Abstract
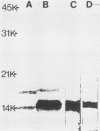
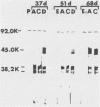
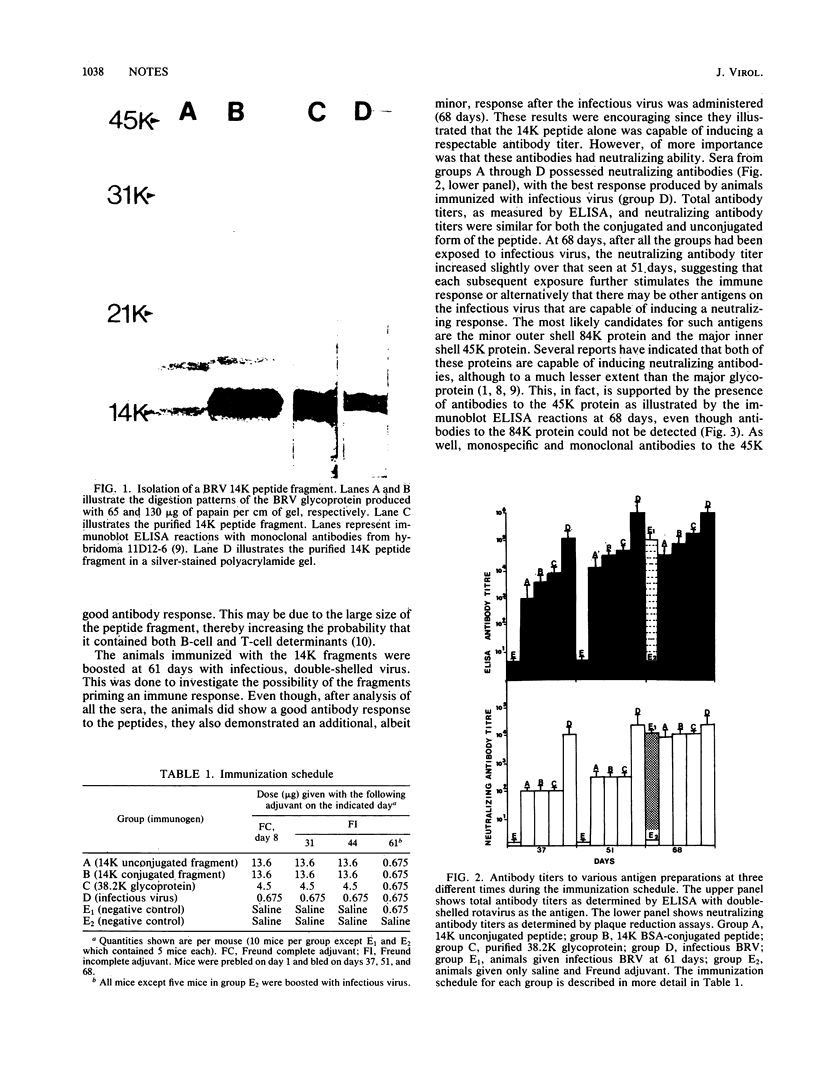
Previous experiments demonstrated that an antigenic site responsible for virus neutralization and cell attachment was located on a 14,000-molecular-weight fragment of the major bovine rotavirus (BRV) glycoprotein (M. Sabara, J. E. Gilchrist, G. R. Hudson, and L. A. Babiuk, J. Virol. 53:58-66, 1985). However, it was necessary to investigate whether this fragment also had the ability to induce the production of neutralizing antibodies. Upon immunization of mice, the bovine serum albumin-conjugated 14,000-molecular-weight fragment, the unconjugated 14,000-molecular-weight fragment, and the native glycoprotein all induced a similar neutralizing antibody response, albeit to a lesser extent than did the infectious, whole virus. In addition, immuno-blot enzyme-linked immunosorbent assay analysis of the reactivity of anti-peptide serum versus anti-glycoprotein serum with the glycoprotein was very comparable. These results suggest that the 14,000-molecular-weight fragment may represent not only a biologically active region but also an immunodominant area of the glycoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F. X., Mandl C., Berger R., Tuma W., Kunz C. Antibody-induced conformational changes result in enhanced avidity of antibodies to different antigenic sites on the tick-borne encephalitis virus glycoprotein. Virology. 1984 Feb;133(1):25–34. doi: 10.1016/0042-6822(84)90422-7. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Deregt D., Babiuk L. A., Misra V. Genetic heterogeneity within individual bovine rotavirus isolates. J Virol. 1982 Dec;44(3):813–822. doi: 10.1128/jvi.44.3.813-822.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. Derivation of neutralizing monoclonal antibodies against rotavirus. J Virol. 1983 Mar;45(3):1143–1146. doi: 10.1128/jvi.45.3.1143-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. The major surface glycoprotein of simian rotavirus (SA11) contains distinct epitopes. Virology. 1984 Apr 30;134(2):318–327. doi: 10.1016/0042-6822(84)90300-3. [DOI] [PubMed] [Google Scholar]