Abstract
The ERM proteins (ezrin, radixin, and moesin) are a group of band 4.1-related proteins that are proposed to function as membrane/cytoskeletal linkers. Previous biochemical studies have implicated RhoA in regulating the association of ERM proteins with their membrane targets. However, the specific effect and mechanism of action of this regulation is unclear. We show that lysophosphatidic acid stimulation of serum-starved NIH3T3 cells resulted in relocalization of radixin into apical membrane/actin protrusions, which was blocked by inactivation of Rho by C3 transferase. An activated allele of RhoA, but not Rac or CDC42Hs, was sufficient to induce apical membrane/actin protrusions and localize radixin or moesin into these structures in both Rat1 and NIH3T3 cells. Lysophosphatidic acid treatment led to phosphorylation of radixin preceding its redistribution into apical protrusions. Significantly, cotransfection of RhoAV14 or C3 transferase with radixin and moesin revealed that RhoA activity is necessary and sufficient for their phosphorylation. These findings reveal a novel function of RhoA in reorganizing the apical actin cytoskeleton and suggest that this function may be mediated through phosphorylation of ERM proteins.
INTRODUCTION
Ezrin, radixin, and moesin (the ERM proteins) are three closely related proteins in the band 4.1 superfamily, members of which are known to serve as membrane–cytoskeletal linkers (Tsukita and Yonemura, 1997; Tsukita et al., 1997). The ERM proteins are found in dynamic plasma membrane/actin interfaces such as ruffling membranes, cleavage furrows, and microvilli; ezrin is a component of microvilli in a number of polarized epithelia (Bretscher, 1983; Hanzel et al., 1991; Berryman et al., 1993; Franck et al., 1993; Winckler et al., 1994; Amieva and Furthmayr, 1995). Furthermore, antisense experiments have demonstrated that these proteins are critical for the formation of surface microvilli in a lymphoma cell line as well as maintenance of cell–cell and cell–substratum adhesion in certain epithelial cells (Takeuchi et al., 1994). Expression of a dominant negative allele of ezrin has also been shown to cause loss of microvilli in polarized epithelial cells (Crepaldi et al., 1997). It was recently reported that ezrin is involved in the reorganization of ICAM-2 into uropods in lymphoblastoma cells, sensitizing them to natural killer cells (Helander et al., 1996). This again suggests that the ERM proteins may help to reorganize both the cytoskeleton and membrane proteins to promote cell–cell adhesion. These proteins are also closely related to the product of the neurofibromatosis type 2 (NF2) tumor suppressor gene merlin, which is mutated in certain cancer tumor types (Rouleau et al., 1993; Trofatter et al., 1993).
Several studies suggest that the ERM proteins have a bipartite structure analogous to that proposed for band 4.1, composed of an amino-terminal domain responsible for binding to integral membrane targets [including the hyaluronic acid receptor CD44 (Tsukita et al., 1994)] and a carboxyl domain that binds to the actin cytoskeleton (Turunen et al., 1994; Pestonjamasp et al., 1995). Studies performed in vitro and in vivo indicate that in resting cells the ERM proteins may exist in a head-to-tail, intramolecular association that masks both the membrane- and actin-binding sites (Berryman et al., 1995; Gary and Brestcher, 1995; Tsukita et al., 1997). Because all three ERM proteins have been shown to be phosphorylated and relocalized to dynamic actin structures in a variety of cell types in response to growth factors (Bretscher, 1989; Urishidani et al., 1989; Fazioli et al., 1993; Nakamura et al., 1995), it has been proposed that phosphorylation may regulate these proteins through disruption of this intramolecular association; however, this has not been demonstrated experimentally.
RhoA is a member of the ras-like GTPase superfamily and has been shown to regulate the actin cytoskeleton and mitogenic signaling in response to extracellular signals (Machesky and Hall, 1996). RhoA has been tied to many cellular functions, including regulation of cell motility (Takaishi et al., 1993; Ridley et al., 1995), polarity (Strutt et al., 1997), cytokinesis (Kishi et al., 1993), and cell–cell (Tominaga et al., 1993) and cell–substratum adhesion (Laudanna et al., 1996). Of note, the ERM proteins and RhoA have been shown to colocalize, and moesin coimmunoprecipitates with RhoGDI, a regulator of Rho (Takaishi et al., 1995; Hirao et al., 1996). RhoA was also shown to regulate the association of ERM proteins with one potential integral membrane target, CD44 (Hirao et al., 1996). Here, we have analyzed the relationship between RhoA and the ERM proteins and describe a novel morphogenetic activity of RhoA in fibroblasts: the formation of apical membrane/actin protrusions. The ERM proteins appear to be critical components of these structures. Further, we report that stimulation of RhoA activity results in phosphorylation of the ERM proteins, which correlates with the formation of these structures.
MATERIALS AND METHODS
Cell Lines and Transfection
NIH3T3 cells were obtained from American Type Culture Collection (Rockville, MD). R12 cells were a generous gift of M. Symons (Onyx Pharmaceuticals, Richmond, CA). R12 cells are a subclone of Rat1 cells stably expressing the tTA tetracycline-repressible transactivator (Resnitzky et al., 1994). All experiments were performed in the absence of tetracycline. For some indicated experiments, two previously characterized NIH3T3 cell lines stably expressing full-length radixin with the hemagglutinin (HA) epitope tag at their carboxyl terminus were utilized (Henry et al., 1995). Lysophosphatidic acid (1-oleoyl) was purchased from Avanti Polar Lipids (Alabaster, AL) and prepared in phosphate-buffered saline (PBS) with 0.6 mM CaCl2, 0.5 mM MgCl2, 1% bovine serum albumin (BSA) (essentially fatty acid free, Sigma, St. Louis, MO) as described by van Corven et al. (1989). All cells were transfected by modified calcium phosphate with glycerol shock as previously described (Henry et al., 1995). For indirect immunofluorescence analysis, exponentially growing NIH3T3 cells or R12 cells were seeded at 1–2 × 105 cells onto ethanol-sterilized glass coverslips in a six-well dish and the next day were transfected with 5 μg total DNA per well. For these experiments, 2.5 μg of each GTPase or empty pcDNA3 were cotransfected with 2.5 μg of each ERM or merlin expression construct. For immunoprecipitation experiments, exponentially growing cells were plated at 1–2 × 106 in a 100-mm dish and then transfected the next day with 20 μg total DNA. For these experiments, 15 μg of RhoAV14, C3, or empty expression plasmid were cotransfected with 5 μg of moesin or radixin expression plasmids. Transfected cells were placed in DMEM supplemented with 10% fetal calf serum (Hyclone, Logan, UT), for R12 cells, or DMEM supplemented with 10% calf serum (for NIH3T3 cells), for 16 h and then rinsed in DMEM and placed in DMEM (0% serum) for 8–12 additional hours. For lysophosphatidic acid (LPA) treatment, cells were starved as described by Buhl et al. (1995); cells were starved 20–24 h in DMEM supplemented with 0.1% fetal bovine serum and then rinsed with DMEM and placed in DMEM alone (0% serum) for 16 h.
Expression Constructs
GTPase constructs (RhoAV14, RhoAN19, Rac1V12, CDC42V12) were cloned into the pcDNA3 mammalian expression vector and were obtained from Lisa Stowers and John Chant (Harvard University, Cambridge, MA). Each of the GTPases carried the myc epitope tag at their amino terminus. Expression plasmids encoding C3 transferase (EFC3) and empty vector (EFplink) were obtained from R. Triesmann (Imperial Cancer Research Fund, London). Full-length radixin and RADC mutant driven by β-actin or tetracycline-repressible promoter were previously described (Henry et al., 1995). Full-length merlin and moesin were generated by reverse-transcriptase-polymerase chain reaction utilizing RNA from murine fetal brain and murine adult lung, respectively. Primer sequences for mouse merlin were 5′-GCCGTCGACGCCGGAGCCATCGCTTCTCG-3′ and the reverse primer 5′-GCCGTCGACGAGTTCTTCAAAGAAGGC-3′. Primer sequences for mouse moesin were 5′-GGCCGTCGACCCCAAAACGATCAGTGTGCG-3′ and the reverse primer 5′-GGCCGTCGACCATGGACTCAAACTCATCAATGCG-3′. Reverse transcriptase-polymerase chain reaction fragments were digested with Sal and directed cloned into pcDNA3 carrying an HA epitope tag directly following the Sal cloning site and ATG directly preceding it, such that the final construct expressed each with the HA tag at their carboxyl terminus.
Primary Antibodies
Monoclonal antibody (mAb) 12CA5 and biotinylated 12CA5 were purchased from Boehringer Mannheim (Indianapolis, IN). mAb 9E10 was purchased from Oncogene Science (Uniondale, NY). pAb CR22 recognizes primarily moesin by indirect immunofluorescence, although it does cross-react with radixin and ezrin as previously described (Tsukita et al., 1994). Anti-CD44 rat monoclonal IM7 was purchased from PharMingen (San Diego, CA).
Immunocytochemistry/Confocal Microscopy
Cells were grown on ethanol-sterilized glass coverslips as described above. Twenty-four to 30 h posttransfection, cells were fixed for 15 min with 3.7% paraformaldehyde in PBS at room temperature, washed three times in PBS, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and washed three times in PBS. Cells were then blocked in 10% normal goat serum in PBS (Vector Laboratories, Burlingame, CA). The cells were then incubated in primary antibody diluted in PBS containing 1% BSA for 30 min at 37°C, washed three times in PBS, and then incubated for 30 min at 37°C with the appropriate secondary antibody diluted in PBS containing 1% BSA: fluorescein isothiocyanate (FITC) or rhodamine-conjugated goat anti-mouse for 12CA5, or FITC goat anti-rat for IM7. In some experiments, during secondary antibody incubation, cells were stained with rhodamine-conjugated phalloidin (Molecular Probes, Eugene, OR) and/or 4,6-diamidino-2-phenylindole (Sigma) to reveal F-actin and DNA, respectively. For 12CA5 and 9E10 double labeling experiments, 9E10 and rhodamine-goat anti-mouse incubations were performed as above; then, after secondary washes, cells were fixed in 2% paraformaldehyde, washed three times in PBS, and then incubated with biotinylated 12CA5 diluted in PBS containing 1% BSA as above, washed, and finally incubated with FITC-streptavidin diluted in PBS containing 1% BSA. Coverslips were mounted onto glass slides using Mowiol mounting medium (Hoescht Celanese, Charlotte, NC) containing the antifade agent 1,4 diaza-bicyclo[2,2,2] octane (Aldrich, Milwaukee, WI) at 15 mg/ml. Cells were examined by conventional microscopy on an Axioplan microscope (Carl Zeiss, Thornwood, NY) using 63 × 1.4 N.A. and 100 × 1.3 N.A. objectives. For confocal microscopy, cells were examined with a MRC600 scanning laser confocal microscope (Bio-Rad Laboratories, Richmond, CA). Images were recorded on Tri-X-Pan400 film or Ektachrome Elite400 film (Eastman Kodak, Rochester, NY).
Scanning Electron Microscopy (SEM)
SEM was performed on transfected RhoAV14, CDC42V12, and pcDNA3 vector control NIH3T3 cells. Transfections were performed as above using 2.5 μg RhoAV14 or CDC42V12 and 2.5 μg pcDNA3 or 5 μg pcDNA3. Three independent transfections were examined by SEM with more than 200 cells examined per transfection. Coverslips from the same transfections were processed for anti-myc immunofluorescence to gauge the transfection efficiency per sample. The percentage of cells demonstrating the phenotype as shown in Figure 5 for RhoV14 or CDc42V12 was nearly identical to the percentage shown to be transfected by these GTPases by anti-myc indirect immunofluorescence on parallel coverslips. Moreover, the exact same phenotype of hundreds of apical projections was observed in nearly all cells of an NIH3T3 cell line stably expressing RhoAV14 and never in the parental NIH3T3 cell line. Cells plated as described above were fixed in 2% gluteraldehyde (EM grade, Sigma) in PBS for 15 min at 0°C, washed three times in PBS, incubated in 1% OsO4 at 0°C for 30 min, washed three times in PBS, and then dehydrated in a graded series of ethanol and dried in a critical point drier after substitution with liquid CO2. Dried samples were coated with approximately 200 Å gold/paladium using a gold sputter coater (Technics, Alexandria, VA) and were examined under a scanning electron microscope (Amray, Bedford, MA).
Figure 5.
SEM of RhoAV14-induced apical membrane structures and CDC42-induced filopodia. SEM was performed on serum-starved vector control (a), RhoAV14-transfected (b), and CDC42V12-transfected (c) NIH3T3 cells. Note the number and length of the apical membrane protrusions induced by the presence of activated RhoA. Three independent transfections were examined by SEM with more than 200 cells examined per transfection. The percentage of cells demonstrating the phenotype as shown for RhoAV14 or CDC42V12 was nearly identical to the percentage shown to be transfected by either GTPase when a parallel coverslip was analyzed by indirect immunofluorescence. Furthermore, <2% of vector-only transfectants had apical structures similar to those seen with RhoAV14, and they were highly reduced in number and size (ca. 20 per cell as opposed to 100–200). Bar, 10 μm.
Immunoprecipitation and Immunoblotting
For LPA treatment, parallel sets of NIH3T3 cells stably expressing HA-radixin were starved as above until the last incubation in DMEM alone. After 10 h in DMEM alone, cells were washed in DMEM without methionine and cysteine or DMEM without phosphate and then placed in these media for 40 min, at which time 500 μCi [35S]methionine, cysteine (New England Nuclear, Boston, MA) or 1 mCi ]32P]orthophosphate (NEN) was added, respectively, and incubated for an additional 5 h. Cells were then treated with vehicle or LPA for indicated times. Treated cells were washed with PBS and lysed in 1 ml of RIPA-PIP buffer (50 mM Tris, pH 7.5, 1% Triton X-100, 0.5% sodium deoxycholate, 10% glycerol, 2 mM EDTA, 150 mM NaCl, 0.5% SDS) with protease and kinase/phosphatase inhibitors 50 mM NaF, 1 mM NaOVO4, 50 μm phenylarsine oxide, 20 mM NaMoO4, 1 μm staurosporine, 100 nM calyculin A. Lysates were rocked at 4°C for 15 min, scraped with a rubber policeman, and microcentrifuged at 14,000 × g for 15 min. An aliquot of the supernatant was then trichloroacetic acid precipitated to equilibrate incorporated counts. Equilibrated supernatants were precleared with normal rabbit serum and 50% protein A-Sepharose beads (Pierce Chemical, Rockford, IL) for 1 h. After preclearing, each lysate were incubated with 1 μg 12CA5 (preincubated for 2 h with 50% protein A-Sepharose). After 3 h incubation with 12CA5/protein A-Sepharose, immunocomplexes were pelleted at 1000 × g and washed three times in RIPA-PIP buffer without inhibitors. Sample buffer (5×) was added, and samples were incubated at 100°C for 5 min. Samples were resolved on 7% SDS-PAGE gels and visualized by autoradiography (after fixation and fluorometric enhancement of the 35S gel).
For Rho/C3 transfection experiments, 16 h posttransfection, cells were washed in DMEM without phosphate and incubated with DMEM without phosphate for 40 min after which 1 mCi [32P]orthophosphate was added to each plate as above and incubated at 37°C for 8 h. Cells were then lysed and immunoprecipitated as above. After resolving on a 7% SDS-PAGE gel, immunoprecipitates were transferred to polyvinyl diflouride membranes (PVDF) as previously described (McClatchey et al., 1997). After Western transfer, the PVDF blot was wrapped in saran wrap, and autoradiography was used to visualize the 32P signal. After a sufficient exposure (2 d in Figure 8), the blot was equilibrated in TBS-T (10 mM Tris, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and immunoblotted with 12CA5, followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody, and the signal was visualized by enhanced chemiluminescence, as previously described (Henry et al., 1995).
Figure 8.

RhoA activity is necessary and sufficient for phosphorylation of moesin. R12 or NIH3T3 cells were transfected with vector only, HA-moesin only, HA-moesin and RhoAV14, or HA-moesin and C3 transferase, as indicated. Cells were then serum starved and metabolically labeled with [32P]orthophosphate; cell lysates were immunoprecipitated as in Figure 7. To ensure that equivalent levels of HA-moesin were expressed and immunoprecipitated, immunoprecipitated samples were transferred to PVDF after SDS-PAGE and immunoblotted with 12CA5 antibody; signal was detected by enhanced chemiluminescence. Moesin is indicated by arrowhead at left. The presence of RhoA increased the basal level of moesin phosphorylation by 7-fold in R12 cells and 2.5-fold in NIH3T3 cells, while C3 treatment reduced the basal level of phosphorylation by at least 50% in both cell types (top panel). Protein levels were approximately equivalent in all conditions tested (bottom panel). The data shown are representative of three independent experiments.
Detergent Extraction Analysis
Parallel plates of NIH3T3 cells were transfected with 15 μg of either pcDNA3 or RhoAV14, along with 5 μg of HA-moesin as described above. At 16 h posttransfection, one set of plated cells was washed in DMEM without phosphate and incubated with DMEM without phosphate for 40 min, and 1 mCi [32P]orthophosphate was added to each plate as described above and incubated at 37°C for 8 h. The other set of cells was washed in DMEM without cysteine or methionine and incubated with DMEM without cysteine or methionine for 40 min, and 0.5 mCi [35S]methionine-cysteine mix was added to each plate and incubated at 37°C for 8 h. To obtain the detergent-soluble and -insoluble fractions, cells were rinsed once in PBS, and then incubated in 1 ml of Triton X-100 lysis buffer (80 mM piperazine-N,N-bis[ethanesulfonic acid], pH 6.4, 5 mM EGTA, 1 mM MgCl2, 0.5% Triton X-100) with protease and kinase/phosphatase inhibitors (see above) for 40 s. Solubilized material was collected as the detergent-soluble fraction. The material remaining on the tissue culture dish was incubated in RIPA-PIP buffer as described above and designated the detergent insoluble fraction. Samples were then immunoprecipitated with 12CA5 antibody (see above).
RESULTS
LPA-induced Relocalization of Radixin Is Blocked by C3 Transferase
LPA induces the formation of focal adhesions and stress fibers in quiescent Swiss3T3 fibroblasts in a manner dependent on RhoA activity (Ridley and Hall, 1992). To investigate the role of Rho in the regulation of ERM proteins, we examined the effect of LPA treatment on radixin localization, utilizing NIH3T3 cells stably expressing full-length radixin, epitope-tagged at its carboxyl terminus (Henry et al., 1995). Serum deprivation of these cells resulted in decreased radixin immunostaining and diffuse localization with a reduction (although not total disappearance) of actin stress fibers (Figure 1A, panels a and b). Within 3–5 min after LPA treatment, radixin was redistributed into short apical membrane protrusions grossly similar to microvilli and to peripheral actin protrusions as well (Figure 1A, panels c–h). This result was confirmed using a polyclonal antibody that recognizes all three ERM proteins endogenously in NIH3T3 cells (Figure 1B, a and c). These apical structures also contained F-actin as visualized by rhodamine phalloidin (see arrows in Figure 1A, panels e–h; Figure 1B, panels c and d).
Figure 1.
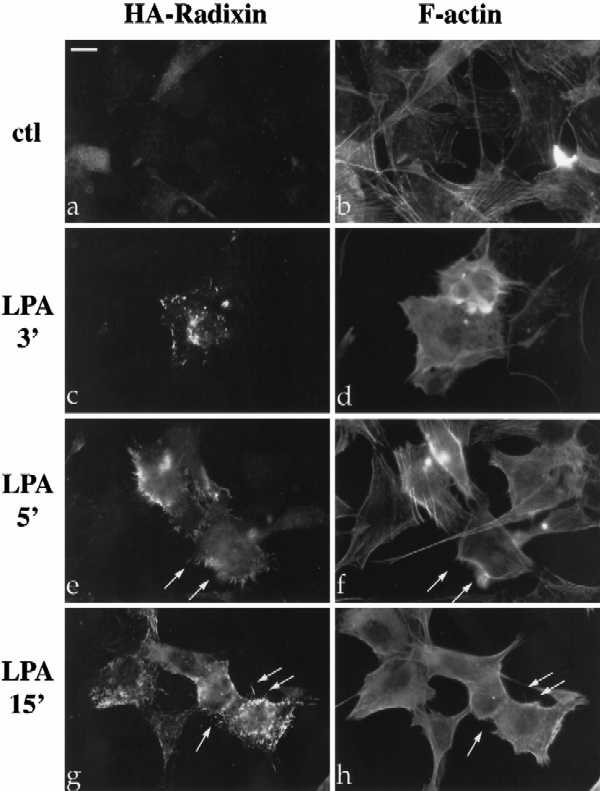
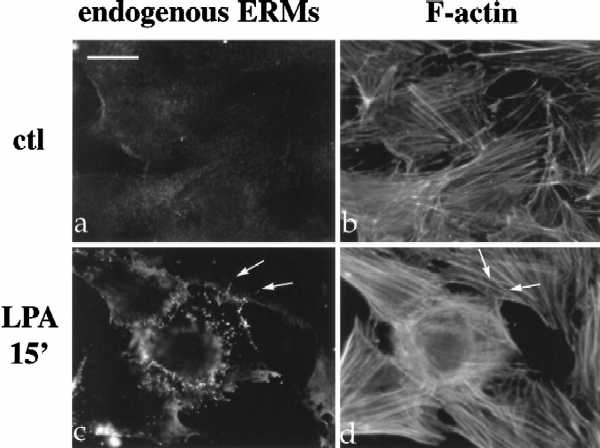
LPA-induced relocalization of radixin into apical membrane/actin protrusions in NIH3T3 cells. (A) Indirect immunofluorescence was performed on NIH3T3 cells stably expressing HA-epitope–tagged radixin. These cells were serum starved, and then treated with vehicle for 15 min (a and b), or with LPA (6 μM) for 3 min (c and d), 5 min (e and f), or 15 min (g and h). HA-radixin localization (using the 12CA5 mAb against the HA epitope) is shown in panels a, c, e, and g, and F-actin localization (using rhodamine phalloidin) is shown in b, d, f, and h. Starting 3–5 min after LPA treatment, radixin was relocalized into peripheral and apical membrane protrusions that also contain actin. These results were observed in three independent stable cell lines. Arrows denote colocalization of HA-radixin and actin in LPA-induced membrane protrusions. (B) NIH3T3 cells were serum starved and then treated with vehicle (a and b) or LPA for 15 min (c and d) and visualized for endogenous ERM proteins (a and c) using the CR22 polyclonal antibody or F-actin using rhodamine phalloidin (b and d). Arrows indicate the colocalization of ERM proteins and actin in membrane protrusions. Similar results were observed when a radixin-specific antibody was used as well. These results demonstrate that endogenous ERM proteins are similarly relocalized after LPA treatment as the exogenous epitope-tagged form of radixin. Bar, 10 μm.
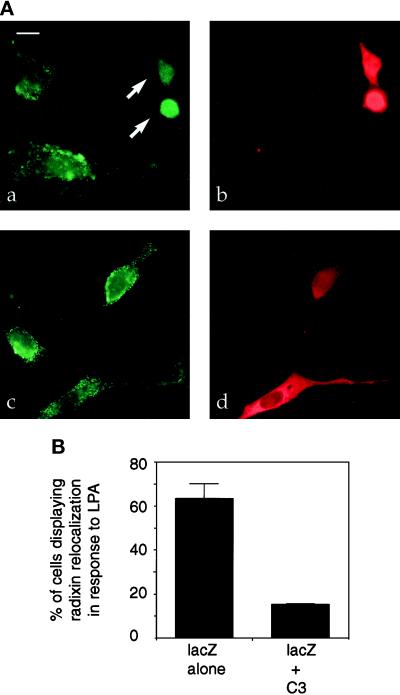
Given that RhoA is a major mediator of LPA-induced actin rearrangement (Ridley and Hall, 1992; Jalink et al., 1994; Chrzanowska-Wodnicka and Burridge, 1996; Kozma et al., 1997), we examined whether inactivation of RhoA by C3 transferase might inhibit the LPA-induced radixin relocalization. C3 transferase catalyzes the ADP ribosylation of RhoA on asparagine 41, which is thought to cause its functional inactivation (Aktories et al., 1989; Sekine et al., 1989). To introduce C3 into the radixin-expressing cell lines, we transiently cotransfected expression plasmids carrying the C3 cDNA and a lacZ cDNA, or the lacZ plasmid with an empty vector into NIH3T3 cells stably expressing HA-radixin. The cells were then serum starved and treated with LPA. Double immunostaining for β-gal and radixin demonstrated that the presence of C3 significantly reduced the localization of radixin in apical structures after LPA treatment; only 15% of the C3-transfected cells showed the relocalization compared with 66% in the lacZ-only control (Figure 2). It should be noted, however, that prolonged C3 treatment leads to cell rounding and loss of adhesion in fibroblasts (Paterson et al., 1990), raising the possibility that the inhibition of radixin relocalization could be a secondary consequence of other changes in cell shape or adhesion. 4,6- Diamidino-2-phenylindole staining of DNA was performed in all these experiments, and examination of the C3-expressing cells revealed that their nuclei are morphologically intact, in contrast to those treated with many other stimuli that induce apoptosis, suggesting that the inhibition of radixin relocalization is not simply a consequence of C3 inducing apoptosis.
Figure 2.
C3 pretreatment blocks LPA-induced relocalization of radixin in NIH3T3 cells. (A) NIH3T3 cells stably expressing HA-radixin were transfected with expression plasmids encoding lacZ and C3 transferase (a and b) or lacZ alone (c and d) and then serum-starved and treated with LPA for 15 min. LacZ (b and d) localization (red) is shown to indicate transfected cells, and HA-radixin localization (green) is shown in panels a and c. C3-transfected cells (arrows in panel a) show a lack of radixin localization into apical protrusions after LPA treatment. Bar, 10 μm. (B) Percentage of cells in lacZ only or lacZ plus C3 transfections displaying relocalization of radixin to apical protrusions after LPA treatment. Results of three independent experiments are shown. One hundred transfected cells were counted for each sample. Errors bars denote SD of the mean.
RhoA Is Sufficient to Relocalize ERM Proteins to Apical Membrane/Actin Protrusions
Given that C3 treatment reduced LPA-induced relocalization of radixin, we next addressed whether activated RhoA might be sufficient for radixin relocalization. Expression plasmids encoding myc epitope-tagged activated (RhoAV14) or dominant negative (RhoAN19) alleles of RhoA were cotransfected with radixin (tagged with the HA epitope) into NIH3T3 cells. The cells were then starved and visualized for RhoA and radixin localization by indirect immunofluorescence using the myc and HA epitope tags, respectively. Radixin alone in serum-starved NIH3T3 cells colocalized with peripheral actin, including a few apical protrusions (Figure 3a). In cells cotransfected with RhoAV14, radixin was highly concentrated in approximately 100–200 apical membrane/actin protrusions in >80% of the transfected cells. These protrusions were morphologically similar to the apical structures induced by LPA treatment (Figure 3, c and d; see also Figure 5b, described below). All cells displaying the radixin relocalization also contained profuse stress fibers, a hallmark feature of activated Rho in fibroblasts (Figure 6d and our unpublished results). Relocalization of endogenous ERM proteins by transfection of RhoAV14 was also observed (our unpublished results). The localization of radixin to apical protrusions was not observed in cells transfected with the dominant-negative RhoAN19 (Figure 3, panels e–f). Importantly, transfection with activated alleles of two other small GTPases in the Rho subfamily, Rac and CDC42, also failed to induce radixin relocalization to apical structures but did alter radixin’s subcellular distribution (Figure 3, panels b, g, and h). Activated CDC42 induced filopodial structures in a large percentage of transfected cells as previously reported (Kozma et al., 1995; Nobes and Hall, 1995), but these projections were larger in length and diameter than the RhoA-induced structures and were always located in the ventral plane of the cell along the substratum, rather than on the apical surface (see Figures 3b and 5c).
Figure 3.
Cotransfection of RhoAV14, but not RhoAN19, RacV12, or CDC42V12, with radixin is sufficient to cause relocalization to apical membrane/actin protrusions in NIH3T3 cells. NIH3T3 cells were transiently transfected with radixin (a and b), radixin and RhoAV14 (c and d), radixin and RhoAN19 (e and f), radixin and RacV12 (g and h), and radixin and CDC42V12 (b). All GTPase constructs were myc epitope tagged. HA-radixin (a, b, c, e, and g) and myc-tagged GTPases (d, f, and h) localizations are shown. Cotransfections followed by immunolocalization were performed in 12 independent experiments. Radixin localization in the presence of activated RhoA was further verified by transfection of radixin into two independent NIH3T3 cell lines stably expressing the RhoAV14 allele. In all experiments, at least 100 transfected cells were examined for each condition, and cells shown are representative of > 80% of the transfected cell population. In the presence of activated RhoA, but not dominant negative RhoA, activated Rac, or activated CDC42, radixin localized into apical membrane protrusions. α-myc and actin staining were used in all experiments to verify functional expression of RhoAV14. Bar, 10 μm.
Figure 6.
Modulation of RhoA-dependent apical protrusions by a radixin carboxyl-terminal domain mutant. R12 cells were transfected with the RADC mutant alone (a and b), or RADC and RhoAV14 (c and d). HA-RADC localization shown in panels a and c, and F-actin as visualized by rhodamine phalloidin are shown in b and d. Note colocalization of RADC with stress fibers in absence of RhoAV14 (arrows in a and b) and localization of actin into profuse stress fibers and long apical processes containing RADC in the presence of RhoAV14 (arrows in c and d). NIH3T3 cells were transfected with RhoAV14 and full-length radixin (e) or RhoAV14 and RADC mutant (f) and then serum-starved and subjected to confocal microscopy analysis. Panels e and f represent combined optical sections of HA-radixin (e) or HA-RADC (f) immunolocalization. NIH3T3 cells were transfected with lacZ and RhoAV14 (g) or lacZ, RhoAV14, and RADC (h) and then serum-starved and subjected to double immunostaining for lacZ and another marker of RhoA-induced apical structures, CD44. CD44 immunolocalization is shown in g and h. LacZ-positive cells (i.e., transfectants) are indicated by arrows. Transfections were performed in four independent experiments. Twenty-five percent of the cells transfected with RADC and RhoAV14 displayed the extremely long apical protrusions as seen in panels c, f, and h, whereas cells transfected with RhoA alone or RhoA plus full-length radixin never showed this phenotype. These results suggest that the presence of a mutant form of radixin can directly alter the size and number of RhoA-induced apical protrusions, but that additional factors (e.g., cell cycle) may control the full expressivity of this phenotype. Bar, 10 μm.
To determine whether the RhoA-induced radixin localization was unique to this protein or was a more general property of the ERM family, we examined the localization of moesin under these conditions. We also tested the effect on the product of the NF2 tumor suppressor gene, merlin, which is a more distantly related member of this family. In addition, we addressed whether this was a unique property of NIH3T3 cells by performing parallel transfections with R12 cells (a Rat1 cell derivative). In both serum-starved NIH3T3 and R12 cells, exogenous moesin and radixin were concentrated in apical actin protrusions when cotransfected with RhoAV14, but not with RhoAN19, RacV12, or CDC42V12, or when transfected without a GTPase (Figure 4). In all experiments, the localization of moesin or radixin was indistinguishable. In contrast, merlin, which localizes very similarly to radixin and moesin when transfected alone in these cells (compare panels a and g of Figure 4), was not present in apical protrusions in the presence of activated RhoA (Figure 4h), suggesting that the redistribution of ERM proteins in these structures is a specific effect. Interestingly, however, merlin became less clearly plasma membrane-associated in cells expressing RhoAV14. Using antiCD44 staining as a marker for the RhoA-induced apical structures (see below), we confirmed that these were still formed in the merlin plus RhoAV14 cotransfectants (our unpublished results). Additionally, experiments using a HA-tagged murine ezrin confirmed that it behaved as moesin and radixin in these experiments (our unpublished results).
Figure 4.
Cotransfection with RhoV14 relocalizes moesin, but not merlin, to apical membrane protrusions in R12 cells. Cells were transiently transfected with moesin (a), moesin and RhoAV14 (b and c), moesin and RhoAN19 (d), moesin and RacV12 (e), moesin and CDC42v12 (f), merlin (g), or merlin and RhoAV14 (h). Moesin and merlin were tagged with HA-epitope tag at their C termini. HA-moesin (a–f) or HA-merlin (g and h) immunolocalization using the 12CA5 mAb is shown. Panels b and c represent two different focal planes of the same cell to better illustrate the nature of the apical protrusions. All cells presented here were also shown to be positive for GTPase expression (where applicable) by α-myc immunostaining. These transfections were performed seven independent times, and at least 100 transfected cells were examined for each condition. Cells shown are representative of >90% of the transfected cell population. Bar, 10 μm.
To characterize the RhoA-induced apical structures more completely, and to clarify the difference between the protrusions induced by CDC42 and RhoA, we performed SEM on NIH3T3 cells transfected with RhoAV14, CDC42V12, or empty vector (see MATERIALS AND METHODS). As shown in Figure 5, SEM revealed that serum-starved cells transfected with empty vector only had a smooth apical surface, while the RhoAV14 transfectants were covered with 100–200 apical membrane protrusions and villus-like blebs. Moreover, the same phenotype, consisting of hundreds of apical projections, was observed in >75% of all cells of an NIH3T3 cell line stably expressing RhoAV14 (our unpublished results). The number and size of the protrusions in RhoAV14-transfected cells observed by SEM correlated extremely closely with the immunostaining of the ERM proteins in the presence of RhoAV14, suggesting that these proteins are localized in the membrane protrusions visualized by this technique. Furthermore, as suggested by indirect immunofluorescence, the CDC42V12 transfectants showed longer, peripherally localized filopodia, and these cells displayed an absence of apical surface structures (Figure 5c).
A Carboxyl Domain of Radixin Modulates the RhoA-dependent Apical Protrusions
Given that activated RhoA induced the localization of ERM proteins to apical protrusions and the previous demonstration that ERM proteins are critical for microvilli formation in polarized epithelial and lymphoma cells (Takeuchi et al., 1994; Crepaldi et al., 1997), we tested whether the ERM proteins may be critical modulators of the membrane protrusions formed in response to Rho activation. We utilized a previously characterized mutant composed of the carboxyl-terminal domain of radixin, denoted RADC, which corresponds roughly to the second half of the protein. This fragment is capable of inducing long apical processes when transfected into HeLa cells and NIH3T3 cells in the presence of full serum (Henry et al., 1995). A similar mutant of ezrin induced analogous processes in other cell types (Martin et al., 1995). This activity of the C-terminal domains was not observed upon transfection of the full-length molecules and was suppressed by the N-terminal domain in trans in some instances, suggesting the protein may normally be found in an inactive intramolecular association (Henry et al., 1995; Martin et al., 1995). This model is further supported by several studies in vitro (Gary and Bretscher, 1995; Magendantz et al., 1995).
First, we investigated whether the RADC polypeptide alone would induce apical membrane protrusions in the absence of serum factors (which activate endogenous Rho and other GTPases) in NIH3T3 or R12 cells. As shown in Figure 6, a and b, this mutant localized to actin stress fibers in these cells, without causing formation of long apical protrusions. However, upon cotransfection of RhoAV14 with RADC, the formation of long apical protrusions was readily observed (Figure 6, c and d). This result demonstrates that activated RhoA is sufficient to reproduce the effect of this mutant in full serum, suggesting that activation of endogenous RhoA by serum may be necessary for the previously reported activity of this mutant. Moreover, we observed that the protrusions induced by RhoA in the presence of this radixin mutant appeared distinct from those normally induced by RhoA. Confocal imaging analysis utilizing combined optical sections revealed a decreased number and increased diameter and length of protrusions induced in the presence of RhoAV14 and RADC, as compared with those caused by RhoAV14 and full-length radixin (Figure 6, e and f).
To examine whether the longer protrusions caused by RhoA and RADC were formed in addition to those induced by endogenous ERM proteins, we utilized immunostaining for CD44, a protein that we have found to be specifically enriched in the Rho-induced apical structures. CD44 has been proposed to be a integral membrane target for the ERM proteins (Tsukita et al., 1994; Hirao et al., 1996). Cells were cotransfected with lacZ, lacZ, and RhoV14, or lacZ, RhoAV14, and RADC, and processed for double immunofluoresence to detect lacZ and CD44. We observed that the fine layer of CD44-rich apical protrusions induced by RhoAV14 alone was disrupted in the presence of the RADC mutant. Instead, fewer, longer CD44-positive processes were present in these cells (Figure 6, panels g and h). These data suggest that by introducing a mutant form of radixin, the character of the RhoA-induced apical membrane protrusions can be altered, both in size and quantity.
Rho Is Necessary and Sufficient for Serine/Threonine Phosphorylation of ERM Proteins
To investigate further whether the ERM proteins are downstream targets of RhoA in the formation of the apical membrane protrusions and to elucidate the mechanism by which this regulation might occur, we examined the phosphorylation of radixin in response to LPA treatment. Parallel plates of NIH3T3 cells stably expressing HA-radixin were metabolically labeled with [35S]methionine or [32P]orthophosphate during serum starvation, and then stimulated with 6 μM LPA. As shown in Figure 7, LPA led to a rapid and prolonged threefold increase in the level of phosphorylation of radixin within one minute after treatment. The increase in radixin phosphorylation clearly preceded relocalization of radixin in these cells (see above, Figure 1A), and the level of phosphorylation remained constant between 1 and 15 min after LPA treatment (Figure 7). There was no increase in the steady-state level of radixin in these experiments, as revealed by 35S-labeled controls (Figure 7). Radixin phosphorylated under these conditions did not react with anti-phosphotyrosine antibodies in Western blots, suggesting that the modification occurred on serine/threonine residues (our unpublished results).
Figure 7.

LPA treatment of NIH3T3 cells induces phosphorylation of radixin within 1 min. Metabolically labeled NIH3T3 cells stably expressing HA-radixin or parental (mock) NIH3T3 cells were serum-starved, then treated with LPA (6 μM) for various timepoints as indicated. Cell lysates were immunoprecipitated with 12CA5 (α-HA) mAb. Radixin is indicated by arrowhead at left. Radixin phosphorylation was increased threefold within 1 min of LPA treatment and remained constant for 15 min. Note background band (*) whose orthophosphate content was not apparently modulated by LPA. Molecular size standards are indicated at right. The data shown are representative of four independent experiments.
To examine whether RhoA activity was necessary and sufficient for ERM phosphorylation, we cotransfected R12 and NIH3T3 cells with HA-tagged radixin or moesin along with C3, RhoV14, or an empty expression vector. The cells were then serum starved and radiolabeled with [32P]orthophosphate, and the transfected ERM proteins were immunoprecipitated and resolved by SDS-PAGE. To ensure that equivalent levels of HA-radixin or moesin were produced and immunoprecipitated under each condition, immunoprecipitates were transferred to PVDF after SDS-PAGE. After exposure of the 32P signal, the blots were probed with anti-HA antisera and the signal was detected by chemiluminescence (Figure 8). In both cell types, coexpression of C3 transferase reduced the level of phosphorylation of both radixin and moesin by at least 50% below the basal level observed in cells transfected with vector alone; protein levels were not affected (Figure 8; our unpublished results). Moreover, cotransfection with RhoAV14 led to strong induction (sevenfold in Rat1 cells; 2.5 fold in NIH3T3 cells) of moesin and radixin phosphorylation, again without affecting protein levels (Figure 8; our unpublished results). These results indicate that RhoA activity is necessary and sufficient to induce phosphorylation of the ERM proteins.
As a first step in addressing the functional consequence of this Rho-dependent phosphorylation, we examined the detergent extractibility of phosphorylated and unphosphorylated moesin. Specifically, we compared the Triton X-100 extractibility of moesin metabolically labeled with either [35S]methionine or [32P]orthophosphate, in the presence or absence of RhoAV14. As shown in Figure 9, in sharp contrast to 35S- labeled moesin, 32P-labeled moesin was found predominantly in the Triton X-100 insoluble fraction. This result is consistent with the phosphorylated form of the protein having a greater affinity for the cytoskeleton. Furthermore, coexpression of RhoAV14 increased the percentage of total (35S-metabolically labeled) moesin found in the insoluble fraction, which presumably reflects the increase in moesin phosphorylation caused by the activated RhoAV14 allele.
Figure 9.

32P-labeled moesin partitions with the Triton X-100 insoluble fraction. NIH3T3 cells were transfected with HA-moesin only or HA-moesin and RhoAV14 as indicated. Cells were then serum starved and metabolically labeled with [32P]orthophosphate or [35S]methionine/cysteine as described in Figure 8, and then subjected to 0.5% Triton X-100 detergent extraction. Soluble (S) and insoluble (I) fractions were immunoprecipitated with 12CA5 antibody. In contrast to total (35S) moesin, 32P-labeled moesin was found almost exclusively in the insoluble fraction. Also, coexpression of RhoAV14 increases the percentage of moesin in the insoluble fraction. The data shown are representative of three independent experiments.
DISCUSSION
Previous studies have demonstrated that RhoA and the ERM proteins colocalize in sites of cell–cell adhesion and in membrane ruffles in epithelial cells (Takaishi et al., 1995). Furthermore, a detailed biochemical analysis has indicated that in cell lysates, RhoA modulates the affinity of the ERM proteins with a membrane fraction and specifically its association with the integral membrane protein CD44 (Hirao et al., 1996). However, the mechanism of action or physiological consequence of the connection between RhoA and the ERM proteins has remained unclear. Here, we have more closely examined the relationship between the ERM family of membrane/cytoskeletal linkers and the RhoA GTPase. We found that in NIH3T3 cells, treatment with LPA induced the rapid phosphorylation and subsequent dramatic relocalization of radixin into peripheral and apical membrane protrusions. Notably, LPA-induced relocalization of the ERM proteins was blocked by C3 transferase. Furthermore, we have shown that RhoA activity is both sufficient and necessary for ERM protein phosphorylation. Under these conditions, RhoA activity was also sufficient for the relocalization of radixin and moesin into apical membrane protrusions in two different cell types. These data suggest that growth factors may mediate the localization of the ERM proteins via a RhoA-dependent kinase, such as protein kinase N, Rho-kinase, or PRK2 (Amano et al., 1996; Leung et al., 1996; Watanabe et al., 1996; Amano et al., 1997; Vincent and Settleman, 1997). One or more of these kinases may serve either alone or in concert with other RhoA-dependent signals to cause ERM proteins to alter their cytoskeletal localization into the microvilli-like protrusions. Furthermore, the fact that a mutant form of radixin was able to alter the number and size of the Rho-dependent protrusions may indicate the ERM proteins are critical components and/or regulators of these structures. Interestingly, we have found that phosphorylation of the related merlin protein is regulated by stimuli known to activate Rho (Shaw et al., submitted), although the localization of this protein in the presence of activated Rho is clearly distinct from that of the ERM proteins. This difference may be explained by the presence of an actin-binding motif in the ERM proteins that is not well conserved in merlin.
The ERM proteins have previously been demonstrated to be essential components of microvillar structures on polarized epithelial as well as lymphocyte cell surfaces (Takeuchi et al., 1994; Crepaldi et al., 1997), and the concentration of these proteins into microvilli-like structures in a diverse array of cell types in response to growth factor activation or viral infection is firmly established (Gould et al., 1986; Bretscher, 1989; Pakkanen and Vaheri, 1989; Hanzel et al., 1991; Dransfield et al., 1997). Our results may implicate RhoA in the growth factor-induced relocalization of ERM proteins into apical structures such as microvilli. In this manner, the ERM proteins may serve as regulatable scaffolding proteins, localizing and perhaps clustering adhesion receptors such as CD44, ICAM-2, and ICAM-3 with signaling enzymes such as protein kinase A and c-yes, which have recently been reported to associate with ERM proteins (Crepaldi et al., 1997; Dransfield et al., 1997), to important sites of action.
Ezrin is known to be serine/threonine phosphorylated concomitant with its localization into microvilli in response to growth factors in a variety of cell types, including gastric parietal cells where it may participate in histamine-mediated secretion (Urishidani et al., 1989; Hanzel et al., 1991). Indeed, it has been argued that phosphorylation may serve as the physiological activator to release the intramolecular association of the inactive ERM proteins, allowing them to bind to both their membrane target and actin (Tsukita et al., 1997). Furthermore, there is a strong correlation between ezrin serine/threonine phosphorylation and its association with the cytoskeleton in the kidney epithelium (Chen et al., 1995). We have demonstrated that phosphorylation of moesin correlates with its association with the Triton X-100 insoluble fraction in vivo, and consistent with this, activated RhoA increases the percentage of total moesin that is detergent insoluble. Therefore, our results provide a connection between a modulator of growth factor signaling (RhoA) and the downstream effects on ERM localization and phosphorylation, again suggesting the two may be functionally linked.
Additional pathways downstream of RhoA may also be involved in the relocalization of ERM proteins. RhoA is known to activate a PI5 kinase activity in fibroblasts leading to increased phosphotidylinositol 4,5-bisphosphate (PIP2) production (Chong et al., 1994; Ren et al., 1996). It has been recently demonstrated that the ERM proteins have a PIP2 binding site (Niggli et al., 1995), and the ability of full-length ERM proteins to interact with CD44 in vitro is dependent on the presence of PIP2 (Hirao et al., 1996). It is possible that synergy between PIP2 binding and RhoA-dependent phosphorylation fully activates the ERM proteins, or perhaps one of the two stimuli serve as a molecular targeting signal for apical protrusions. Indeed, it has recently been shown that pleckstrin can induce membrane protrusions in COS cells, in a manner dependent on its ability to bind PIP2 via one of its PH domains (Ma et al., 1997). Moreover, phosphorylation was demonstrated to regulate the membrane association and protrusion-inducing activity of the PH domain of pleckstrin. Perhaps the ERM proteins are regulated in a similar manner, whereby serine/threonine phosphorylation downstream of Rho activation causes a conformation change unmasking the PIP2 binding site in the amino terminus. Clearly, the identification of the sites of RhoA-dependent phosphorylation is necessary to further explore these possibilities.
Several studies have indicated that the ERM proteins may be regulated by a head-to-tail intramolecular association, which serves to mask the affinity of both the N- and C-termini from their respective targets (Gary and Bretscher, 1995). It has been proposed that upon disruption of the intramolecular association, the ERM proteins can form homodimers, heterodimers, and oligomers that may be critical for their role in microvilli formation in the placenta (Berryman et al., 1995). It would be very interesting to determine whether such oligomeric species were induced in a RhoA-dependent manner in the fibroblasts employed here. In fact, the mechanism by which the carboxyl terminal fragment of radixin alters the size of the Rho-A-induced apical membrane structures may be by altering the lattice of oligomers, which could contribute directly to the size of the protrusions. Interestingly, vinculin, another molecule whose localization is regulated by RhoA, is also thought to be controlled by a similar head-to-tail intramolecular association (Johnson and Craig, 1995; Gilmore and Burridge, 1996). It has been suggested that a combination of phosphorylation and PIP2 binding may unmask vinculin from its inactive conformation (Gilmore and Burridge, 1996; Jockusch and Rudinger, 1996). Indeed, the effects of RhoA on the cytoskeleton may be mediated through a common mechanism of phosphorylation and PIP2 binding leading to the enhanced activity of a diverse group of cytoskeletal effectors, including pleckstrin, vinculin, and the ERM proteins.
Recently, two reports have further suggested connections between Rho and the ERM proteins. Mackay and colleagues (1997) utilized a biochemical screen for proteins necessary for RhoA- and Rac-induced actin rearrangement and identified the ERM proteins, although the mechanism by which the ERM proteins act in this permeabilized cell system is unclear at present. Conversely, Takai and colleagues have suggested that Rho may lie upstream of ERM proteins in a biochemical pathway, as the amino terminus of the ERM proteins have been shown to bind and negatively regulate Rho-GDI, a protein that keeps Rho in its inactive, GDP-bound state (Takahashi et al., 1997). While the precise nature of ERM modulation of Rho-dependent pathways remains to be determined, our results strongly suggest that the ERM proteins can serve as downstream effectors of RhoA in its modulation of the apical actin cytoskeleton in fibroblasts. Perhaps the ERM proteins also act to localize Rho via RhoGDI to a subset of its potential effectors at these specialized apical membrane cytoskeleton sites.
Taken altogether, these results suggest a novel function of RhoA in the induction of apical membrane/actin protrusions and introduce the possibility that through RhoA-dependent phosphorylation, the ERM proteins may be critical regulators and components of these structures. The ability of ERM proteins to target specific adhesion molecules to unique cytoskeletal regions such as uropods in T lymphocytes ( Helander et al., 1996; Serrador et al., 1997) or differentiated microvilli in epithelial cells may be similarly induced by a pathway downstream of RhoA or a related GTPase.
ACKNOWLEDGMENTS
The authors thank Karen Cichowski for many helpful suggestions; Andi McClatchey, John Chant, Lisa Stowers, Rick Cerione, John Erickson, Sheila Thomas, Jeff Settleman, and Richard Hynes for helpful discussions during the course of this study and/or for comments on the manuscript; Sachiro Tsukita for the kind gift of CR22 polyclonal antibody; Ed Seling at Harvard University Museum of Comparative Zoology for helping with the SEM; and Richard Triesmann, Marc Symons, and John Chant for kindly supplying reagents.
REFERENCES
- Aktories K, Braun U, Rosener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho- kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Amieva M, Furthmayr H. Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment, and cell-cell contacts. Exp Cell Res. 1995;219:180–196. doi: 10.1006/excr.1995.1218. [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Berryman M, Gary R, Bretscher A. Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol. 1995;131:1231–1242. doi: 10.1083/jcb.131.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of isolated microvillus cytoskelton, and its localization in non-muscle cells. J Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- Chen J, Cohn JA, Mandel LJ. Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc Natl Acad Sci USA. 1995;92:7495–7499. doi: 10.1073/pnas.92.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4- phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di Fiore PP. The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene. 1993;8:1335–1345. [PubMed] [Google Scholar]
- Franck Z, Gary R, Bretscher A. Moesin, like ezrin, colocalizes with actin in the cortical cytoskeleton in cultured cells, but its expression is more variable. J Cell Sci. 1993;105:219–231. doi: 10.1242/jcs.105.1.219. [DOI] [PubMed] [Google Scholar]
- Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl- inositol-4–5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Gould KL, Cooper JA, Bretscher A, Hunter T. The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J Cell Biol. 1986;102:660–669. doi: 10.1083/jcb.102.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli [published erratum appears in EMBO J 1991 Dec, 10(12), 3978–81] EMBO J. 1991;10:2363–73. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander TS, Carpen O, Turenen O, Kovanen P, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as target for killer cells. Nature. 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- Henry MD, Gonzalez Agosti C, Solomon F. Molecular dissection of radixin: distinct and interdependent functions of the amino- and carboxy-terminal domains. J Cell Biol. 1995;129:1007–1022. doi: 10.1083/jcb.129.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Rudinger M. Crosstalk between cell adhesion molecules: vinculin as a paradigm for regulation by conformation. Trends Cell Biol. 1996;6:311–315. doi: 10.1016/0962-8924(96)10022-2. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains [see comments] Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AD, Brass LF, Abrams CS. Pleckstrin associates with plasma membranes and induces the formation of membrane projections: requirements for phosphorylation and the NH2-terminal PH domain. J Cell Biol. 1997;136:1071–1079. doi: 10.1083/jcb.136.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Esch F, Furthmayr H, Hall A. Rho- and Rac- dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magendantz M, Henry MD, Lander A, Solomon F. Interdomain interactions of radixin in vitro. J Biol Chem. 1995;270:25324–25327. doi: 10.1074/jbc.270.43.25324. [DOI] [PubMed] [Google Scholar]
- Martin M, Andreoli C, Sahuquet A, Montcourrier P, Algrain M, Mangeat P. Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J Cell Biol. 1995;128:1081–1093. doi: 10.1083/jcb.128.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–1265. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270:31377–31385. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- Niggli V, Andreoli C, Roy C, Mangeat P. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 1995;376:172–176. doi: 10.1016/0014-5793(95)01270-1. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Pakkanen R, Vaheri A. Cytovillin and other microvillar proteins of human choriocarcinoma cells. J Cell Biochem. 1989;41:1–12. doi: 10.1002/jcb.240410102. [DOI] [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestonjamasp K, Amieva MR, Strassel CP, Nauseef WM, Furthmayr H, Luna EJ. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol Biol Cell. 1995;6:247–259. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Bokoch GM, Traynor-Kaplan A, Jenkins GH, Anderson RA, Schwartz MA. Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol Biol Cell. 1996;7:435–442. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, Pulst S, Lenior G, Bijlsma E, Fashold R, Dumanski J, deJong P, Parry D, Eldrige R, Aurias A, Delattre O, Thomas G. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Sekine A, Fujiwara M, Narumiya S. Asparigine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sanchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409–1423. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Tsukita S, Takai Y. Direct interaction of the RhoGDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, Haase VH, Ambrose CM, Munroe D, Bove C, Haines JL, Martuza R, McDonald ME, Seizinger BR, Short MP, Buckler AJ, Gusella JF. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S, Tsukita S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–8. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urishidani T, Hanzel DK, Forte JG. Characterization of an 80 kD phosphoprotein involved in parietal cell stimulation. Am J Physiol. 1989;256:G1070–G1081. doi: 10.1152/ajpgi.1989.256.6.G1070. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- Vincent S, Settleman J. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol Cell Biol. 1997;17:2247–2256. doi: 10.1128/mcb.17.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Winckler B, Gonzalez Agosti C, Magendantz M, Solomon F. Analysis of a cortical cytoskeletal structure: a role for ezrin-radixin-moesin (ERM proteins) in the marginal band of chicken erythrocytes. J Cell Sci. 1994;107:2523–2534. doi: 10.1242/jcs.107.9.2523. [DOI] [PubMed] [Google Scholar]