Abstract
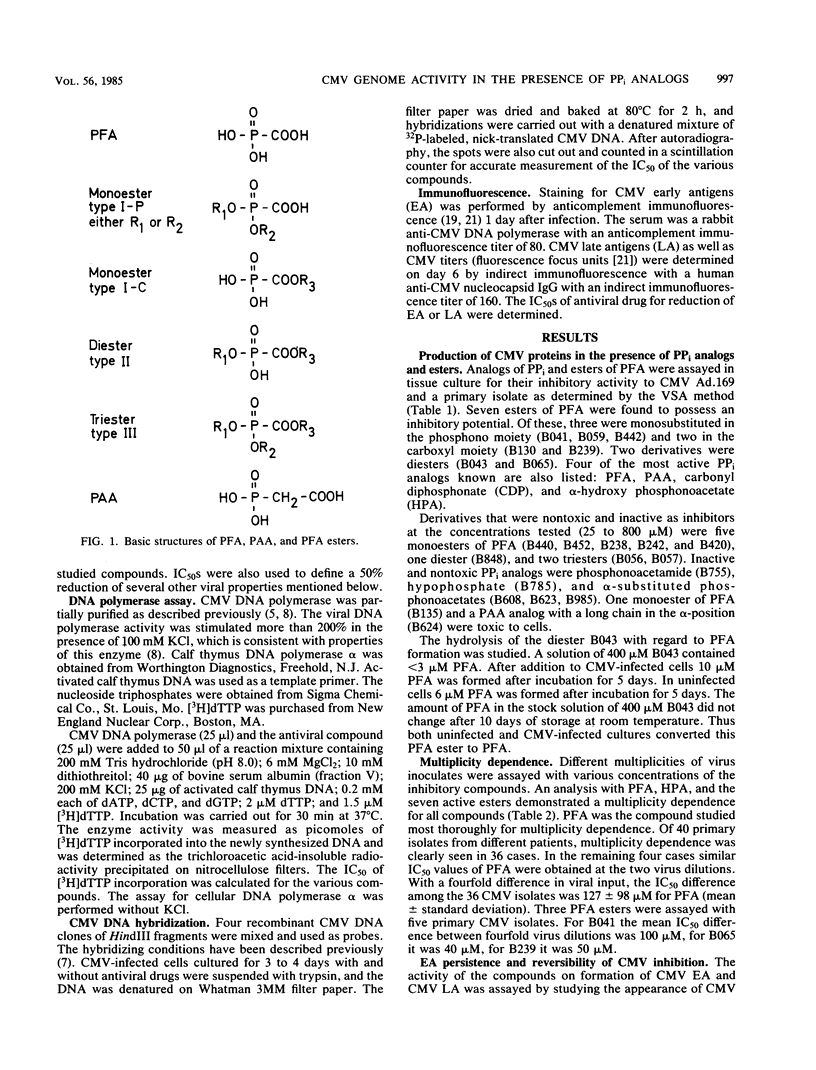
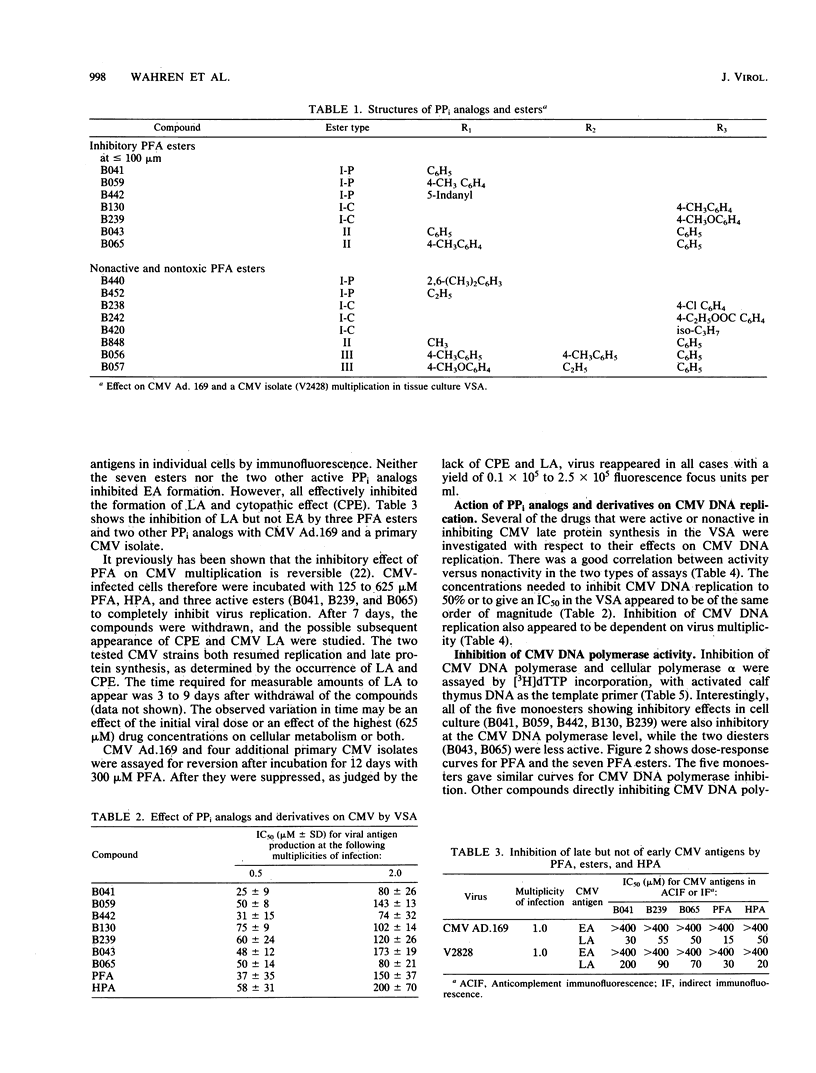
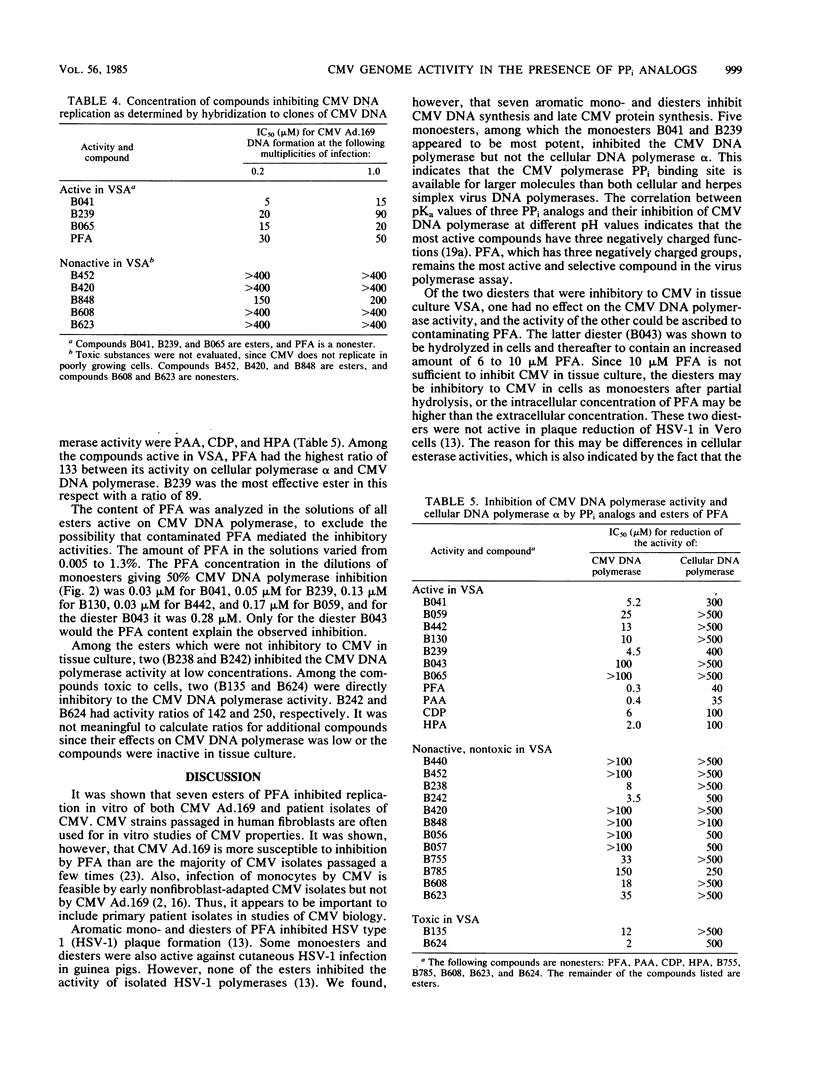
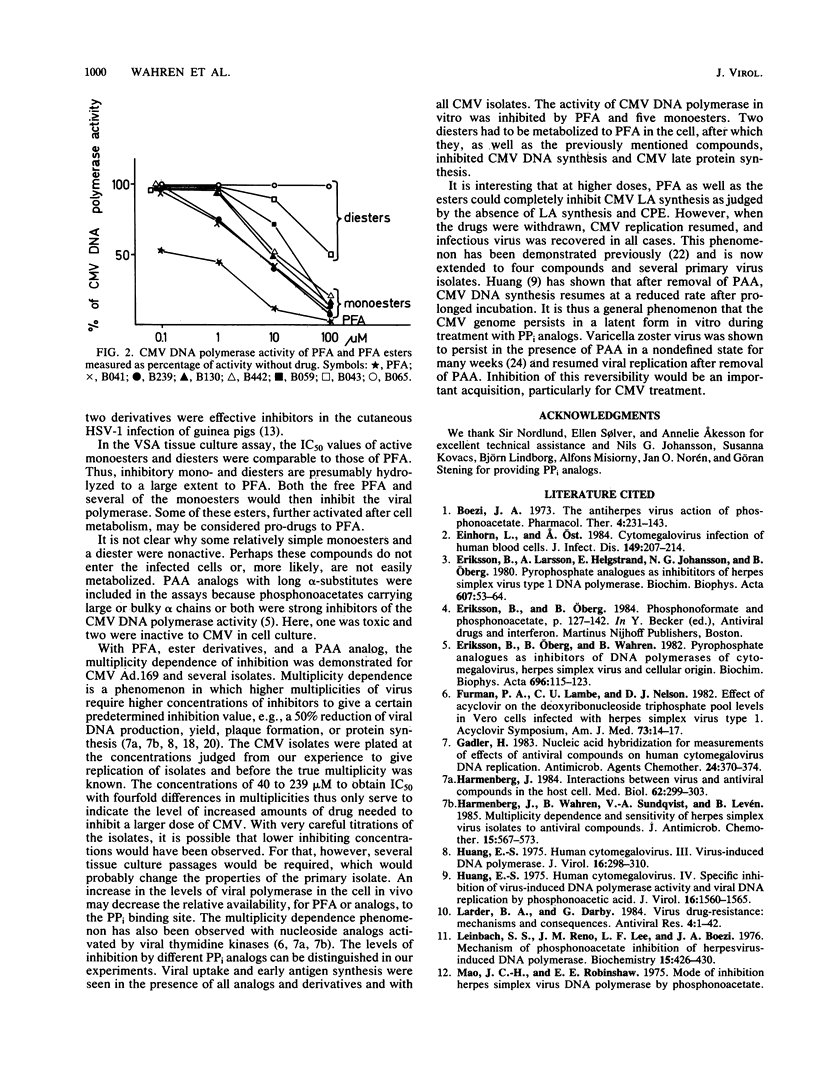
PPi analogs and esters of these were studied for their effect on cytomegalovirus (CMV) multiplication. Five aromatic monoesters of phosphonoformate esterified either in the phosphono or the carboxylic group and two diesters were demonstrated to inhibit CMV DNA synthesis and late viral protein synthesis. In a direct assay, the monoesters but not the diesters inhibited CMV DNA polymerase activity. The production of early CMV antigens was not inhibited by any of the compounds. After incubation with either drug for periods up to 7 days, renewed viral production occurred on withdrawal of the compound. All inhibitory esters as well as PPi analogs showed a CMV multiplicity dependence. This was demonstrated both for CMV strain Ad.169 and for all tested CMV isolates. Evidence was found that the esters are hydrolyzed to phosphonoformate and, therefore, may be of importance as useful prodrugs in the specific therapy of CMV infections. The general phenomenon of reversibility to the productive state and the multiplicity dependence of CMV are important factors in any treatment schedule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boezi J. A. The antiherpesvirus action of phosphonoacetate. Pharmacol Ther. 1979;4(1):231–243. doi: 10.1016/0163-7258(79)90021-4. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984 Feb;149(2):207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Larsson A., Helgstrand E., Johansson N. G., Oberg B. Pyrophosphate analogues as inhibitors of herpes simplex virus type 1 DNA polymerase. Biochim Biophys Acta. 1980 Mar 28;607(1):53–64. doi: 10.1016/0005-2787(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Oberg B., Wahren B. Pyrophosphate analogues as inhibitors of DNA polymerases of cytomegalovirus, herpes simplex virus and cellular origin. Biochim Biophys Acta. 1982 Feb 26;696(2):115–123. doi: 10.1016/0167-4781(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Lambe C. U., Nelson D. J. Effect of acyclovir on the deoxyribonucleoside triphosphate pool levels in Vero cells infected with herpes simplex virus type 1. Am J Med. 1982 Jul 20;73(1A):14–17. doi: 10.1016/0002-9343(82)90056-0. [DOI] [PubMed] [Google Scholar]
- Gadler H. Nucleic acid hybridization for measurement of effects of antiviral compounds on human cytomegalovirus DNA replication. Antimicrob Agents Chemother. 1983 Sep;24(3):370–374. doi: 10.1128/aac.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmenberg J. Interactions between virus and antiviral compounds in the host cell. Med Biol. 1984;62(6):299–303. [PubMed] [Google Scholar]
- Harmenberg J., Wahren B., Sundqvist V. A., Levén B. Multiplicity dependence and sensitivity of herpes simplex virus isolates to antiviral compounds. J Antimicrob Chemother. 1985 May;15(5):567–573. doi: 10.1093/jac/15.5.567. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Virus drug-resistance: mechanisms and consequences. Antiviral Res. 1984 Apr;4(1-2):1–42. doi: 10.1016/0166-3542(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Norén J. O., Helgstrand E., Johansson N. G., Misiorny A., Stening G. Synthesis of esters of phosphonoformic acid and their antiherpes activity. J Med Chem. 1983 Feb;26(2):264–270. doi: 10.1021/jm00356a028. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Duff R. G., Mao J. C. Antiviral potential of phosphonoacetic acid. Ann N Y Acad Sci. 1977 Mar 4;284:310–320. doi: 10.1111/j.1749-6632.1977.tb21966.x. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Wahren B. An interchangeable ELISA for cytomegalovirus antigen and antibody. J Virol Methods. 1981 Apr;2(5):301–312. doi: 10.1016/0166-0934(81)90029-x. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Vahlne A., Lycke E. Inhibition of herpes simplex virus infection in tissue culture by trisodium phosphonoformate. Proc Soc Exp Biol Med. 1979 Jun;161(2):115–118. doi: 10.3181/00379727-161-40502. [DOI] [PubMed] [Google Scholar]
- Wahren B., Einhorn L., Gadler H. Early interactions between human cytomegalovirus and cells. Arch Virol. 1984;79(1-2):55–65. doi: 10.1007/BF01314303. [DOI] [PubMed] [Google Scholar]
- Wahren B., Eriksson B. Cytomegalovirus DNA polymerase inhibition and kinetics. Adv Enzyme Regul. 1985;23:263–274. doi: 10.1016/0065-2571(85)90051-2. [DOI] [PubMed] [Google Scholar]
- Wahren B., Harmenberg J., Sundqvist V. A., Levén B., Sköldenberg B. A novel method for determining the sensitivity of herpes simplex virus to antiviral compounds. J Virol Methods. 1983 Mar;6(3):141–149. doi: 10.1016/0166-0934(83)90026-5. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Inhibition of cytomegalovirus late antigens by phosphonoformate. Intervirology. 1980;12(6):335–339. doi: 10.1159/000149093. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Reversible inhibition of cytomegalovirus replication by phosphonoformate. Intervirology. 1980;14(1):7–15. doi: 10.1159/000149156. [DOI] [PubMed] [Google Scholar]
- Wahren B., Wahren P., Harmenberg J., Sundqvist V. A. Computer-based virus sensitivity assay and neutralization method. Applications for herpesviruses. J Virol Methods. 1983 May;6(5):271–282. doi: 10.1016/0166-0934(83)90042-3. [DOI] [PubMed] [Google Scholar]


