph4 cells. In contrast, using Raichu-Rac1 as a probe for Rac1 activation, no difference in the spatiotemporal activation of Rac1 was detected between the ZO1(wt)/2(wt) (Supplemental Figure 3 and Supplemental Movies 11 and 12) and ZO1(ko)/2(kd) Eph4 cells (Supplemental Figure 3 and Supplemental Movies 13 and 14).
Tentative Model for the ZO-1/2–dependent Formation of Epithelial ZA
In the present study, by comparing ZO1(ko)/2(kd) Eph4 cells with ZO1(wt)/2(wt) Eph4 cells, we have revealed the ZO-1/2- and RhoA-dependent junctional integration of myosin-2 into ZA in epithelial sheet cells. Based on these findings, we have developed a synergetic model for formation of the ZA as shown schematically in Figure 7. In this model, the process unfolds as follows: 1) As described previously, when E-cadherin-based cell–cell adhesion is initially formed (Yonemura et al., 1995; Vasioukhin et al., 2000), punctate AJs formed complexes with associated components, including catenins and actin (Irie et al., 2004). 2) Punctate AJs develop into prezonula-AJs, which are partially linearized and associated with E-cadherin and actin, but without myosin-2. (Steps 1 and 2 are thought not to be dependent on, but accelerated by ZO-1 and -2.) 3) In ZO1(wt)/2(wt) Eph4 cells in which ZO-1/2 are expressed, the formation of prezonula-AJ and the integration of myosin-2 with prezonula-AJ to establish ZA are likely to proceed simultaneously in such a way that it seems that punctate AJs are directly organized into the ZA. 4) Synergistically, the ZO is formed just above the ZA, thus establishing an epithelial cell sheet with TJ paracellular barrier function (Tsukita et al., 2001; Matter and Balda, 2003). As shown here, the study of ZO1(ko)/2(kd) Eph4 cells have led us to a better understanding of the formation of the ZA as well as of the functions of ZO-1/2, although the exact time course of formation of ZA and ZO is still to be elucidated.
Figure 7.
Schematic drawing of tentative model for the process of ZO-1/2–dependent formation of ZA. When confluent epithelial cells initiate cell–cell adhesion to form epithelial sheets through the raising of calcium concentrations from calcium free to a normal level (2 mM), spot-like AJs are initially formed at sites of cell–cell adhesion. In ZO1(ko)/2(kd) Eph4 cells prezonula-AJs are formed such that they are immunofluorescently positive for E-cadherin/actin but negative for myosin-2. Myosin-2 is distributed in the cortical layer with actin, distinctly but along prezonula-AJ. In contrast in ZO1(wt)/2(wt) Eph4 cells, ZA is simultaneously established, being associated with positive immunofluorescence signals for E-cadherin/myosin-2/ZO-1/ZO-2 and with fluorescence for actin. In ZO1(wt)/2(wt) Eph4 cells, after establishment of the ZA, the TJ is formed along the ZA to establish the ZO in which ZO-1 and ZO-2 are preferentially localized.
DISCUSSION
To date, studies on ZO-1/2 have shown that they are essential molecules involved in the formation of tight junctions on the premise that the ZA composed of AJs has been established, and claudins are expressed in epithelial cells (Umeda et al., 2006). Subsequently, it has been suggested that ZO-1/2 are molecules important not only for TJ formation but also AJ to be organized in a epithelial-typed linearized arrangement (Ikenouchi et al., 2007). However, it has not been fully clarified how ZO-1/2 play an essential role in ZA formation. In this study, we were able to verify this by focusing on the myosin-2 architecture.
In ZO-1-knockout ZO-2-knockdown Eph4 cells [ZO1(ko)/2(kd) Eph4 cells], or ZO-1-knockout ZO-2-knockout F9 cells [ZO1(ko)/2(ko) F9 cells] that have differentiated into epithelial-type, the immunofluorescence of myosin-2 extended in the cytoplasm, indicating that myosin-2 had not been well-integrated into AJs. Close observation showed that these AJs of ZO-1/2–deficient epithelial-typed cells were E-cadherin/actin positive but myosin-2 negative. They were clearly distinguishable from the normal, E-cadherin/actin/myosin-2–positive ZA and should be designated prezonula-AJ. On close observation of E-cadherin staining, AJs clearly seemed fragmented in places rather than smoothly continuous. This observation may suggest that, in the absence of ZO-1/2, the circumferential rings have not been integrated into the AJs, and the continuous, ring-shaped ZA has not been fully formed. It remains to be determined whether the diffuse staining pattern of myosin-2 in the cortical layer of ZO1(ko)/2(kd) Eph4 sheet cells, as well as more clear precircumferential ring-like myosin-2 staining pattern in fully confluent cells, reflects the potential circumferential ring, although it is most likely the case. A similar diffuse staining pattern was also observed during the formation of ZA in wild-type cells. The formation of such a cortical arrangement of myosin-2 and actin along the cell periphery may be essential for the continuity of ZA. Noteworthy is that the junctional integration of myosin-2 with the prezonula-AJ to establish ZA we showed, to our knowledge, is the first observations of the prezonula-AJ state. Thus, the association of actin and subsequent integration of myosin-2 with the prezonula-AJ likely preceded the establishment of the ZA of ZO1(wt)/2(wt) Eph4 cells, which was more highly organized architecture as shown by the more insusceptibility to extracellular trypsin treatment.
The AJ-related actin exists in two forms, colocalized and noncolocalized with myosin-2, and the fact that latrunculin A and blebbistatin induces myosin-2 disintegration in ZO-1(ko)/2(kd) Eph4 cells seems to indicate the importance of cytoskeletal role of actin without myosin-2 and also the importance of actomyosin interaction, respectively, in the integration of myosin-2 into the ZA. The distinct roles of actin organization associated with the above-described two forms are probably important.
ZO-1/2 seem to have two functional aspects: structural proteins and signaling molecules. As structural proteins, ZO-1/2 bind directly to cell adhesion molecules (α-catenin; Itoh et al., 1997), claudin (Itoh et al., 1999a), and occludin (Itoh et al., 1999b) and cytoskeletal actin, thereby forming the cell adhesion–cytoskeletal complex. As signaling molecules, ZO-1/2 dynamically control the organization of the cell–cell contacts, and they were previously suggested to control the temporal activity of Rac1 (Ikenouchi et al., 2007). Using the FRET technique in this study, we showed that ZO-1/2–dependent RhoA activation occurred at the time of ZA formation in a spatiotemporally regulated manner, as shown in ZO1(wt)/2(wt) Eph4 cells. The importance of RhoA in the integration of myosin-2 into the ZA was also confirmed by the results of myosin-2 integration when ZO1(ko)/2(kd) Eph4 cells were transfected with the dominant-active-form of RhoA or stimulated by LPA, an activator of RhoA. In addition, the treatment of the ROCK inhibitor of ZO1(wt)/2(wt) Eph4 cells resulted in myosin-2 disintegration from the ZA, suggesting the importance of RhoA signaling to keep the integration of myosin-2. However, it is still unknown what ROCK targets for myosin-2 integration into the ZA. Because the phospho-mimic mutants of myosin-2 did not effect the integration of myosin-2 into ZA, at least myosin-2-phosphorylation was not dominantly involved in myosin-2 integration into the ZA.
A recent study made use of FRET imaging to show that, during the initiation and expansion of cell–cell adhesion between two epithelial cells, the activation of Rac1 and RhoA was restricted to the edges of cell–cell contact (Yamada and Nelson, 2007). In the present study of cell–cell adhesion in confluent epithelial cells of high cell density after calcium replacement, we found ZO-1/2–dependent generation of the activation zones of RhoA, but not of Rac1, at various points of cell–cell contact around ZO1(wt)/2(wt) Eph4 cells. In addition, this finding differs from previous findings that the activation of Rac1 was dependent on ZO-1/2. This inconsistency might be attributable to differences in cell density under the experimental conditions. Although it was suggested that Rac1 activation was mainly associated with lamellipodia in the initiation of E-cadherin-mediated cell–cell adhesion (Yamada and Nelson, 2007), in the present study the cell–cell adhesion was not likely to be mainly associated with marked lamellipodia motility, due to the higher cell density in the fully confluent cell cultures used in this study, compared with that in the previous study (Ikenouchi et al. 2007).
It was likely that RhoA activation should be spatiotemporally regulated for integration of myosin-2 to ZA. Hence, the results that transfection of RhoA-DA induced the integration of myosin-2 to ZA in ZO1(ko)/2(kd) Eph4 cells might be presumably due to that ZO-2 expression was suppressed by knockdown but was persistent, even if at a low level. If this was the case, restoration of myosin-2 integration should not occur in epithelial-typed ZO1(ko)/2(ko) F9 cells after transfection with RhoA-DA, presumably because ZO-2 failed to provide RhoA-DA with information on its location as a cytoskeletal component. In fact, in our trials no restoration of myosin-2 integration occurred in epithelial-typed ZO1 (ko)/2(ko) F9 cells after transfection with RhoA-DA (data not shown). In addition, it was noteworthy that RhoA-DA and ZO-1 needed to be expressed on both sides of the cell–cell adhesive areas for integration of myosin-2 to ZA, suggesting a cell–cell adhesion-based mechanism of integration of myosin-2 to ZA.
It was shown that the circumferential ring existed as a structure to maintain actomyosin contractility (Owaribe and Masuda, 1982), possibly due to cell–cell adhesion-related signaling process. The presence of such a circumferential ring is peculiar to epithelial cells, and it is probably formed during the process of myosin-2 integration into the ZA. Although these processes usually occur in epithelial cells in a synergetically progressive manner, it seems that the stage before myosin-2 integration into the ZA was observed in this study in epithelial cells with no expression of ZO-1/2. Further detailed studies of the molecular architecture of precircumferential ring will lead to the elucidation of the formation mechanism of the epithelial cell-specific circumferential ring.
In conclusion, the epithelial cells lacking the expression of ZO-1/2 have revealed that ZO-1/2 play dual roles, structural and signaling, and integrate both roles in the final stage of the synergitic formation of ZA and ZO. In this study, we have presented a tentative model for the ZO-1/2–dependent formation of ZA (Figure 7). Because it is plausible that ZO-1/2 bind to various kinds of structural and signaling molecules, one of the most potent ways to further reveal the functional roles of ZO-1/2 should be to identify the binding partners in detail by every possible method, including immunoprecipitation and two-hybrid assays. To examine the role of ZO-1/2 binding partners, the application of Eph4 and F9 cells without expression of ZO-1/2 should be highly potent. Studies along these lines are being conducted in our laboratory.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the members of our laboratory and Dr. J. Ikenouchi for helpful discussion. We especially appreciate the great technical assistance of Ms. Yuka Miyake and also thoughtful discussion with her.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0352) on July 2, 2008.
REFERENCES
- Adachi M., Inoko A., Hata M., Furuse K., Umeda K., Itoh M., Tsukita Sh. Normal establishment of epithelial tight junctions in mice and cultured cells lacking expression of ZO-3, a tight-junction MAGUK protein. Mol. Cell. Biol. 2006;26:9003–9015. doi: 10.1128/MCB.01811-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M. Zonula occludens (ZO)-1 and ZO-2, Membrane-associated guanylate kinase homologues (MAGUKs) of the tight junction. Biochem. Soc. Trans. 1995;23:470–475. doi: 10.1042/bst0230470. [DOI] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. Myosin-dependent junction remodeling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Fanning A. S., Little B. P., Rahner C., Utepbergenov D., Walther Z., Anderson J. M. The unique-5 and -6 motifs of zo-1 regulate tight junction strand localization and scaffolding properties. Mol. Biol. Cell. 2007;18:721–731. doi: 10.1091/mbc.E06-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez S., Munguia B. C., Gonzalez-Mariscal L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp. Cell Res. 2007;313:1533–1547. doi: 10.1016/j.yexcr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. D. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J. Cell Sci. 2005;118:5191–5203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J., Umeda K., Tsukita Sa., Furuse M., Tsukita Sh. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J. Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko A., Itoh M., Tamura A., Matsuda M., Furuse M., Tsukita Sh. Expression and distribution of ZO-3, a tight junction MAGUK protein, in mouse tissues. Genes Cells. 2003;8:837–845. doi: 10.1046/j.1365-2443.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- Irie K., Shimizu K., Sakisaka T., Ikeda W., Takai Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004;15:643–656. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita Sh. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999a;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R. E., Kurokawa K., Ohba Y., Yoshizaki H., Mochizuki N., Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Morita K., Tsukita Sh. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occluding and a catenin. J. Bio. Chem. 1999b;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita Sh. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to a catenin and actin filaments. J. Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Yonemura S., Kitani-Yasuda T., Tsukita Sa., Tsukita Sh. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J. Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T., M., Uehata I., Tamechika J., Keel K., Nonomura M., Maekawa S., Narumiya Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- Ivanov A. I., Hunt D., Utech M., Nusrat A., Parkos C. A. Differential roles for actin polymerization and a myosin 2 motor in assembly of epithelial apical junctional complex. Mol. Biol. Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno T., et al. Deficiency of zonula occludens-1 cause embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol. Biol. Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K. Tight junctions, membrane-associated guanylate kinases and cell signaling. Curr. Opin. Cell Biol. 1995;7:641–649. doi: 10.1016/0955-0674(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Kitajiri S, et al. Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J. Cell Sci. 2004;117:5087–5096. doi: 10.1242/jcs.01393. [DOI] [PubMed] [Google Scholar]
- Matter K., Balda D. S. Signaling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- McNeil E., Capaldo C. T., Macara I. G. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2006;17:1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Aoki T. K., Matsuda M. Monitoring spatio-temporal regulation of Ras and Rho GTPase with GFP-based FRET probes. Methods. 2005;37:146–153. doi: 10.1016/j.ymeth.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Owaribe K., Masuda H. Isolation and characterization of circumferential microfilament bundles from retinal pigmented epithelial cells. J. Cell Biol. 1982;95:310–315. doi: 10.1083/jcb.95.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan A. M., Maddugoda M., Kraemer A., Stehbens S. J., Verma S., Kovacs E. M., Yap A. S. Myosin 2 is a key Rho kinase target necessary for local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Utepbergenov D. I., Mueller S. L., Beyermann M., Schneider-Mergener J., Krause G., Blasig I. E. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1, potential mechanism of tight junction regulation. Cell Mol. Life Sci. 2004;61:1354–1365. doi: 10.1007/s00018-004-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Nose A., Iwasaki K., Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct. Funct. 1986;11:245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1, a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita Sa., Furuse M., Tsukita Sh. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Uchimura T., Fumoto K., Yamamoto Y., Ueda K., Hosoya H. Spatial localization of mono- and diphosphorylated myosin 2 regulatory light chain at the leading edge of motile HeLa cells. Cell Struct. Funct. 2002;27:479–486. doi: 10.1247/csf.27.479. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Yin M., Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Woods D. A., Bryant P. J. ZO-1, DlgA and PSD95/SAP 90, Homologous proteins in tight, septate and synaptic cell junctions. Mech. Dev. 1993;44:85–89. doi: 10.1016/0925-4773(93)90059-7. [DOI] [PubMed] [Google Scholar]
- Xu J., Kausalya P. J., Phua D. C., Ali S. M., Hossain Z., Hunziker W. Early embryonic lethality of mice lacking ZO-2, but not ZO-3, reveals critical and nonreduntant roles for individual zonula occludens proteins in mammalian development. Mol. Cell Biol. 2008;281:1669–1678. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Nelson W. J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Harada N., Kano K., Taya S., Canaani E., Matsuura Y., Mizoguchi A., Ide C., Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J. Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Itoh M., Nagafuchi A., Tsukita Sh. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- Yoshizaki H., Ohba Y., Kurokawa K., Itoh R. E., Nakamura T., Mochizuki N., Nagashima K., Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J., Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.