Abstract
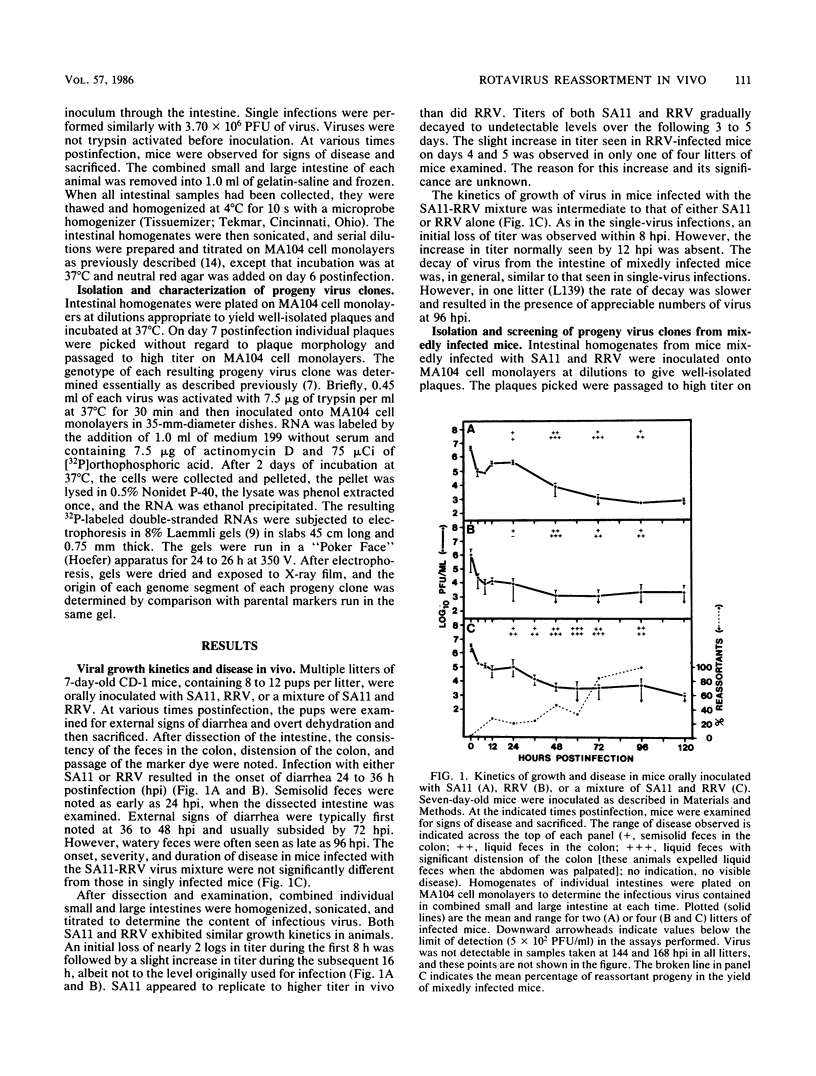
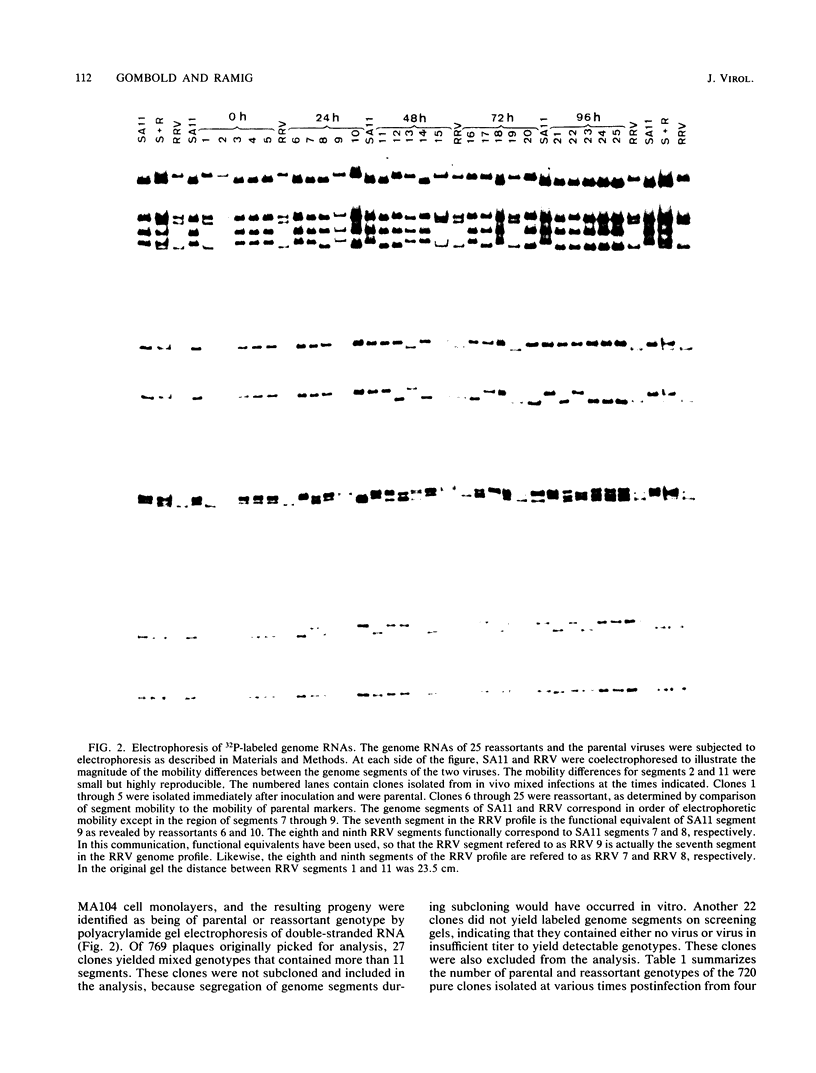
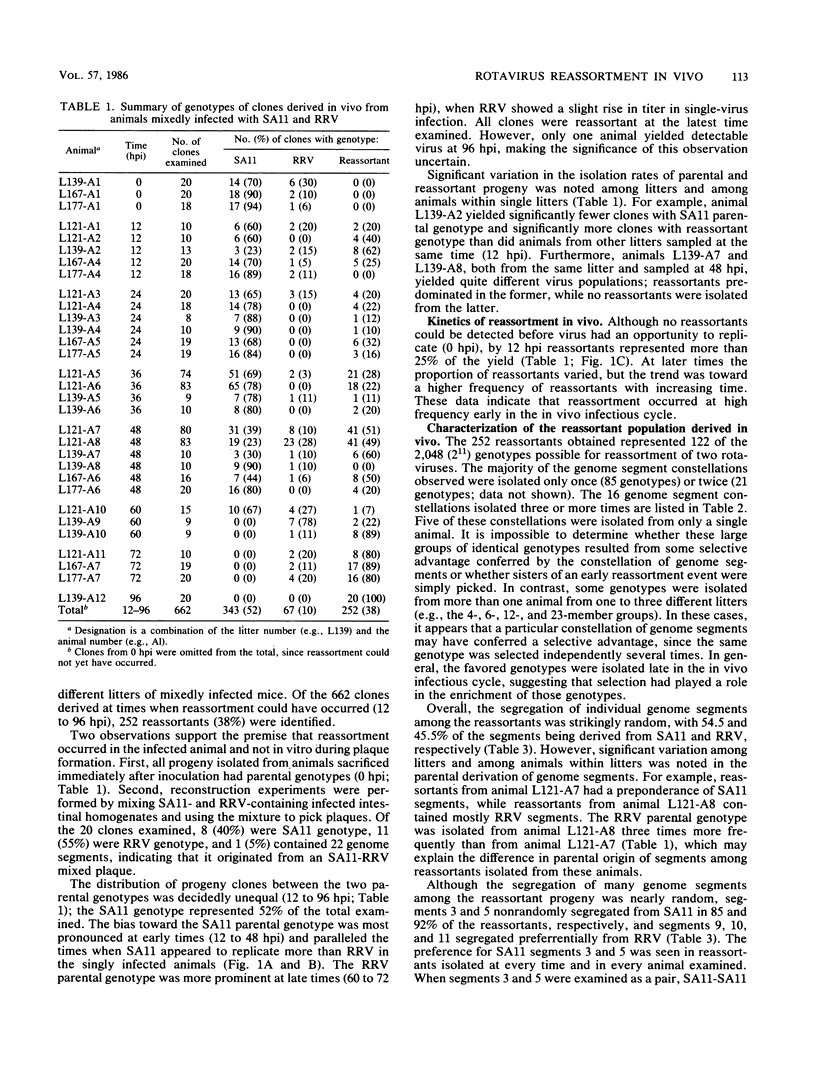
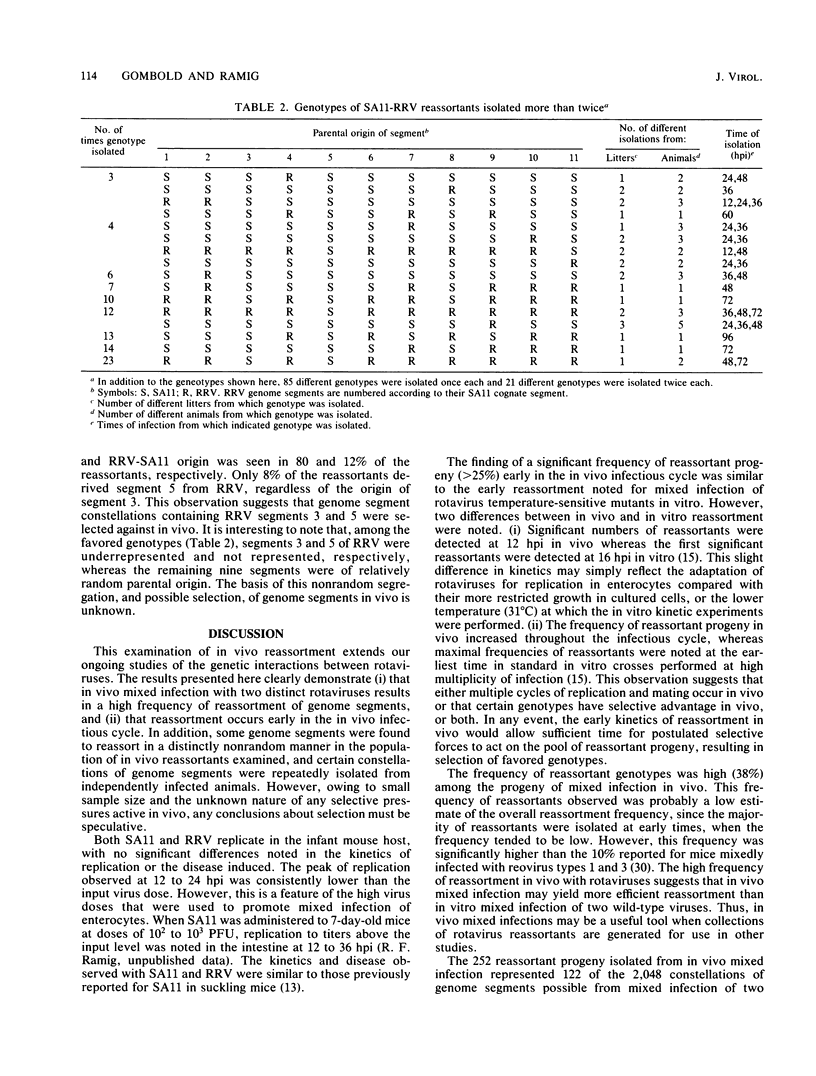
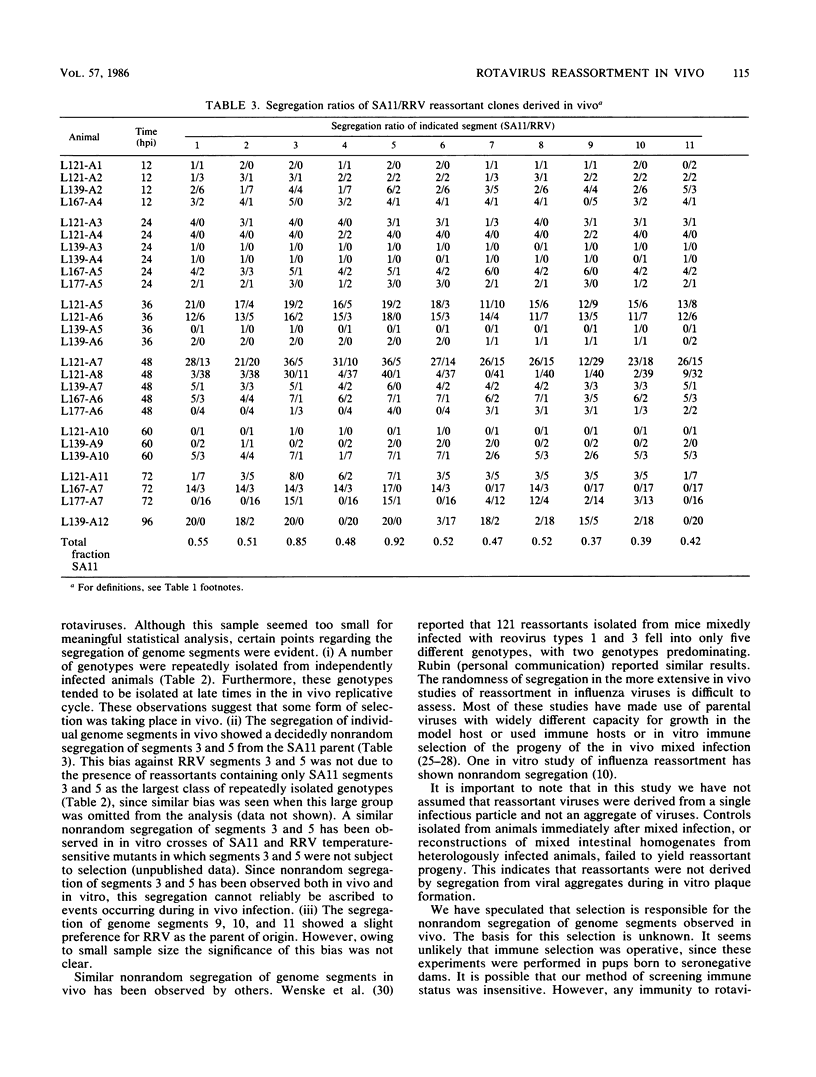
Seven-day-old CD-1 mice born to seronegative dams were orally inoculated with a mixture of wild-type simian rotavirus SA11 and wild-type rhesus rotavirus RRV. At various times postinfection, progeny clones were randomly isolated from intestinal homogenates by limiting dilution. Analysis of genome RNAs by polyacrylamide gel electrophoresis was used to identify and genotype reassortant progeny. Reassortment of genome segments was observed in 252 of 662 (38%) clones analyzed from in vivo mixed infections. Kinetic studies indicated that reassortment was an early event in the in vivo infectious cycle; more than 25% of the progeny clones were reassortant by 12 h postinfection. The frequency of reassortant progeny increased to 80 to 100% by 72 to 96 h postinfection. A few reassortants with specific constellations of SA11 and RRV genome segments were repeatedly isolated from different litters or different animals within single litters, suggesting that these genotypes were independently and specifically selected in vivo. Analysis of segregation of individual genome segments among the 252 reassortant progeny revealed that, although most segments segregated randomly, segments 3 and 5 nonrandomly segregated from the SA11 parent. The possible selective pressures active during in vivo reassortment of rotavirus genome segments are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H. Genetic potential of bunyaviruses. Curr Top Microbiol Immunol. 1979;86:1–33. doi: 10.1007/978-3-642-67341-2_1. [DOI] [PubMed] [Google Scholar]
- Chanock S. J., Wenske E. A., Fields B. N. Human rotaviruses and genome RNA. J Infect Dis. 1983 Jul;148(1):49–50. doi: 10.1093/infdis/148.1.49. [DOI] [PubMed] [Google Scholar]
- Desselberger U., Nakajima K., Alfino P., Pedersen F. S., Haseltine W. A., Hannoun C., Palese P. Biochemical evidence that "new" influenza virus strains in nature may arise by recombination (reassortment). Proc Natl Acad Sci U S A. 1978 Jul;75(7):3341–3345. doi: 10.1073/pnas.75.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Desselberger U. Cocirculation of different rotavirus strains in a local outbreak of infantile gastroenteritis: monitoring by rapid and sensitive nucleic acid analysis. J Med Virol. 1983;11(1):39–52. doi: 10.1002/jmv.1890110106. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Estes M. K., Ramig R. F. Assignment of simian rotavirus SA11 temperature-sensitive mutant groups B and E to genome segments. Virology. 1985 May;143(1):309–320. doi: 10.1016/0042-6822(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Palese P., Schulman J. L. Nonrandom association of parental genes in influenza A virus recombinants. Virology. 1979 May;95(1):269–274. doi: 10.1016/0042-6822(79)90430-6. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Kalica A. R., Kono R. Isolation of a recombinant between simian and bovine rotaviruses. J Gen Virol. 1980 May;48(1):253–256. doi: 10.1099/0022-1317-48-1-253. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Kornstein M. J., Plotkin S. A. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984 Jul;51(1):233–236. doi: 10.1128/jvi.51.1.233-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F. Factors that affect genetic interaction during mixed infection with temperature-sensitive mutants of simian rotavirus SA11. Virology. 1983 May;127(1):91–99. doi: 10.1016/0042-6822(83)90374-4. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants of simian rotavirus SA11. Virology. 1982 Jul 15;120(1):93–105. doi: 10.1016/0042-6822(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Deregt D., Babiuk L. A., Misra V. Genetic heterogeneity within individual bovine rotavirus isolates. J Virol. 1982 Dec;44(3):813–822. doi: 10.1128/jvi.44.3.813-822.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C. Influenza virus genetics. Adv Genet. 1979;20:1–36. doi: 10.1016/s0065-2660(08)60544-1. [DOI] [PubMed] [Google Scholar]
- Spencer E. G., Avendaño L. F., García B. I. Analysis of human rotavirus mixed electropherotypes. Infect Immun. 1983 Feb;39(2):569–574. doi: 10.1128/iai.39.2.569-574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K., Bishop D. H., Roy P. Analyses of the genomes of bluetongue viruses recovered in the United States. I. Oligonucleotide fingerprint studies that indicate the existence of naturally occurring reassortant BTV isolates. Virology. 1981 Oct 15;114(1):210–217. doi: 10.1016/0042-6822(81)90266-x. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Clerx-Van Haaster C. M., Bishop D. H. Analyses of Patois group bunyaviruses: evidence for naturally occurring recombinant bunyaviruses and existence of immune precipitable and nonprecipitable nonvirion proteins induced in bunyavirus-infected cells. Virology. 1981 Apr 30;110(2):318–332. [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., D'Hondt E., André F. E., Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983 Oct 8;2(8354):807–811. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H., Granoff A. The "in vivo" production of "new" influenza A viruses. I. Genetic recombination between avian and mammalian influenza viruses. Virology. 1971 May;44(2):317–328. doi: 10.1016/0042-6822(71)90263-7. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H., Granoff A. The "in vivo" production of "new" influenza viruses. 3. Isolation of recombinant influenza viruses under simulated conditions of natural transmission. Virology. 1973 Jan;51(1):149–162. doi: 10.1016/0042-6822(73)90375-9. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H. Studies on the origin of pandemic influenza. IV. Selection and transmission of "new" influenza viruses in vivo. Virology. 1974 Dec;62(2):404–413. doi: 10.1016/0042-6822(74)90402-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H. The in vivo production of "new" influenza A viruses. II. In vivo isolation of "new" viruses. Virology. 1972 May;48(2):528–536. doi: 10.1016/0042-6822(72)90063-3. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Chanock S. J., Krata L., Fields B. N. Genetic reassortment of mammalian reoviruses in mice. J Virol. 1985 Nov;56(2):613–616. doi: 10.1128/jvi.56.2.613-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]