Abstract
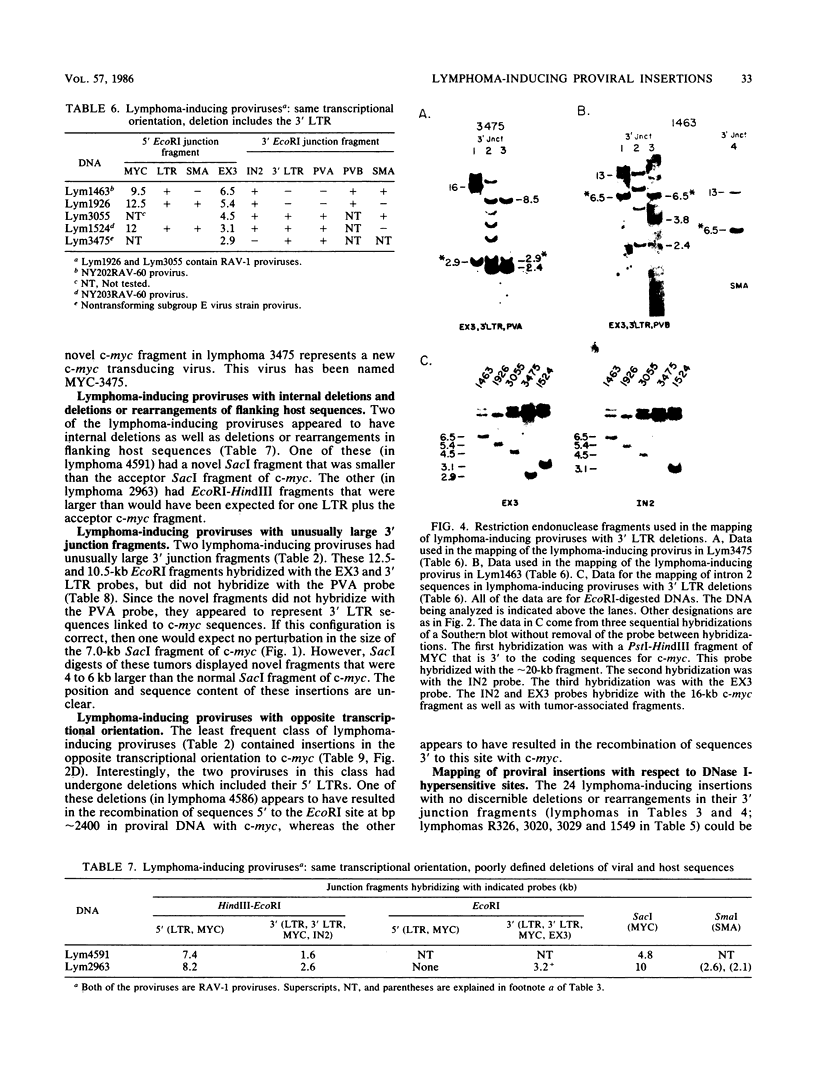
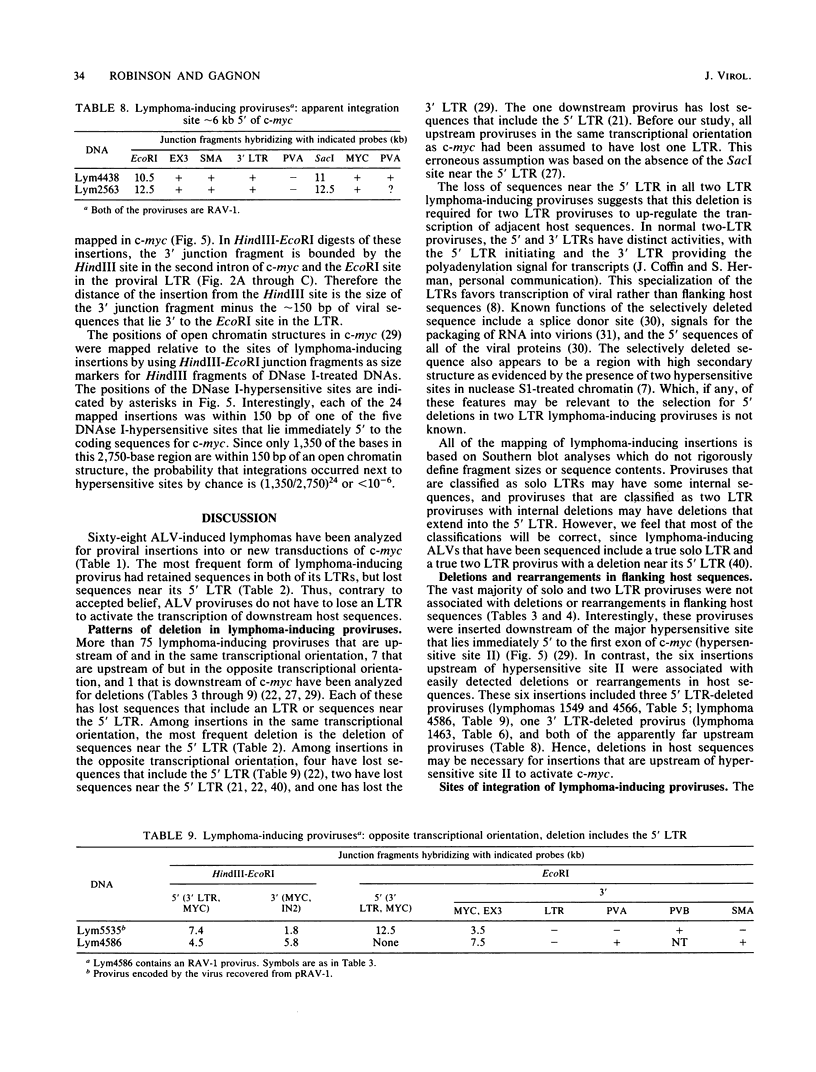
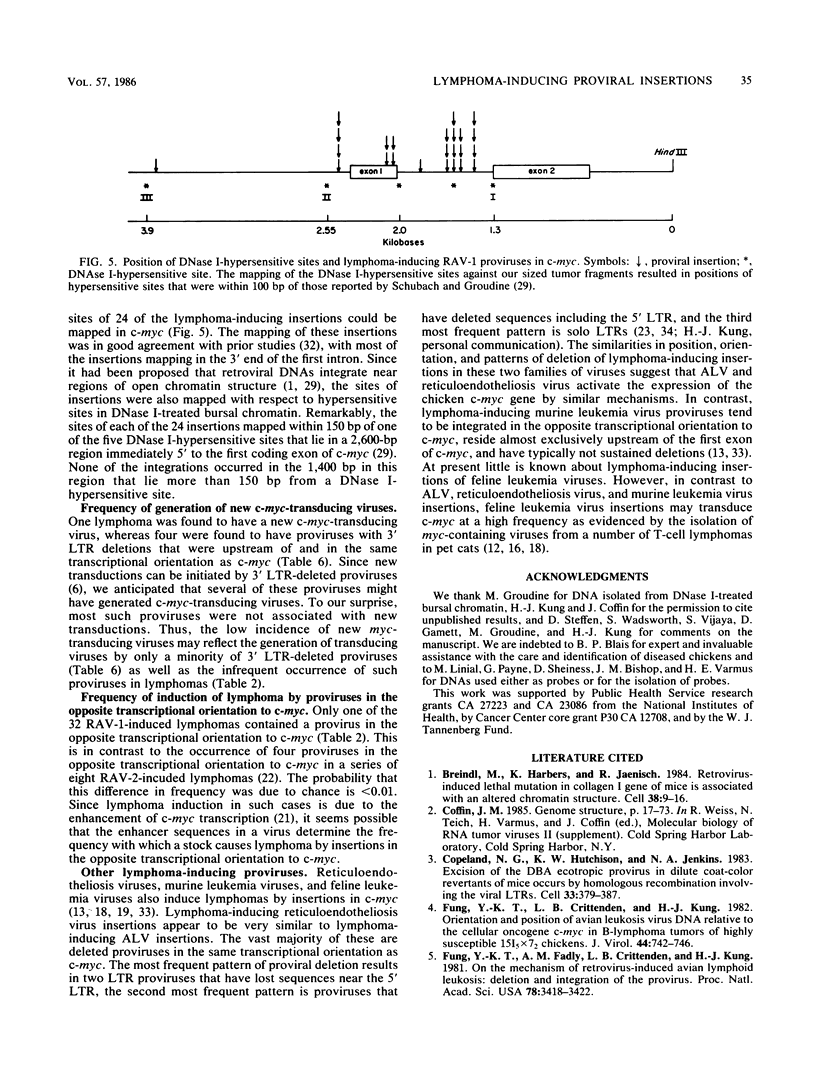
Sixty-eight lymphomas induced by eight different avian leukosis viruses have been analyzed on Southern blots for virus-induced mutations in the chicken c-myc gene. Sixty-six of the lymphomas exhibited a proviral insertion in c-myc, whereas one exhibited a new transduction of c-myc. Sixty-four of the proviral insertions were in the same transcriptional orientation as c-myc. Two were in the opposite transcriptional orientation. All of the insertions were upstream of the protein-coding sequences of c-myc, with most residing in the first exon or the first intron of c-myc. All of the lymphoma-inducing proviruses had deletions that included either sequences near the 5' long terminal repeat (LTR) or an LTR. The most frequent lymphoma-inducing provirus appeared to have retained both of its LTRs, but had lost sequences near its 5' LTR. The second and third most frequent lymphoma-inducing proviruses consisted of solo LTRs or of proviruses that had lost the 5' LTR as well as some internal sequences. Twenty-four insertions were mapped in c-myc. Each of these mapped to within 150 base pairs of one of the five DNase I-hypersensitive sites that occur in a 3-kilobase region immediately 5' to the protein-coding sequences of c-myc. One lymphoma contained a new c-myc transducing virus. This virus, MYC-3475, caused rapid-onset myelocytomatosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M., Harbers K., Jaenisch R. Retrovirus-induced lethal mutation in collagen I gene of mice is associated with an altered chromatin structure. Cell. 1984 Aug;38(1):9–16. doi: 10.1016/0092-8674(84)90521-x. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Crittenden L. B., Kung H. J. Orientation and position of avian leukosis virus DNA relative to the cellular oncogene c-myc in B-lymphoma tumors of highly susceptible 15I5 X 7(2) chickens. J Virol. 1982 Nov;44(2):742–746. doi: 10.1128/jvi.44.2.742-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Conkin K. F. Chromatin structure and de novo methylation of sperm DNA: implications for activation of the paternal genome. Science. 1985 May 31;228(4703):1061–1068. doi: 10.1126/science.2986289. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Boone L., Skalka A. M. Isolation and characterization of recombinant DNA clones of avian retroviruses: size heterogeneity and instability of the direct repeat. J Virol. 1980 Mar;33(3):1026–1033. doi: 10.1128/jvi.33.3.1026-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Gardner M. B., Casey J. W. Isolation of a feline leukaemia provirus containing the oncogene myc from a feline lymphosarcoma. 1984 Apr 26-May 2Nature. 308(5962):853–856. doi: 10.1038/308853a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Groudine M. Transcription of three c-myc exons is enhanced in chicken bursal lymphoma cell lines. Proc Natl Acad Sci U S A. 1985 Jan;82(1):53–57. doi: 10.1073/pnas.82.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Hughes D., McFarlane R., Wilkie N. M., Onions D. E., Lees G., Jarrett O. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias. 1984 Apr 26-May 2Nature. 308(5962):814–820. doi: 10.1038/308814a0. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Pachl C., Schubach W., Eisenman R., Linial M. Expression of c-myc RNA in bursal lymphoma cell lines: identification of c-myc-encoded proteins by hybrid-selected translation. Cell. 1983 Jun;33(2):335–344. doi: 10.1016/0092-8674(83)90415-4. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- RUBIN H., VOGT P. K. An avian leukosis virus associated with stocks of Rous sarcoma virus. Virology. 1962 May;17:184–194. doi: 10.1016/0042-6822(62)90096-x. [DOI] [PubMed] [Google Scholar]
- Ridgway A. A., Swift R. A., Kung H. J., Fujita D. J. In vitro transcription analysis of the viral promoter involved in c-myc activation in chicken B lymphomas: detection and mapping of two RNA initiation sites within the reticuloendotheliosis virus long terminal repeat. J Virol. 1985 Apr;54(1):161–170. doi: 10.1128/jvi.54.1.161-170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Jensen L., Coffin J. M. Sequences outside of the long terminal repeat determine the lymphomogenic potential of Rous-associated virus type 1. J Virol. 1985 Sep;55(3):752–759. doi: 10.1128/jvi.55.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Pearson M. N., DeSimone D. W., Tsichlis P. N., Coffin J. M. Subgroup-E avian-leukosis-virus-associated disease in chickens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1133–1141. doi: 10.1101/sqb.1980.044.01.122. [DOI] [PubMed] [Google Scholar]
- Schubach W., Groudine M. Alteration of c-myc chromatin structure by avian leukosis virus integration. Nature. 1984 Feb 23;307(5953):702–708. doi: 10.1038/307702a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. K., Linial M., Goodenow M. M., Hayward W. S. Nucleotide sequence 5' of the chicken c-myc coding region: localization of a noncoding exon that is absent from myc transcripts in most avian leukosis virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4697–4701. doi: 10.1073/pnas.81.15.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R. A., Shaller E., Witter R. L., Kung H. J. Insertional activation of c-myc by reticuloendotheliosis virus in chicken B lymphoma: nonrandom distribution and orientation of the proviruses. J Virol. 1985 Jun;54(3):869–872. doi: 10.1128/jvi.54.3.869-872.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., Reddy E. P., Duesberg P. H., Papas T. S. Nucleotide sequence analysis of the chicken c-myc gene reveals homologous and unique coding regions by comparison with the transforming gene of avian myelocytomatosis virus MC29, delta gag-myc. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2146–2150. doi: 10.1073/pnas.80.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Payne G., Varmus H. E. Proviral deletions and oncogene base-substitutions in insertionally mutagenized c-myc alleles may contribute to the progression of avian bursal tumors. Proc Natl Acad Sci U S A. 1984 Feb;81(3):843–847. doi: 10.1073/pnas.81.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]