Abstract
Pseudomonas exotoxin (PE) is a cytotoxin which, after endocytosis, is delivered to the cytosol where it inactivates protein synthesis. Using diaminobenzidine cytochemistry, we found over 94% of internalized PE in transferrin (Tf) -positive endosomes of lymphocytes. When PE translocation was examined in a cell-free assay using purified endocytic vesicles, more than 40% of endosomal 125I-labeled PE was transported after 2 h at 37°C, whereas a toxin inactivated by point mutation in its translocation domain was not translocated. Sorting of endosomes did not allow cell-free PE translocation, whereas active PE transmembrane transport was observed after > 10 min of endocytosis when PE and fluorescent-Tf were localized by confocal immunofluorescence microscopy within a rab5-positive and rab4- and rab7-negative recycling compartment in the pericentriolar region of the cell. Accordingly, when PE delivery to this structure was inhibited using a 20°C endocytosis temperature, subsequent translocation from purified endosomes was impaired. Translocation was also inhibited when endosomes were obtained from cells labeled with PE in the presence of brefeldin A, which caused fusion of translocation-competent recycling endosomes with translocation-incompetent sorting elements. No PE processing was observed in lymphocyte endosomes, the full-sized toxin was translocated and recovered in an enzymatically active form. ATP hydrolysis was found to directly provide the energy required for PE translocation. Inhibitors of endosome acidification (weak bases, protonophores, or bafilomycin A1) when added to the assay did not significantly affect 125I-labeled PE translocation, demonstrating that this transport is independent of the endosome-cytosol pH gradient. Nevertheless, when 125I-labeled PE endocytosis was performed in the presence of one of these molecules, translocation from endosomes was strongly inhibited, indicating that exposure to acidic pH is a prerequisite for PE membrane traversal. When applied during endocytosis, treatments that protect cells against PE intoxication (low temperatures, inhibitors of endosome acidification, and brefeldin A) impaired 125I-labeled PE translocation from purified endosomes. We conclude that PE translocation from a late receptor recycling compartment is implicated in the lymphocyte intoxication procedure.
INTRODUCTION
Pseudomonas exotoxin A (PE) is a 66-kDa single-chain protein composed of three major structural domains, termed I, II, III, from the amino- to the carboxyl terminus. They are responsible for cell binding, translocation, and catalytic activity, respectively (Pastan et al., 1992). Sensitivity to this toxin varies over a wide range among mammalian cells. Most of them are moderately PE sensitive and liver cells show intermediate sensitivity. The reasons why mouse fibroblasts (LM cells, Swiss 3T3, and L929 cells) are highly PE sensitive, i.e., 100- to 300-fold more sensitive than moderately sensitive cells, are not yet entirely understood (Mucci et al., 1995).
Cell intoxication by PE begins by its binding at the plasma membrane to the α2-macroglobulin (α2-M) receptor/(LRP), a multiligand receptor (Kounnas et al., 1992). The toxin then enters cells by receptor-mediated endocytosis (Morris et al., 1983; Morris and Saelinger, 1986; Jinno et al., 1989). Electron microscopic examination indicated that PE-gold, a multivalent ligand ultimately reaching lysosomes, is first transferred from early to mature endocytic elements in the perinuclear Golgi region (Morris et al., 1983; Morris and Saelinger, 1986). The receptor recycling compartment is found in this pericentriolar region of the cell (Hopkins et al., 1994; Ghosh and Maxfield, 1995).
Receptors such as the transferrin (Tf) receptor are, during their fast cycle, returned to the plasma membrane directly from the sorting endosome, a rab4+, rab5+, rab7− (Ullrich et al., 1996) structure with a peripheral distribution in most cell types (Gruenberg and Maxfield, 1995). During their long cycle they can also be recycled from the long-lived recycling compartment where rab5, but not rab7 and rab4 (Ullrich et al., 1996), is present and which has a pericentriolar localization close to the trans-Golgi network (TGN) (Gruenberg and Maxfield, 1995). Late endosomes (rab4−, rab5−, rab7+), through which ligands destined for lysosomal degradation are routed from sorting endosomes, also exhibit a perinuclear distribution (Gruenberg and Maxfield, 1995). The exact route followed by internalized PE within the endosomal system remains to be examined using monovalent PE as well as specific markers of the different elements which might be involved in its uptake.
Within highly sensitive cells, a small fraction (<10%) of endocytosed PE can be processed by an endosomal furin-like protease able to release a 28-kDa fragment that was identified as the amino-terminal binding domain from a 37-kDa fragment jointly composed of translocation and enzymatic domains. This processing event seems to be required for optimum intoxication of highly PE-sensitive cells (Ogata et al., 1990; Pastan et al., 1992), even though mutated PEs which were more efficiently cleaved intracellularly showed less toxicity (Zdanovsky et al., 1993; Chiron et al., 1996). After reduction of the disulfide bond linking the two fragments, the 37-kDa carboxyl-terminal fragment could be translocated after further transport to the TGN or the endoplasmic reticulum (ER) (Zdanovsky et al., 1993; Miesenböck and Rothman, 1995). A moderate (two- to threefold) enhancement of cytotoxicity was observed upon replacement of the PE-native carboxyl-terminal sequence by KDEL (Seetharam et al., 1991), indicating that this chimera was recognized by the KDEL receptor which enables retrograde transport of lumenal proteins from the TGN to the ER (Miesenböck and Rothman, 1995). This does not seem to be the case for the native toxin since, even after removal of the terminal lysine from the REDLK carboxyl-terminal sequence of PE, no binding of PE derivatives to the purified KDEL receptor has been observed (Kreitman and Pastan, 1995). These data were confirmed in vivo since, upon transfection with a single-chain antibody gene fused with a PE truncated from domain I; the resulting immunotoxin was secreted and not significantly retained within the ER (Chen et al., 1997).
Hence, if internalized PE is routed to the ER, this would occur via a relatively inefficient mechanism not involving KDEL-mediated retrieval, perhaps similar to that enabling shiga toxin to reach the ER (Sandvig et al., 1992). Contrary to shiga toxin, which was visualized in the Golgi and ER by electron microscopy upon uptake by butyric acid-treated cells (Sandvig et al., 1992), and to cholera toxin, which was also observed within these structures after endocytosis using both electron and confocal immunofluorescence microscopy (Bastiaens et al., 1996; Majoul et al., 1996), internalized PE has not been observed elsewhere other than in the endocytic pathway (Morris et al., 1983; Morris and Saelinger, 1986; Jinno et al., 1989; Mucci et al., 1995). Nevertheless, PE translocation could take place from an intracellular site where the toxin concentration is too low to be detected using available microscopic techniques.
This translocation step, kinetically limiting for cytotoxicity (Hudson and Neville, 1987), enables PE to reach the cytosol where it will finally kill cells by catalytically inactivating protein synthesis. This inactivation is obtained by ADP ribosylation of elongation factor 2 (EF2) (Pastan et al., 1992).
Several treatments affecting endocytic uptake and routing were found to protect cells from PE intoxication. Inhibitors of endosome acidification, such as weak bases and monensin, prevent PE cytotoxicity (Sundan et al., 1984; Morris and Saelinger, 1986), and uptake into acid endocytic elements seems to be required for PE translocation. Nevertheless, low pH was not sufficient to induce PE translocation through the plasma membrane, as was the case for diphtheria toxin (DT) (Olsnes and Sandvig, 1988), and PE translocation therefore has other requirements that are only fulfilled once endocytosis has taken place.
Temperatures below 22°C, which impair ligand egress from sorting endosomes (Futter et al., 1996), also protect cells from PE intoxication (Morris and Saelinger, 1986).
Brefeldin A (BFA) is a fungal antibiotic known to inhibit recruitment of ADP-ribosylating factor to membranes and induce the fusion of early endosomes with the TGN as well as mixing of the other Golgi elements with the ER. Albeit transport between these two hybrid organelles is inhibited, most endocytic uptake and recycling continues through the early endosome–TGN network (Hunziker et al., 1992; Wood and Brown, 1992). When BFA-sensitive cells were treated with BFA, they were protected 2- to 30-fold from PE cytotoxicity (Seetharam et al., 1991; Yoshida et al., 1991). Nevertheless, among all of the elements of the endocytic pathway affected by BFA, it is not easy to identify those which are primarily responsible for the protection.
In this report, we studied PE uptake by lymphocytes which are moderately PE-sensitive cells and the target of many PE-based immunotoxins (Brinkmann and Pastan, 1994). We observed internalized PE almost exclusively within Tf-positive endocytic elements and obtained evidence on the potential of PE to translocate across the membrane of purified endosomes using a cell-free assay and a translocation-deficient PE as control. Exposure to acidic pH during endocytosis was required for this toxin to acquire translocation competence, which was obtained 10–20 min after the onset of PE endocytosis, when the toxin was delivered to the pericentriolar receptor recycling compartment.
Involvement of endosomal translocation in the intoxication process was further indicated through treatments which are known to protect cells against PE intoxication (Morris et al., 1983; Sundan et al., 1984; Morris and Saelinger, 1986; Seetharam et al., 1991; Yoshida et al., 1991); when performed during PE endocytosis they impaired subsequent PE translocation from purified endosomes.
MATERIALS AND METHODS
Reagents
Chemicals and radiochemicals were obtained from Sigma (Les Ulis, France) and Amersham (Arlington Heights, IL), respectively. Purified PE was purchased from the Swiss Serum and Vaccine Institute (Bern, Switzerland). Goat anti-PE antibodies (List Biological laboratories, Campbell, CA) were purified on PE immobilized on N-hydroxy-succinimide-activated Sepharose (Pharmacia, Uppsala, Sweden). Affinity-purified rabbit antibodies anti-rab5B and anti-rab4 were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-rab7 (Méresse et al., 1995) was provided by Dr. P. Chavrier (Marseille, France). EF2 was partially purified from rat reticulocyte lysates by ammonium sulfate precipitation and ion-exchange chromatography on DEAE-cellulose (Chung and Collier, 1977). Fractions containing EF2 were localized by DT ADP ribosylation activity.
Expression and Purification of PE343Q
Escherichia coli BL21 (λDE3) cells were transfected with pET3d-ProOmpA-PE343Q, a plasmid containing the OmpA signal sequence, allowing secretion into the periplasmic space (Siegall et al., 1991). Expression was induced at OD595 ∼0.4 by adding 1 mM isopropyl-β-d-thiogalactoside. After 2 h, bacteria were fractionated and PE343Q was purified to homogeneity from the periplasm (Douglas et al., 1987) by ion-exchange chromatography, first on Q-Sepharose, and then on a Mono-Q column (Pharmacia) (Seetharam et al., 1991).
Protein Labeling
PE, PE343Q, and Tf were labeled with fluorescein isothiocyanate (FITC) using FITC-coated celite (Beaumelle et al., 1992). The resulting PE-FITC and PE343Q-FITC were as toxic as native toxins to L929 cells. Gold conjugates of PE and ricin were prepared using 10-nm particles obtained by the tannic acid method (Beaumelle et al., 1990). Radiolabeling of proteins was performed at room temperature using 100 μg of protein, 10 μg of Iodo-Gen (Fraker and Speck, 1978), and 0.5 mCi 125INa. The reaction was stopped after 10 min by adding 1 mg/ml tyrosine. Endocytosis of gold-, FITC-, or 125I-labeled PE (1 μg/ml) was inhibited (>95%) by excess PE (0.5 mg/ml).
Cell Culture, Labeling, and Fractionation
Mouse [BW 5147 (Beaumelle and Hopkins, 1989)] or human [CCRF-CEM (Beaumelle et al., 1992)] lymphocytes and L929 mouse fibroblasts (Ogata et al., 1990) were cultured as respectively indicated.
Endosomes were purified from mouse BW cells as detailed previously (Beaumelle and Hopkins, 1989). Briefly, after 30 min of labeling at 37°C with the tracer, in DMEM supplemented with 0.1 mg/ml bovine serum albumin (BSA) and 0.15 mg/ml human LDL, cells were washed with DMEM/BSA then cooled on ice. After adding ricin-gold (40 min at 2°C and several washes), cells were lysed under hypotonic conditions using nitrogen cavitation and Potter homogenization. Isotonicity was restored and the cell lysate was centrifuged for 5 min at 800 × g. The supernatant was mildly trypsinized and then crude membranes were isolated by ultracentrifugation and layered on a discontinuous sucrose gradient (40%/30%/20% sucrose). After ultracentrifugation, purified endosomes were obtained from the 30%/20% interface. Sucrose was diluted out and then endosomes were recovered by ultracentrifugation and finally resuspended in translocation buffer (110 mM KCl, 15 mM MgCl2, 1 μg/ml penicillin G, 20 mM PIPES, pH 7.1). This isolation method is based on specific lightening of endosomes with coendocytosed LDL and burdening of plasma membrane vesicles with ricin-gold. Mild trypsinization of the low-speed supernatant increases the efficiency of the lightening procedure (Beaumelle and Hopkins, 1989).
Measurement of 125I-PE Uptake
Cells (2.107/ml) were incubated for 30 min in DMEM/BSA containing 125I-labeled PE (1 μg/ml). Nonbound ligand was then removed by three washes with ice-cold phosphate-buffered saline (PBS). Cells were resuspended in Pronase (0.3% in PBS), left for 45 min on ice, and then spun at 20,800 × g for 3 min through a cushion of dibutylphthalate. Internalization was calculated as the fraction of cell-associated label becoming resistant to Pronase scraping (Beaumelle and Hopkins, 1989).
Electron Microscopy and 3′,3′-Diaminobenzidine (DAB) Cytochemistry
The Tf-horseradish peroxidase (HRP) conjugate (M ratio Tf/HRp = 1) (Beaumelle and Hopkins, 1989) showed endocytosis and recycling kinetics identical to those of 125I-labeled Tf in BW lymphocytes.
DAB cytochemistry was performed on both cells and isolated endosomes using a slight modification of a previously described procedure (Futter et al., 1996). Cells were labeled in DMEM/BSA/LDL for 30 min at 37°C with Tf-HRP (50 μg/ml) and 500 ng/ml of either 125I-labeled Tf or 125I-labeled PE before two washes with DMEM/BSA and one with PBS. They were then either fractionated to obtain endosomes as described above or treated for 30 min on ice with proteinase K (1 mg/ml in PBS) to remove ligands at the plasma membrane before adding 1 mM phenylmethylsulfonyl fluoride to arrest proteolysis (van Weert et al., 1995). Preliminary experiments showed that this treatment was efficient for selectively releasing ligands at the plasma membrane, whereas endocytosed molecules remained inaccessible. Cells or endosomes in PBS or translocation buffer, respectively, received 200 μg/ml DAB before splitting the suspension in half. Up to 0.02% H2O2 was added to one of them. After 30 min on ice, samples received 1% Triton X-100 and 0.1% NaN3 to stop DAB polymerization and dissolve membranes before centrifugation for 5 min at 160,000 × g on a 17% sucrose cushion. Cross-linking efficiency was calculated using H2O2-induced disappearance of 125I-ligand from the detergent-soluble fraction (Futter et al., 1996).
To examine Tf and PE endocytosis by electron microscopy, cells were labeled with Tf-HRP (20 μg/ml) and PE-gold (1 μg protein/ml) in DMEM/BSA/LDL for 30 min at 37°C and washed with DMEM/BSA, then with PBS. They were either fixed directly before revealing HRP activity and processing for electron microscopic examination or first fractionated for endosome preparation as described above, except that the 4°C cell treatment with ricin-gold was omitted for purposes of clarity. This step frees the endosome preparation from any contamination by elements derived from the plasma membrane (Beaumelle and Hopkins, 1989). Hence, in this particular experiment, some plasma membrane vesicles were present in the endosome preparation. These vesicles are much larger than endosomes (Beaumelle et al., 1990) and no PE-gold was observed within them.
Cell-Free Translocation Assays
The point-mutated PE343Q (Siegall et al., 1991) was used as control nontranslocating toxin throughout this study (see RESULTS). Other negative controls were Tf and HRP (Beaumelle et al., 1992, 1993).
To monitor PE-FITC translocation, cells were labeled for 30 min at 37°C with PE-FITC (2 μg/ml), PE343Q-FITC (2 μg/ml), or Tf-FITC (25 μg/ml). Endosomes were then isolated and incubated at 37°C in translocation buffer supplemented with 10 mM ATP/10 mM MgCl2, except when otherwise indicated. Fluorescein–fluorescence measurements were performed at 520 nm after the indicated period of time using an endosome preparation without fluorescent label as control, and pH was calculated from the 495/450-nm excitation ratio (Beaumelle et al., 1993).
Translocation of 125I-labeled PE from BW endosomes was monitored through a cell-free assay (Taupiac et al., 1996). After cell labeling with the indicated concentration of radiolabeled ligand (125I-labeled PE, 1 μg/ml; 125I-labeled PE343Q, 1 μg/ml; 125I-labeled Tf, 250 ng/ml; 125I-labeled HRP, 3 μg/ml) endosomes were purified and resuspended in translocation buffer with or without 10 mM ATP/10 mM MgCl2 before incubation for the indicated period of time at 37°C. Translocation was then stopped by cooling on ice. The ATP concentration remained stable over the time of the assay. Control samples were kept on ice. Ultracentrifugation (for 5 min at 160,000 × g in a Beckman airfuge or 42.2 Ti rotor) on a 17% sucrose cushion was used to separate translocated material from endosomes. Quantification was based on the (cpm supernatant)/(cpm endosomes) ratio increase (Beaumelle et al., 1992).
Confocal Immunofluorescence Microscopy
Cells (BW mouse lymphocytes) in DMEM/BSA were labeled for 30 min at 37°C with PE (2 μg/ml) and when indicated with Tf-FITC (25 μg/ml). They were then cooled to 4°C and washed twice with DMEM/BSA and twice with PBS. Cells were then fixed, permeabilized, labeled with antibodies, mounted, and examined under a Leica confocal microscope (optical section ∼0.5 μm) as described (Hémar et al., 1995). For rabbit anti-rab protein antibodies, the secondary antibody was FITC-labeled swine anti-rabbit IgG (Nordic Immunological Laboratories, Tilburg, the Netherlands). Goat anti-PE antibodies were revealed using a mouse monoclonal anti-goat IgG (Sigma) and a tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse IgG (Sigma) as the second and third antibody, respectively. No immunofluorescence staining was observed in the absence of primary antibody. No TRITC fluorescence was obtained in the absence of endocytosed PE.
Assay for PE Catalytic Activity
The ADP ribosylation activity of PE was tested using partially purified EF2 (Chung and Collier, 1977). Each assay contained 50 μl of buffer (10 mM dithiothreitol, 4 mM EDTA, 100 mM Tris-HCl, pH 8.2), 20 μl of purified EF2, 20 μl of 32P-labeled NAD (70 μM; 100,000 cpm), and 10 μl of enzyme. Control tubes contained everything but enzyme. Incubation at 30°C for 6 to 8 h was stopped by adding 1 vol of 20% trichloroacetic acid (TCA) before filtration onto glass-fiber filters. Before testing their activity, translocation media received 10 mg/ml BSA as carrier protein and were dialyzed overnight against 2 mM dithiothreitol, 1 mM EDTA, and 20 mM Tris-HCl (pH 8.2) to eliminate both ATP and MgCl2 which are potent ADP ribosylation inhibitors (Chung and Collier, 1977).
Other Methods
Toxin cytotoxicity to BW cells was tested by measuring the rate of protein synthesis as the amount of [35S]methionine incorporated into TCA-insoluble material (Beaumelle et al., 1992). A luciferin/luciferase kit (Boehringer Mannheim, Mannheim, Germany) was used to assay ATP concentrations. Radiolabeled proteins on gels were quantified by using storage phosphor screens and a Storm apparatus (Molecular Dynamics, Sunnyvale, CA). Errors are shown as SEM (n = 3–5).
RESULTS
Internalized PE Is Localized in Tf-positive Endosomes
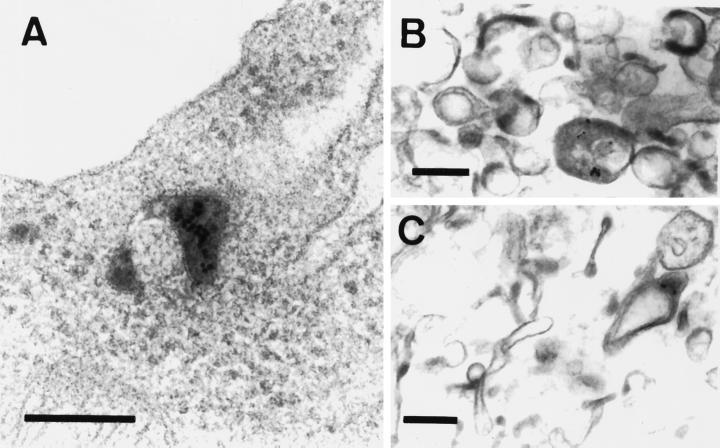
We investigated the PE intracellular pathway in mouse BW lymphocytes using Tf as early endosome marker (Gruenberg and Maxfield, 1995). The initial internalization pathway was examined using PE-gold (Morris et al., 1983; Morris and Saelinger, 1986). When endocytosed along with Tf-HRP for 30 min at 37°C, PE-gold was localized by electron microscopy in peroxidase-positive compartments, both inside the cell (Figure 1A) and within isolated endosomes (Figure 1, B and C). Tf is more actively endocytosed than PE and some endocytic elements were only labeled with Tf-HRP.
Figure 1.
Electron micrographs of BW 5147 cells and the endosome preparation after labeling with PE-gold and Tf-HRP. Cells were labeled for 30 min at 37°C with 1 μg/ml PE-gold and 20 μg/ml Tf-HRP, washed, and then either fixed directly or first fractionated to obtain endosomes. Whole cells (A) or the endosome preparation (B and C) were treated to reveal HRP activity for electron microscopy. Sections were examined after staining with uranyl acetate and lead citrate. Reaction products for Tf-HRP are contained within endocytic vesicles and PE-gold is observed within Tf-HRP-positive compartments. Bar, 0.2 μm.
Gold conjugates are multivalent and gold-toxin could be handled by cells differently from monovalent toxin (van Deurs et al., 1986). We thus examined the degree of colocalization of PE and Tf using DAB cytochemistry (van Weert et al., 1995; Futter et al., 1996). To investigate the efficiency of the procedure, cells were first allowed to internalize Tf-HRP (50 μg/ml) and 125I-labeled Tf (500 ng/ml). Maximum cross-linking occurred after 30 min at 2°C and plateaued at 65 ± 2% of endocytosed 125I- labeled Tf. The same value (67 ± 2%) was obtained using entire cells (after digestion of plasma membrane-bound ligands). These results are in close agreement with the finding that 70% of Tf receptors could be cross-linked by Tf-HRP in HepG2 homogenates (Stoorvogel et al., 1989).
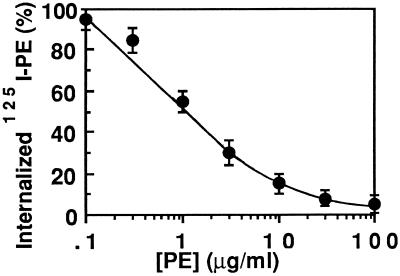
To examine whether 125I-labeled PE could be used to follow PE entry into BW cells, we monitored the inhibition of uptake of the radiolabeled toxin by increasing concentrations of cold toxin. Figure 2 highlights that 125I-labeled PE and PE competed for internalization. The fraction of 125I-labeled PE uptake that could not be inhibited by a 100-fold excess of PE was below 5% of the total. These results show the validity of using 125I-labeled PE to study PE internalization by these cells.
Figure 2.
Inhibition of 125I-labeled PE uptake by PE. BW cells were labeled with 1 μg/ml 125I-labeled PE for 30 min at 37°C in the presence of the indicated PE concentration. Internalization was followed as the fraction of cell-associated 125I-labeled PE resistant to Pronase treatment (0.3% in PBS) for 45 min at 2°C. Twenty to 25% of cell-associated 125I-labeled PE (5, 500 cpm/23,000 cpm) was intracellular after uptake at 37°C in the absence of competing PE. The results are expressed as a percentage of this value.
When cointernalized with Tf-HRP, 61 ± 1% and 65.5 ± 1% of endocytosed 125I-labeled PE was cross-linked by DAB in cells and purified endosomes, respectively. With reference to 125I-labeled Tf cross-linking efficiency, this shows that 94 and 98% of endocytosed 125I-labeled PE was localized in Tf-positive structures within cells and the endosome preparation, respectively. Intracellular PE was therefore almost exclusively endosomal in BW lymphocytes.
We then used a well-characterized endosome preparation obtained from mouse lymphocytes to examine PE translocation. With reference to the cell homogenate, endosomes are highly enriched in this preparation, which contains 40–44% endosomal 125I-labeled Tf and only minor amounts of ER- or Golgi-derived elements (5–6% NADH cytochrome C reductase for the ER, 2–3% galactosyl transferase, and <1% sialyl transferase for the trans-Golgi and the TGN) (Beaumelle and Hopkins, 1989). Both early and late endocytic structures are present according to localization of the respective markers, the Tf receptor and, the cation-independent mannose-6-phosphate receptor (Beaumelle et al., 1990). The fractionation procedure, based on a double-density shift on a discontinuous sucrose gradient (see MATERIALS AND METHODS), produced endosome preparations of reproducible purity.
Replacement of alanine 343 by glutamine within the PE translocation domain produced PE343Q whose cytotoxicity is 100-fold lower than that of native PE. Since this point mutant exhibits unaffected cell-binding and catalytic activities (Siegall et al., 1991), its translocation ability is selectively impaired and we used it as a control, nontranslocating PE for this study. Other PEs mutated within their translocation domain also showed complete inhibition of translocation activity (Taupiac et al., manuscript in preparation).
Fluorescent PE Can Cross Endosome Membrane
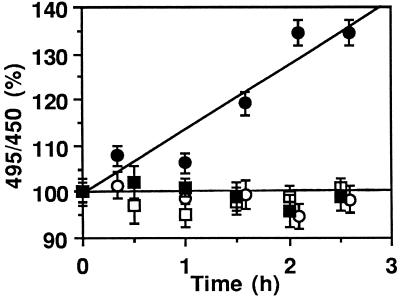
Fluorescence of endosomes loaded with PE-FITC, PE343Q-FITC, or Tf-FITC was monitored during a 37°C incubation in a cell-free assay. At the beginning of the experiment, using the fluorescein:fluorescence excitation ratio (495:450 nm) to monitor endosomal pH, the probes produced similar pH values (5.3–5.5) in the endosomes.
Figure 3 shows that PE343Q-FITC fluorescence remained stable over the 3-h incubation in the presence or absence of ATP, indicating that endosome integrity and pH were preserved throughout the assay. This was also the case when endosomes were loaded simultaneously with Tf-FITC and PE (5 μg/ml), showing that PE does not disrupt the endosome membrane or induce proton leaks (our unpublished observations).
Figure 3.
Fluorescent PE can cross the endosome membrane. BW cells were incubated for 30 min at 37°C with 2 μg/ml PE-FITC (• and ○) or PE343Q-FITC (▪ and □). Translocation was assayed as described in MATERIALS AND METHODS using isolated endosomes in the presence (closed symbols) or absence (open symbols) of 10 mM ATP + 10 mM MgCl2. Fluorescein fluorescence excitation was obtained at 450 or 495 nm and measured at 520 nm. Upon addition of 5 μM nigericin, a pH level corresponding to the buffer value was obtained for all fluorescent tracers.
In the presence of ATP (Figure 3), an increase in the 495:450 ratio was observed over time for PE-FITC, demonstrating that some toxin molecules became exposed to the medium (pH 7.1).
Cell-Free Translocation of 125I-labeled PE
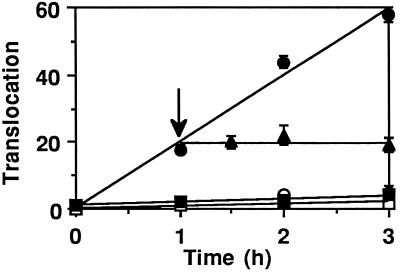
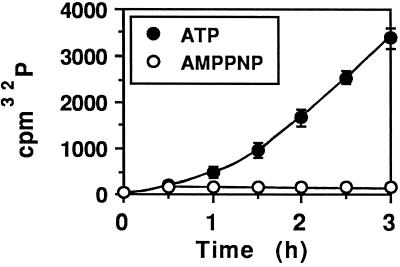
Radiolabeled PE provided further evidence of a time- and ATP-dependent translocation of PE from BW endosomes. Control experiments first showed that endosomes loaded with PE (5 μg/ml) and 125I-labeled TF or 125I-labeled HRP did not release any radioactivity during the translocation assay (performed with or without ATP), further demonstrating that endosome tightness was preserved even in the presence of internalized PE. Accordingly, no liberation of endosomal 125I-labeled PE343Q was observed during the assay, with or without ATP present (Figure 4), whereas an ATP-dependent linear release of 125I-labeled PE into the medium was observed over time. No further 125I-labeled PE translocation was observed when an ATP-depleting system was added 1 h after starting the assay, confirming that this transmembrane transport is a specific energy-driven process. Similar results were obtained using CEM human lymphocyte endosomes (our unpublished results).
Figure 4.
Translocation of 125I-labeled PE across the endosome membrane. After labeling for 30 min at 37°C with 1 μg/ml 125I-labeled PE (▴, •, and ○) or 125I-labeled PE343Q (▪ and □), BW cells were fractionated for endosome isolation. Translocation was assayed in the presence (closed symbols) or absence (open symbols) of 10 mM ATP and was terminated by cooling on ice. (▴) An ATP-depleting system (15 mM glucose, 50 U/ml hexokinase) was added 1 h after the beginning of the assay (arrow). Ultracentrifugation for 5 min at 160,000 × g on a 17% sucrose cushion separated the translocation medium from the endosomes. The radioactivity ratio (medium/endosomes) was used to directly monitor translocation. At the beginning of the experiment, 100–250 cpm and 2000–5000 cpm were routinely present in the medium and the endosomes, respectively.
According to the translocation activity (>40% of endosomal PE was translocated after 2 h at 37°C) and to the 98% localization of PE within endosomes as quantified using DAB cytochemistry, it can be ruled out that contaminants present in the endosome preparation, such as ER- or Golgi-derived vesicles, were a significant source of translocated PE in the assay.
ATP Hydrolysis Is Required for PE Translocation
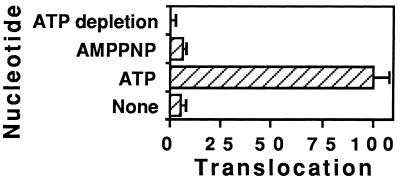
Residual ATP concentration in the endosome preparation is within the nanomolar range and essentially no translocation was observed in the absence of any added nucleotide (Figure 4). ATP hydrolysis is required for this movement since a nonhydrolyzable analogue (AMPPNP) did not support translocation (Figure 5). The Km value we obtained for ATP (4.2 ± 0.5 mM) is very close to the ATP concentration of lymphocyte cytosol [5 mM (Rink et al., 1982)]. The energy requirements for significant PE translocation from endosomes are thus fulfilled within the cell.
Figure 5.
Nucleotide requirement for PE translocation. After labeling BW endosomes with 125I-labeled PE (or 125I-labeled PE343Q as control), translocation was assayed as described in the legend of Figure 4 in the presence of the indicated nucleotide.
A 10-Min Delay after Onset of Endocytosis Is Required to Establish PE Translocation Competence
Cell intoxication by PE is prevented under conditions where delivery of endocytosed tracers to mature endosomal structures is inhibited (Morris and Saelinger, 1986). We thus examined changes in PE translocation activity upon progression along the endocytic pathway.
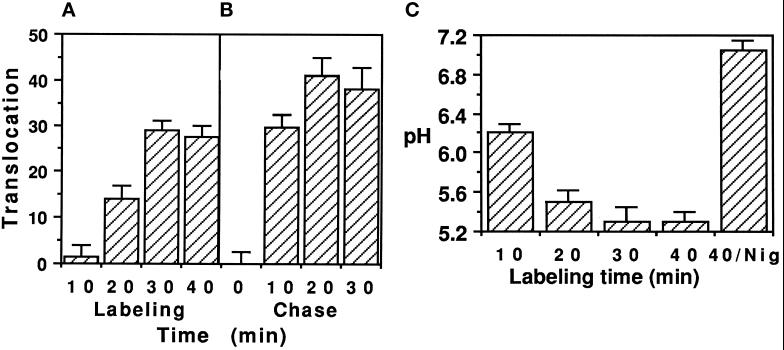
When endosomes were isolated from cells after different labeling times at 37°C with 125I-labeled PE, translocation was the most active after 30 min of endocytosis (Figure 6A). There was no significant exit of 125I-labeled PE from sorting endosomes (labeled for 10 min). Similar results were obtained using a chase protocol (Figure 6B). Hence, a 10- to 20-min delay was necessary after the onset of endocytosis before any PE translocation could be observed, thus restricting this process to mature endocytic elements which could be either late or recycling endosomes (see below).
Figure 6.
PE translocation is restricted to mature endosomes. (A) Cells were labeled with 1 μg/ml 125I-labeled PE at 37°C for the indicated times before endosome isolation and translocation assay. (B) 125I-labeled PE endocytosis was stopped after 10 min at 37°C. Excess ligand was washed out and a chase was performed at 37°C in DMEM/BSA/LDL supplemented with PE (100 μg/ml) for the indicated period of time. (C) After labeling with 2 μg/ml PE-FITC for the indicated times at 37°C, endosomes were isolated and resuspended in translocation buffer supplemented with 2 mM ATP. Endosome pH was measured within 5 min using the 495:450 nm excitation ratio for fluorescein fluorescence. A calibration curve was plotted using PE-FITC dilutions in appropriate buffers. When indicated (Nig), 5 μM nigericin were added to the translocation buffer.
Studies using model membranes demonstrated that PE insertion depended on acidification (Jiang and London, 1990; Sanyal et al., 1993). It was therefore of interest to investigate the potential involvement of acidification in establishing PE translocation competence by measuring pH levels encountered by PE during its progression along the endocytic pathway. We could not use a chase protocol because of the moderate fluorescence in PE-FITC-labeled endosomes after 10 min of endocytosis. Continuous labeling experiments showed that after 10 min of uptake, PE-FITC was in mildly acidic (pH ∼6.2) sorting endosomes (Figure 6C). It then gradually moved to more acidic structures (pH 5.3 after 30 min). Maximum acidification (−1.5 pH units) was observed within the first 20 min of endocytosis (Figure 6C). Although the pH reached at that point was higher than the pH 5 required to trigger PE insertion into model membranes (Jiang and London, 1990), the correlation between the kinetics of establishment of translocation competence (Figure 6A) and of acidification (Figure 6C) already suggests a relationship between the two events.
Identity of Endosomal Elements Enabling PE Translocation
Cells were labeled with Tf-FITC and PE for 30 min at 37°C, conditions which enabled the most active PE translocation as measured using isolated endosomes (Figure 6A). Upon examination by confocal fluorescence microscopy (Figure 7), Tf-FITC was essentially localized at one pole of the cell in the pericentriolar region, as previously observed in several cell types (Yamashiro et al., 1984; Hopkins et al., 1994) including the lymphocyte (Hémar et al., 1995). This structure only became labeled after more than 10–15 min of labeling (Hémar et al., 1995 and our unpublished results), indicating that these elements are recycling endosomes.
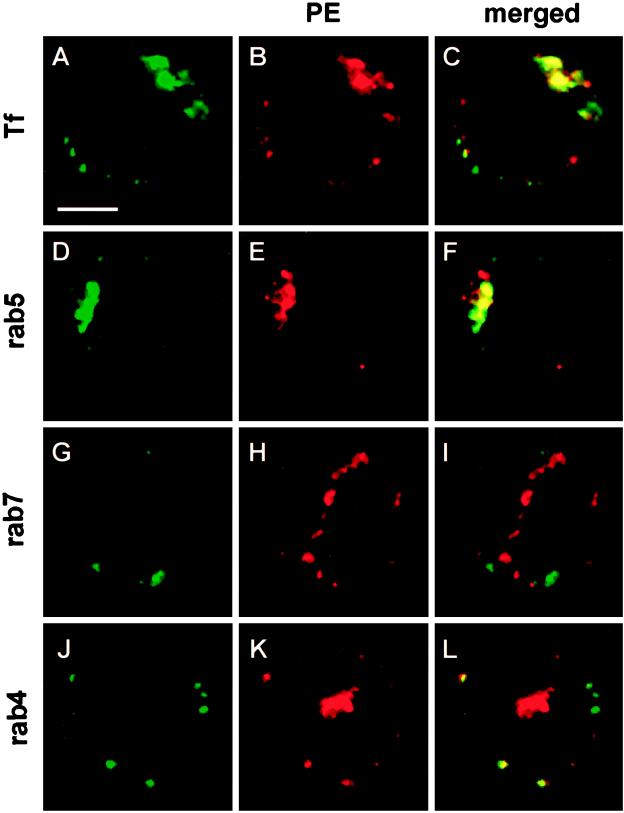
Figure 7.
Localization of internalized PE in BW lymphocytes by confocal immunofluorescence microscopy. Cells were incubated with PE (2 μg/ml) for 30 min at 37°C and then washed, fixed, permeabilized, and processed for detection of PE using affinity-purified goat anti-PE, mouse monoclonal anti-goat and TRITC-labeled goat anti-mouse antibodies. When indicated, rab proteins were labeled using affinity-purified rabbit antibodies and FITC-labeled swine anti-rabbit antibodies. (A–C) Tf-FITC (25 μg/ml) was coendocytosed with PE. (D–F) Localization of rab5 and PE. In these sections (A–F), the label is concentrated at one pole of the cell in the pericentriolar region. (G–I) Localization of rab7 and PE. (J–L) Localization of rab4 and PE. In these cells (G–L), PE and recycling endosomes are observed within a valley between two lobes of the nucleus, whereas neither rab7 (and late endosomes) nor rab4 (and sorting endosomes) are found in this area. Representative medical optical sections recorded using the FITC and the TRITC channels, and the combination of both images are presented. Bar, 5 μm.
Using immunofluorescence (Figure 7), PE was found along with Tf within this compartment whose identity was further examined using rab5 and rab7 as selective markers for “early” and “late” endosomes, respectively (Gruenberg and Maxfield, 1995, Méresse et al., 1995). Rab4 staining was used to selectively label sorting elements among early endosomes (Ullrich et al., 1996). The structures in which PE was observed after 30 min of endocytosis were a subset of rab5-positive endocytic elements, whereas PE never colocalized with rab7. Colocalization of rab4 and PE was only observed in peripheral sorting endosomes. Together with results from chase experiments (Figure 6B), these data show that the compartment enabling the most active PE translocation among endosomal elements is the bona fide rab4−, rab5+, rab7−, and Tf+ recycling endosome (Ullrich et al., 1996).
Biological Activity of Translocated Toxin
We then investigated whether the ATP-dependent PE translocation from endosomes as observed in our cell-free assay could be implicated in PE cytotoxicity. If endosomes are a productive translocation site during the intoxication procedure, cell-free translocated PE has to be recovered under an enzymatically active form.
A time-dependent release of EF2-ADP-ribosylating material from PE-labeled endosomes was observed when hydrolyzable ATP was present in the cell-free translocation assay (Figure 8), indicating that translocated PE is catalytically active and confirming that its transmembrane transport requires ATP hydrolysis.
Figure 8.
Translocated PE is enzymatically active. Cells were incubated with PE for 30 min at 37°C before fractionation and endosome isolation. Translocation was assayed for the indicated times in the presence of the indicated nucleotide, and translocation media were obtained by ultracentrifugation. Their ADP ribosylation activity was tested after dialysis using partially purified EF2 and 32P-labeled NAD.
Native PE requires treatments such as freezing and thawing or using urea along with a reducing agent to release constraints on domain III (Allured et al., 1986) and express catalytic activity (Vasil et al., 1977). The finding that translocated PE was enzymatically active indicates that activation occurred during endocytotic or transmembrane transport. The second possibility appears more likely, since translocation undoubtedly requires major conformational changes.
Temperature Dependence of PE Routing to Translocation Sites
We then examined the extent to which the different treatments protecting cells from PE cytotoxicity interfered either directly with PE translocation, or indirectly, i.e., during PE uptake. Cells maintained at low temperatures (19–22°C) are resistant to PE, whereas toxin binding and catalytic activities are only weakly affected in these conditions (Morris and Saelinger, 1986).
In an attempt to identify the stage of the intoxication procedure that might be responsible for the protection by low temperatures, we separately examined their effects on three steps preceding PE delivery to the cytosol as follows: 1) Endocytosis was quantified as the fraction of cell-associated 125I-labeled PE becoming resistant to Pronase. 2) Establishment of translocation competence was measured as translocation activity observed at 37°C from endosomes obtained from cells labeled with 125I-labeled PE at the indicated temperature. 3) Translocation temperature dependence was studied after cell labeling at 37°C.
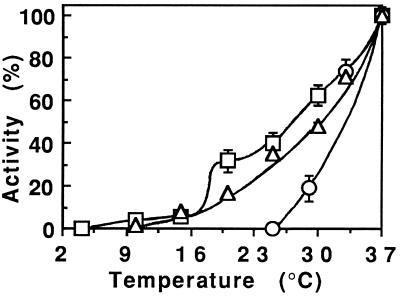
Toxin endocytosis efficiency decreased regularly as the temperature dropped (Figure 9). As could be anticipated for transmembrane transport, translocation activity gradually declined from 37°C to phase transition (∼20°C), below which the membrane was in the gel state and translocation was concomitantly abolished. The results of these experiments (Figure 9) revealed that establishment of translocation competence was the only stage of the intoxication process that was arrested at the protective temperature (below 25°C). Due to limited PE uptake at this temperature, we could not examine its intracellular localization. Nevertheless, PE is also likely endocytosed with Tf at this temperature; we previously studied endocytosis of the Tf receptor under these conditions and localized it in vacuoles which, according to their morphology and protein composition [similar to that of vesicles from coated pits (Beaumelle et al., 1990)] are probably sorting and not recycling endosomes. These observations are in agreement with electron microscopic observations of CEF cells indicating that at 20°C endocytosis is restricted to sorting endosomes (Futter et al., 1996).
Figure 9.
Temperature dependence of 125I-labeled PE routing to the cytosol. ▵, Endocytosis was measured (after 30 min at the indicated temperature) as the fraction of cell-associated label resistant to Pronase scraping (see Figure 2). ○, Acquisition of translocation competence was deduced from translocation measurements performed at 37°C on endosomes isolated from cells labeled with 1 μg/ml 125I-labeled PE for 30 min at the indicated temperature. □, Translocation temperature dependence was observed using endosomes prepared from cells labeled with 125I-labeled PE for 30 min at 37°C. All results are expressed as percentages of the 37°C value.
These results suggest that cells are protected from PE intoxication at 20°C because the toxin is trapped in sorting elements and cannot be transferred to translocation sites such as recycling endosomes. Inhibition of PE delivery to lysosomes by low temperatures, as observed previously using PE-gold (Morris and Saelinger, 1986), would therefore not be involved in protection. This protection also probably does not result from impaired toxin insertion into the endosome membrane below 25°C, since endosomes from cells labeled at this protective temperature did not allow any translocation even after 2 h at 37°C (Figure 9).
Endosome-Cytosol pH Gradient Is Not Required for PE Translocation
Weak bases and protonophores neutralize the endosome lumen and protect cells from PE cytotoxicity (Morris et al., 1983; Sundan et al., 1984; Morris and Saelinger, 1986). By analogy with DT, it was generally concluded that the endosome-cytosol pH gradient was the driving force for PE translocation.
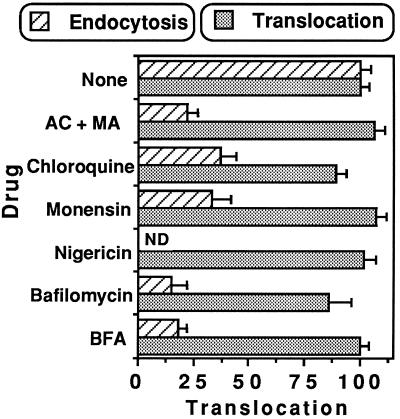
When added to the cell-free assay, weak bases such as ammonium chloride or chloroquine, protonophores such as monensin or nigericin, and bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase (V-ATPase) (Clague et al., 1994), all equilibrated endosome pH at the buffer value (Figure 6C for nigericin). As experienced before using Tf-PE conjugates (Taupiac et al., 1996), none of these drugs significantly altered PE translocation (Figure 10); the endosome-cytosol pH gradient is therefore not required for PE transport through the membrane of endocytic elements.
Figure 10.
When present during endocytosis, drugs protecting cells from PE intoxication impaired endosomal 125I-labeled PE translocation. The following concentrations were used: NH4Cl/methylamine (AC/MA), 20 mM each; chloroquine, 50 μM; monensin, 50 μM; nigericin, 5 μM; bafilomycin A1, 0.5 μM; BFA, 5 μg/ml. Effect on translocation: cells were labeled with 1 μg/ml 125I-labeled PE (in the absence of drug) before endosome isolation; translocation was assayed in the presence of the indicated molecules (n = 6). Effect during endocytosis, i.e., on acquisition of translocation competence: cells were preincubated for 10 min at 37°C in the presence of the indicated molecules before adding 1 μg/ml 125I-labeled PE and 150 μg/ml LDL. After 30 min at 37°C, cells were fractionated to isolate endosomes. Translocation was assayed in the absence of drug (n = 4). ND, not determined.
Inhibitors of Endosome Acidification Impair PE to Acquire Translocation Competence
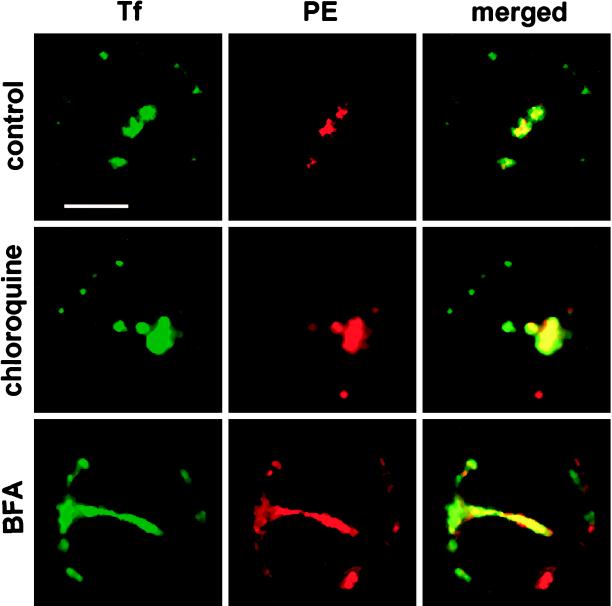
Preliminary experiments demonstrated that, when present during endocytosis, inhibitors of endosomes acidification did not alter the efficiency of ligand (Tf or PE) uptake nor the subsequent fractionation procedure, since both the distribution of endocytosed tracers over the sucrose gradient used to purify endosomes and the endosome yield remained unaffected. Double-label confocal microscopic examination showed that both Tf and PE were delivered to recycling endosomes in cells treated with chloroquine, ammonium chloride and methylamine, monensin, or bafilomycin A1 (Figure 11 for chloroquine). The morphology of this compartment was not notably affected by these drugs (Figure 11 for chloroquine). These results are in agreement with previous studies, indicating that weak bases or bafilomycin A1 do not significantly affect 125I-labeled Tf overall uptake or recycling, or receptor sorting, or endosome localization on density gradients but rather impair ligand delivery to late endosomes and lysosomes (Umata et al., 1990; Clague et al., 1994; van Weert et al.,1995).
Figure 11.
The morphology of the recycling compartment was markedly affected by BFA but not by chloroquine. Lymphocytes were incubated with BFA (5 μg/ml) or chloroquine (50 μM) as indicated for 10 min at 37°C before the addition of Tf-FITC (25 μg/ml) and PE (2 μg/ml). After 30 min at 37°C, cells were washed, fixed, and stained for PE using a TRITC-labeled tertiary antibody as described in the legend of Figure 7. Samples were then examined under a confocal microscope. Both medical optical sections recorded using the FITC and the TRITC channels and their overlays are presented. Bar, 5 μm.
When cell labeling with 125I-labeled PE was performed in the presence of endosome acidification inhibitors, subsequent translocation from isolated endosomes was considerably impaired (Figure 10). In the light of the innocuity of these inhibitors on PE translocation per se (Figure 10) and on its routing to translocation-competent recycling endosomes (Figure 11), exposure to acidic pH is a prerequisite for PE translocation. This is consistent with the correlation between acidification and establishment of translocation competence (Figure 6) and with the low pH-induced PE insertion into model membranes (Jiang and London, 1990).
We conclude that the protective effect of inhibitors of endosome acidification against PE intoxication (Morris et al., 1983; Sundan et al., 1984; Morris and Saelinger, 1986) results from inhibition of PE insertion into the endosome membrane. Previous insertion into the membrane appears to be a compulsory prerequisite for establishing PE translocation competence.
BFA Affects Recycling Compartment
The endosomal system is affected by BFA, which rapidly induces fusion of early endosomes and the TGN, resulting in a large tubular network. Nevertheless, this hybrid organelle enables ligand uptake and recycling (Hunziker et al., 1992; Wood and Brown, 1992). Consistent with these previous observations, a large endocytic network extending from the pericentriolar to the peripheral region and stained with internalized Tf and PE was observed in lymphocytes treated with BFA (Figure 11), indicating that extensive fusion of the sorting and recycling compartments took place. In some cells, this network could be seen in serial optical sections entirely surrounding the nucleus, indicating how large it can be and the extensive dilution of recycling endosomes in this new structure.
Studies performed on BFA-sensitive cells indicated that PE cytotoxicity is reduced 2- to 30- fold by BFA, depending on the cell type (Seetharam et al., 1991; Yoshida et al., 1991). When present during the translocation assay only, BFA did not affect 125I-labeled PE translocation, whereas endosome labeling with 125I-labeled PE in the presence of BFA (5 μg/ml) resulted in more than 80% inhibition of 125I-labeled PE translocation (Figure 10).
Previous studies documented normal ligand uptake (Hunziker et al., 1992) and unaltered buoyant densities of endocytic elements from BFA-treated cells (Sandvig et al., 1991). Accordingly, the endosome yield from lymphocytes was not affected when endocytosis was performed in the presence of BFA, indicating that PE translocation inhibition by BFA was not a result of modification of the endosome isolation procedure.
We therefore concluded that extensive dilution of the translocation-competent recycling compartment into translocation-incompetent sorting endosomes caused by BFA modified the environment normally found by PE in this organelle and resulted in translocation inhibition.
Full-Length PE Translocates Through Endosome Membrane
Processing of PE by furin-like proteases to generate an enzymatically active 37-kDa carboxyl-terminal fragment was reported to be important for optimum cytotoxicity in highly PE-sensitive cells (Ogata et al., 1990; Pastan et al., 1992; Zdanovsky et al., 1993).
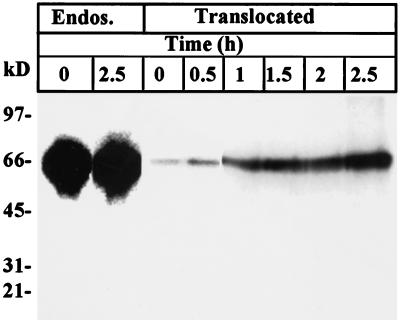
We therefore investigated the requirement for PE proteolytic activation before translocation from lymphocyte endosomes. Reducing SDS-PAGE analysis did not reveal any processing of 125I-labeled PE (Figure 12) in BW endosomes after cell labeling for 30 min at 37°C, even on overexposed films. Accordingly, translocated material from 125I-labeled PE-labeled endosomes exclusively showed the full-sized toxin (Figure 12). Human CEM lymphocytes labeled in the same conditions also did not process endosomal 125I-labeled PE, and identical results were obtained when Western blots of translocated PE were analyzed using enhanced chemiluminescence techniques. Moreover, when translocated material was separated by SDS-PAGE before gel slicing for EF2 ADP ribosylation assays (Beaumelle et al., 1992), all enzymatic activity was found at the level of the intact toxin (our unpublished results).
Figure 12.
Full-length PE translocates from purified BW endosomes. After cell labeling with 1 μg/ml 125I-labeled PE for 30 min at 37°C, endosomes were isolated and translocation was assayed for the indicated times in the presence of 10 mM ATP. Endosomal (Endos.) and translocated proteins (after TCA precipitation) were analyzed using reducing SDS/PAGE followed by autoradiography.
Hence, PE can reach the cytosol from BW lymphocyte endosomes without requiring preliminary cleavage, whether endocytosed via its own receptor (this study) or the Tf receptor (Taupiac et al., 1996).
DISCUSSION
Some immunotoxins prepared using PE are already utilized in human therapy (Brinkmann and Pastan, 1994). Nevertheless, all steps involved in cell intoxication by PE are not yet clearly defined. Binding to a specific receptor followed by endocytosis and translocation are the major events enabling this toxin to reach its cytosolic target. The translocation step is probably the most obscure (Pastan et al., 1992).
In this study we obtained evidence of PE translocation from the receptor recycling compartment and showed that this process is likely involved in lymphocyte intoxication by PE. We also report some requirements for PE membrane traversal. These data were obtained using a cell-free translocation assay which previously enabled us to examine DT and ricin translocation (Beaumelle et al., 1992, 1993).
PE Can Cross Endosome Membrane
Endocytic uptake of PE has been shown, using PE-gold, to occur via early endosomes before transfer to endocytic structures in the pericentriolar Golgi region and finally to lysosomes (Morris and Saelinger, 1986). Such routing to degradation was likely induced by the multivalence of the ligand (van Deurs et al., 1986). Here, we extended earlier studies of PE endocytosis (Morris et al., 1983; Morris and Saelinger, 1986; Jinno et al., 1989) and localized 94–98% of endocytosed PE in Tf-positive elements after 30 min at 37°C. We identified these structures as the pericentriolar, rab4- and rab7-negative but Tf- and rab5-positive receptor recycling compartment by confocal immunofluorescence microscopy.
Our observations are in agreement with biochemical data indicating that PE binding to immobilized LRP is maximum at low pH (pH 5.0–5.5) (Thompson et al., 1991). Hence, contrary to another LRP ligand, α2-M, which was found to rapidly dissociate from the LRP within the acidic endosome lumen and is delivered to the lysosome with high efficiency (Yamashiro et al., 1989), PE probably remained bound to the LRP before translocation. It was therefore not surprising to find PE in the receptor recycling compartment.
Toxins such as cholera toxin, which is subjected to retrograde transport following endocytosis, were predominantly visualized in the Golgi apparatus and in the ER by electron (Majoul et al., 1996) and confocal microscopy (Bastiaens et al., 1996) after 30 min. Examination of PE uptake by fibroblasts (Jinno et al., 1989) and lymphocytes (this study) failed to demonstrate that PE can undergo retrograde transport. Nevertheless, a few molecules of these toxins in the cytosol will kill the cells (Olsnes and Sandvig, 1988), and it is still possible that PE could also translocate from a nonendosomal compartment such as the TGN or the ER (Pastan et al., 1992) where the fraction of PE which did not colocalize with Tf might be present, but at a concentration too low to be detected. This event would likely be less quantitatively significant than translocation from endosomes.
Using different labels (fluorescent and radioactive) and cell-free assays, we obtained evidence of direct, active, energy-dependent translocation of full-length PE through the endosome membrane. A control PE, inactivated in its translocation domain and activity, was not transported. Identification of the endosome as a likely doorstep for PE entry into the cytosol was further indicated by the catalytic activity of the translocated toxin and by extensive PE translocation inhibition when endocytosis was performed under any condition affording cell protection against the toxin.
Requirements for PE Translocation
The minimum 10-min delay required to establish translocation competence after the onset of PE endocytosis is likely explained by the need for a minimum pH to ensure membrane insertion; this pH threshold seems to be only reached after more than 10 min of endocytic transport, which enables the toxin to reach pericentriolar recycling endosomes and pH ∼5.5. This relationship between receptor routing to this compartment and acidification in lymphocyte is consistent with data demonstrating that the pH encountered by Tf-FITC in Hep G2 cells decreases during uptake and transfer from sorting to recycling endosomes (van Weert et al., 1995), whereas in the case of Chinese hamster ovary cells, sorting endosomes are more acidic than recycling endosomes (Yamashiro et al., 1984).
The correlation between establishment of translocation competence (Figure 6A) and acidification of the endosome lumen (Figure 6C) is coherent with results demonstrating the following: first, that acidification below pH 5.5 triggered PE conformational changes in a transforming growth factor-α and PE40 conjugate (Sanyal et al., 1993); second, that mutant Chinese hamster ovary cells cross-resistant to DT and PE were deficient in ATP-dependent endosomal acidification (Robbins et al., 1984); and, third, that inhibitors of endosome acidification such as weak bases [ammonium chloride, chloroquine (Morris et al., 1983; Sundan et al., 1984)] or ionophores [monensin (Morris et al., 1983; Sundan et al., 1984; Morris and Saelinger, 1986)] offered protection from PE intoxication. None of these molecules were found to directly affect PE translocation, delivery to the translocation compartment, or the morphology of the latter. Nevertheless, when present during PE endocytosis, they impaired subsequent PE translocation from isolated endosomes. Together these data provide compelling evidence indicating that transit through acidic compartments is required to establish PE translocation competence, but not for translocation per se. Moreover, PE conformational modification and membrane insertion induced by low endosomal pH appeared to be irreversible since subsequent cell-free translocation can proceed from neutralized endosomes (Figure 10).
Low temperatures (below 25°C) likely protect cells by inhibiting egress of PE from sorting endosomes and its delivery to the translocation-competent recycling compartment.
Endosomes from cells treated with BFA enabled weak but significant PE translocation (∼15% as compared with control conditions). This was probably due to the fact that the toxin was still delivered to recycling endosomes, which are part of a large network where they were diluted into translocation-incompetent sorting endosomes. Why is translocation restricted to the recycling compartment in the endocytic pathway? The low pH is probably involved in combination with the fact that, contrary to the sorting endosome, recycling endosomes are long-lived structures (Hopkins et al., 1994, Ghosh and Maxfield, 1995) and will give more chances to PE to undergo its kinetically limiting translocation. Cellular components specific to this compartment, whose protein composition has not yet been characterized, might also be involved. Alternatively, a molecule able to inhibit PE transmembrane transport could be specifically localized within sorting endosomes.
As for the energy source for PE translocation, the absence of any effect of endosomal acidification inhibitors, including the specific inhibitor of V-ATPase bafilomycin A1 when added to the PE cell-free translocation assay, demonstrated that contrary to DT (Olsnes and Sandvig, 1988; Beaumelle et al., 1992) the driving force for PE membrane traversal is not the endosome-cytosol pH gradient; this energy is supplied by ATP hydrolysis probably via a different ATPase. PE translocation would thus require the sequential action of two endosomal ATPases: the V-ATPase induces lumen acidification, leading to PE insertion into the membrane, and then another ATPase allows translocation per se. Similarities in the observed Km for ATP (∼4 mM) to support ricin (Beaumelle et al., 1993) and PE translocation suggest that the same enzyme might be implicated in the transfer of both toxins to the cytosol.
PE Does Not Strictly Require Processing for Translocation from Lymphocyte Endosomes
Experiments performed on highly PE-sensitive cells (Pastan et al., 1992), and confirmed using cell-free assays (Chiron et al., 1996), identified furin as the cell protease processing PE during endocytosis and generating the 28-kDa and 37-kDa PE fragments, corresponding to the receptor-binding domain (28 kDa) and the translocation and ADP-ribosylating domains (37 kDa). Full-sized PE was found along with the 37-kDa fragment in the cytosol of intoxicated L929 cells (Ogata et al., 1990).
Studies of several PEs mutated within the processed loop failed to provide a direct correlation between PE cleavage and intoxication efficiency since some mutants which were more efficiently processed than native PE were less cytotoxic (Zdanovsky et al., 1993; Chiron et al., 1996). Furin-deficient cells are resistant to PE, but this resistance at least partly arises from a deficiency in LRP, which also requires processing by furin-like proteases for transport to the cell surface after biosynthesis (Gu et al., 1996).
The BW 5147 (mouse) and CEM (human) lymphocytes used in this study are, as most cell types (Mucci et al., 1995), moderately sensitive to PE (IC50 ∼10 nM). After 30–35 min of endocytosis, we did not observe any PE processing within lymphocyte (Figure 12 for BW cells) endosomes and full-length PE was translocated in the cell-free assay. These cells only cleaved PE after 4 h labeling (our unpublished data). After such a long labeling time, most toxin molecules have reached lysosomes, many cells are dead, and processing at this point is probably not related to cell intoxication events. This absence of cleavage during endocytosis contrasts with the results obtained by Ogata et al. (1990), who observed proteolysis as early as 10 min after adding radiolabeled PE to highly PE-sensitive cells (IC50 ∼1 pM for 3T3 and L929 mouse fibroblasts).
Hence, early proteolytic processing (10–15 min after the initiation of endocytosis) could be associated with high sensitivity to PE intoxication. After insertion into the membrane of acidic endosomes, the 37-kDa carboxyl-terminal fragment [produced by proteolysis then released from the receptor-binding domain by disulfide reduction (Pastan et al., 1992)] likely translocates more efficiently than native PE whose affinity for the LRP is maximum at low pH (Thompson et al., 1991). The membrane boundaries enabling translocation of the 37-kDa PE fragment generated in highly PE-sensitive cells such as mouse fibroblasts are yet to be identified.
It is difficult to determine the order of the events, both in time and importance, preceding PE translocation: processing, membrane insertion, and reduction of the disulfide (cys265–287) hooking the two fragments. This disulfide bond is not essential for PE cytotoxicity (Madshus and Collier, 1989), and since we found that proteolysis was not strictly required, the only essential requirement for establishing PE translocation competence would be low pH exposure to trigger its insertion into the endosome membrane. Further work will be required before a more refined model for PE transmembrane transport can be drawn up.
ACKNOWLEDGMENTS
We are grateful to Monique Caysac, Brigitte N’Guyen, and Paul Mangeat for help in various experiments, to Dr. Carol Pitcher and Professor J.M. Lord (University of Warwick, Warwick, United Kingdom) for providing PE and PE343Q clones, to Dr. P. Chavrier (Marseille, France) for anti-rab7 antibodies, and to Drs. A. Dautry-Varsat (Institut Pasteur, Paris, France) and Clare Futter (University College, London, United Kingdom) for advice on lymphocyte confocal microscopy and DAB cytochemistry, respectively. This work was supported by grants from the Association pour la Recherche sur le Cancer and the Ligue Nationale contre le Cancer.
Footnotes
Abbreviations used: BFA, brefeldin A; DAB, 3,3′-diaminobenzidine; DT, diphtheria toxin; EF2, elongation factor 2; HRP, horseradish peroxidase; IC50, 50% inhibitory concentration; α2-M, α2-macroglobulin; LDL, low-density lipoproteins; LRP, low-density lipoprotein receptor-related protein; PBS, phosphate-buffered saline; PE(-FITC), Pseudomonas exotoxin(-fluorescein); Tf(-FITC), transferrin(-fluorescein); TGN, trans-Golgi network; TCA, trichloroacetic acid; V-ATPase, vacuolar-type H+-ATPase.
REFERENCES
- Allured V, Collier RJ, Caroll SF, McKay DB. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0 Angstrom resolution. Proc Natl Acad Sci USA. 1986;83:1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens PI, Majoul IV, Verveer PJ, Söling HD, Jovin TM. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 1996;15:4246–4253. [PMC free article] [PubMed] [Google Scholar]
- Beaumelle B, Alami M, Hopkins CR. ATP-dependent translocation of ricin across the membrane of purified endosomes. J Biol Chem. 1993;268:23661–23669. [PubMed] [Google Scholar]
- Beaumelle B, Bensamar L, Bienvenüe A. Selective translocation of the A chain of Diphtheria Toxin across the membrane of purified endosomes. J Biol Chem. 1992;267:11525–11531. [PubMed] [Google Scholar]
- Beaumelle BD, Gibson A, Hopkins CR. Isolation and preliminary characterization of the major membrane boundaries of the endocytic pathway in lymphocytes. J Cell Biol. 1990;111:1811–1823. doi: 10.1083/jcb.111.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumelle BD, Hopkins CR. High yield isolation of functionally competent endosomes from mouse lymphocytes. Biochem J. 1989;264:137–149. doi: 10.1042/bj2640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U, Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Chen SY, Yang A-G, Chen J-D, Kute T, King CR, Collier J, Cong Y, Yao C, Huang XF. Potent antitumour activity of a new class of tumour-specific killer cells. Nature. 1997;385:78–80. doi: 10.1038/385078a0. [DOI] [PubMed] [Google Scholar]
- Chiron MF, Ogata M, FitzGerald D. Pseudomonas exotoxin exhibits increased sensitivity to furin when sequences at the cleavage site are mutated to resemble the arginine-rich loop of diphtheria toxin. Mol Microbiol. 1996;22:769–778. doi: 10.1046/j.1365-2958.1996.d01-1721.x. [DOI] [PubMed] [Google Scholar]
- Chung DW, Collier RJ. The mechanism of ADP-ribosylation of elongation factor 2 catalyzed by fragment A from diphtheria toxin. Biochim Biophys Acta. 1977;483:248–257. doi: 10.1016/0005-2744(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbé S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Douglas CM, Guidi-Rontani C, Collier RJ. Exotoxin A of Pseudomonas exotoxin: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987;169:4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, Speck JC. Protein and cell membrane iodination with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence of nonvectorial, retrograde transferrin trafficking in the early endosomes of Hep2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gu M, Gordon VM, Fitzgerald DJP, Leppla SH. Furin regulates both the activation of Pseudomonas exotoxin A and the quantity of the toxin receptor expressed on target cells. Infect Immun. 1996;64:524–527. doi: 10.1128/iai.64.2.524-527.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hémar A, Subtil A, Lieb E, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of IL2-receptors in human T lymphocytes. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C, Gibson A, Shipman M, Strickland DK, Trowbridge I. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TH, Neville DM. Temporal separation of protein toxin translocation from processing events. J Biol Chem. 1987;262:16484–16494. [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Brefeldin A and the endocytic pathway. Possible implications for membrane traffic and sorting. FEBS Lett. 1992;307:93–96. doi: 10.1016/0014-5793(92)80908-y. [DOI] [PubMed] [Google Scholar]
- Jiang JX, London E. Involvement of denaturation-like changes in Pseudomonas exotoxin A hydrophobicity and membrane penetration determined by characterization of pH and thermal transitions. J Biol Chem. 1990;265:8636–8641. [PubMed] [Google Scholar]
- Jinno Y, Ogata M, Chaudhary VK, Willingham MC, Adhya S, Fitzgerald DJ, Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989;264:15953–15959. [PubMed] [Google Scholar]
- Kounnas MZ, Morris RE, Thompson MR, FitzGerald DJ, Strickland KD, Saelinger CB. The α2 macroglobulin receptor/low density lipoprotein receptor binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992;267:12420–12423. [PubMed] [Google Scholar]
- Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus IH, Collier RJ. Effects of eliminating a disulfide bridge within domain II of Pseudomonas aeruginosa exotoxin A. Infect Immun. 1989;57:1873–1878. doi: 10.1128/iai.57.7.1873-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul IV, Bastiaens PIH, Sölling HD. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in vero cells. J Cell Biol. 1996;133:777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méresse S, Gorvel J-P, Chavrier P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci. 1995;108:3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, Rothman JE. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995;129:309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RE, Manhart MD, Saelinger CB. Receptor-mediated entry of Pseudomonas toxin: methylamine blocks clustering step. Infect Immun. 1983;40:806–811. doi: 10.1128/iai.40.2.806-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RE, Saelinger CB. Reduced temperature alters Pseudomonas exotoxin entry into the mouse LM cells. Infect Immun. 1986;52:445–453. doi: 10.1128/iai.52.2.445-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci D, Forristal J, Strickland D, Morris R, FitzGerald D, Saelinger CB. Level of receptor-associated protein moderates cellular susceptibility to Pseudomonas exotoxin A. Infect Immun. 1995;63:2912–2918. doi: 10.1128/iai.63.8.2912-2918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Chaudhary VK, Pastan I, FitzGerald DJ. Processing of Pseudomonas Exotoxin by a cellular protease results in the generation of a 37,000-D toxin fragment that is translocated to the cytosol. J Biol Chem. 1990;265:20678–20685. [PubMed] [Google Scholar]
- Olsnes S, Sandvig K. How protein toxins enter and kill cells. In: Frankel AE, editor. Immunotoxins. Dordecht, the Netherlands: Kluwer Academic Publishers; 1988. pp. 39–73. [DOI] [PubMed] [Google Scholar]
- Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg++ in lymphocytes. J Cell Biol. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins AR, Oliver C, Bateman JL, Krag SS, Galloway CJ, Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984;99:1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Garred O, Prydz K, Kovlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Prydz K, Hansen SH, van Deurs B. Ricin transport in Brefeldin A-treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991;115:971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal G, Marquis-Omer D, O’Brien Gress J, Middaugh CR. A transforming growth factor-α-Pseudomonas exotoxin hybrid protein undergoes pH-dependent conformational changes conductive to membrane interaction. Biochemistry. 1993;32:3488–3497. doi: 10.1021/bi00064a037. [DOI] [PubMed] [Google Scholar]
- Seetharam S, Chaudhary VK, FitzGerald DJ, Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991;266:17376–17381. [PubMed] [Google Scholar]
- Siegall CB, Ogata M, Pastan I, FitzGerald D. Analysis of sequences in domain II of Pseudomonas exotoxin A which mediate translocation. Biochemistry. 1991;30:7154–7159. doi: 10.1021/bi00243a016. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Griffith JM, Schwartz AL, Strous GJ. Relations between the intracellular pathways of the receptors for transferrin, asialoglycoprotein, and mannose 6-phosphate in human hepatoma cells. J Cell Biol. 1989;108:2137–2148. doi: 10.1083/jcb.108.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundan A, Sandvig K, Olsnes S. Calmodulin antagonists sensitize cells to Pseudomonas toxin. J Cell Physiol. 1984;119:15–22. doi: 10.1002/jcp.1041190104. [DOI] [PubMed] [Google Scholar]
- Taupiac MP, Alami M, Beaumelle BD. Translocation of full-length Pseudomonas exotoxin from endosomes is driven by ATP hydrolysis but requires prior exposure to acidic pH. J Biol Chem. 1996;271:26170–26173. doi: 10.1074/jbc.271.42.26170. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Forristal J, Kauffmann P, Madden T, Kozak K, Morris RE, Saelinger CB. Isolation and characterization of Pseudomonas aeruginosa exotoxin A binding glycoprotein from mouse LM cells. J Biol Chem. 1991;266:2390–2396. [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbé S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umata T, Moriyama Y, Futai M, Mekada E. The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase. J Biol Chem. 1990;265:21940–21945. [PubMed] [Google Scholar]
- van Deurs B, Tonnessen TI, Petersen OW, Sandvig K, Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986;102:37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert A, Dunn KW, Geuze HJ, Maxfield FR, Stoorvogel W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil ML, Kabat D, Iglewski BH. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977;16:353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Brown WJ. The morphology but not the function of endosomes and lysosomes is altered by brefeldin A. J Cell Biol. 1992;119:273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;137:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Kinetics of α2-macroglobulin endocytosis an degradation in mutant and wild type Chinese hamster ovary cells. J Cell Physiol. 1989;139:377–382. doi: 10.1002/jcp.1041390221. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Chen C, Zhang M, Wu HC. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin and Pseudomonas toxin. Exp Cell Res. 1991;192:389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Zdanovsky AG, Chiron M, Pastan I, FitzGerald DJ. Mechanism of action of Pseudomonas exotoxin. J Biol Chem. 1993;268:21791–21799. [PubMed] [Google Scholar]