Abstract
The purpose of this study was to compare the effectiveness of intramuscular and intranasal midazolam used as a premedication before intravenous conscious sedation. Twenty-three children who were scheduled to receive dental treatment under intravenous sedation participated. The patients ranged in age from 2 to 9 years (mean age, 5.13 years) and were randomly assigned to receive a dose of 0.2 mg/kg of midazolam premedication via either intramuscular or intranasal administration. All patients received 50% nitrous oxide and 50% oxygen inhalation sedation and local anesthetic (0.2 mL of 4% prilocaine hydrochloride) before venipuncture. The sedation level, movement, and crying were evaluated at the following time points: 10 minutes after drug administration and at the times of parental separation, passive papoose board restraint, nitrous oxide nasal hood placement, local anesthetic administration, and initial venipuncture attempt. Mean ratings for the behavioral parameters of sedation level, degree of movement, and degree of crying were consistently higher but not significant in the intramuscular midazolam group at all 6 assessment points. Intramuscular midazolam was found to be statistically more effective in providing a better sedation level and less movement at the time of venipuncture than intranasal administration. Our findings indicate a tendency for intramuscular midazolam to be more effective as a premedication before intravenous sedation.
Keywords: Midazolam, Dentistry, Sedation, Pediatrics, Intramuscular, Intranasal
The induction of intravenous conscious sedation in pediatric patients undergoing extensive dental treatment may be a challenge, particularly during parental separation and venipuncture. The use of sedative premedication may help reduce the anxiety and minimize psychological trauma in these patients. Midazolam (Versed, Hoffman-La Roche Inc, Nutley, NJ) is an example of such a preoperative sedative agent. As a water-soluble benzodiazepine, midazolam is nonirritating and has anxiolytic, sedative, hypnotic, and amnesic properties. Midazolam has been used as a preoperative sedative agent via the intramuscular,1,2 intranasal,3,4 oral,5 and rectal6 routes. A relatively slower onset of action is a disadvantage of both the oral and rectal routes of administration. Tolksdorf and Eick7 found the oral route to be less predictable than the other routes studied, with patients experiencing nausea and vomiting postoperatively. Wilton et al4 noted that oral midazolam also prolongs the recovery time when compared with other routes. Lejus et al8 pointed out that although rectal midazolam is an effective premedication, a drawback relates to modesty issues associated with administering the drug rectally to older children.
Intramuscular sedation is popular due to its ease of administration, rapid onset of action, better absorption, and greater predictability of the length of the latent period and duration of action. According to Malamed,9 intramuscular midazolam is an effective preoperative sedative in children and patients with disabilities. However, one disadvantage of this route relates to the fear of injections, which can be associated with pain.
Intranasal administration of preinductive agents such as midazolam and sufentanil has been previously investigated. Karl et al10 concluded that intranasal midazolam and sufentanil are both effective preinduction sedatives, but midazolam is preferable to sufentanil for most pediatric patients because it possesses a lesser degree of respiratory depression (98% of the patients had peripheral oxygen saturation greater than 95%). Lejus et al8 reported that intranasal midazolam is an effective and rapid route of premedication, yet one that is poorly accepted by patients.
The different routes of administration of midazolam as a sedative premedication have been previously investigated, and its use intranasally has been compared with the oral, sublingual, and rectal routes.8,11,12 However, only one study by de Santos et al13 has compared the intranasal and intramuscular routes of administration of midazolam. Patients received 0.2 mg/kg of midazolam and 0.015 mg/kg of atropine 30–40 minutes before surgery by either the intramuscular or intranasal route. These authors concluded that there were no significant differences in the onset of sedation, degree of sedation, and response to venipuncture when either intramuscular or intranasal midazolam was administered.13 The purpose of the present study was to compare the intramuscular versus intranasal routes of midazolam administration as a premedication and to determine the effectiveness of each route in induction of intravenous sedation.
Methods
Approval of the University of Southern California Institutional Review Board was obtained. All proposed procedures were explained and informed consent was obtained from the parents before inclusion of participants in the study.
Study Sample
Twenty-three healthy, American Society of Anesthesiologists' classification I (ASA I) pediatric patients between the ages of 2 and 9 years participated in the pilot study. The study sample consisted of 7 girls and 15 boys. The mean patient age was 5.13 years (range, 2–9 years). The mean weight was 21.74 kg (range, 12–30 kg). Patients were conveniently selected from a patient pool within the University of Southern California Pediatric Dentistry Department scheduled for restorative dental treatment under intravenous sedation. Patients were scheduled for intravenous sedation if they met the following criteria during the initial examination appointment: extremely apprehensive or uncooperative and unlikely to tolerate treatment in the dental clinic with or without nitrous oxide conscious sedation, healthy (ASA I), severe caries that involved 2 or more quadrants of the mouth, likely to require 2 or more oral conscious sedation visits, and parental consent to have children receive intravenous sedation.
Study Procedure
All patients were required to have nothing by mouth after midnight the night before the appointment, and parents were instructed to give only clear liquids and no milk up to 4–6 hours before the appointment. Patients were weighed and chests were auscultated for possible respiratory congestion before sedative drug induction. The sedation was cancelled if the patient had a cold or flu, had an upper respiratory tract infection, or violated the nothing by mouth restrictions.
Each patient was randomly assigned to have intramuscular or intranasal administration of 0.2 mg/kg of midazolam, as recommended by previous investigators.7,8 Of the 23 patients, 12 received intramuscular midazolam, and 11 received intranasal midazolam. The mean dose was 4.35 mg (range, 2.4–6 mg). For the intramuscular route, the drug was injected into the vastus lateralis muscle of either leg. For the intranasal route, the child was placed on the parent's lap and given drops of midazolam from a needleless syringe into the nose.
Following drug administration, the child remained with the parent in a quiet area away from the treatment room for 15 minutes. The patient was then separated from the parent and taken into the treatment room. The patient was placed in the dental chair in a supine position and secured in a papoose board. A nasal hood was then placed over the nose and a mixture of 50% nitrous oxide and 50% oxygen was administered. The dental anesthesiologist then examined the antecubital fossa of each arm for an adequate vein. Once a suitable vein was found, a tourniquet was placed superior to the venipuncture site. The antecubital fossa area was then cleansed with an alcohol swab and a small volume of 4% prilocaine hydrochloride (0.2 mL or 8 mg) was then injected subcutaneously at the venipuncture site to anesthetize the skin. Venipuncture was then accomplished at the anesthetized site with a 22-gauge indwelling catheter. Heart rate, respiratory rate, blood pressure, and oxygen saturation were monitored continuously following intravenous drug administration.
For consistency, the dental anesthesiologist (S.F.M.) administered the sedative drug, injected the local anesthetic, and performed the venipuncture in all study participants. Pediatric dental residents performed all subsequent dental treatment for the patients in conjunction with the administration of intravenous conscious sedation. All participants were videotaped throughout the experimental period, beginning 5 minutes after administration of the midazolam premedication and concluding after venipuncture.
Behavior Assessment
Previous studies have reported the occurrence of behavior changes 10–15 minutes after drug administration involving both the intramuscular14 and intranasal4 routes. In the current study, changes in behavior were evaluated based on the modified Houpt et al rating scale.15 The behavioral parameters of sedation level, movement, and crying were assessed at each of the following 6 time points: 10 minutes after drug administration, parental separation, securing the patient inside a papoose board, placement of the nasal hood for nitrous oxide and oxygen administration, injection of local anesthetic at the site of venipuncture, and initial venipuncture attempt. An overall global rating of each patient's behavior was also determined at the conclusion of the venipuncture.
Two evaluators independently assessed the behavior parameters as noted herein from the videotapes. The evaluators had no prior knowledge of which premedication route had been used. Before actual evaluation, the 2 evaluators were calibrated using the modified Houpt et al rating scale.
Statistical Analysis
The independent variable in the study was the choice of drug route (intramuscular or intranasal). The dependent variables in assessment of the effectiveness of each route were the sedation level, degree of movement, and degree of crying. Because ordinal data were obtained with the rating scale, the nonparametric Mann-Whitney U test at the 95% significance level was used to compare the effectiveness of the 2 routes of midazolam administration. Interobserver reliability was assessed using the Spearman's rank correlation coefficient.
Results
Interobserver Reliability
Interobserver reliability for the 2 evaluators was generally very high (rs = 0.9991). The Spearman's rank correlation coefficients were also very high (rs = 0.9995) for specific ratings of sedation, movement, and crying.
Evaluation of Level of Sedation
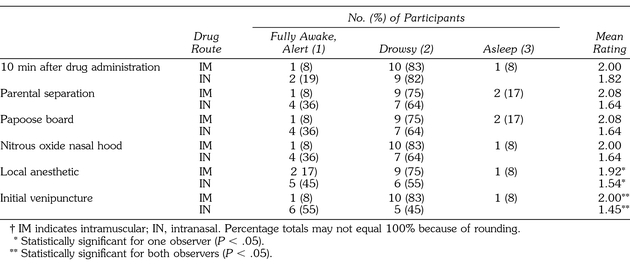
Table 1 provides a summary of sedation level ratings at each of the assessment time points. The Mann-Whitney U test indicated a statistical difference in sedation level at the time of local anesthetic administration by observer B, z = −1.976 (P < .048), and at the time of venipuncture by both observers, z = −2.435 (P < .015). Patients who were given intramuscular midazolam were more deeply sedated at the time of local anesthetic administration (observer B) and at the time of venipuncture (both observers) than those receiving intranasal midazolam. Eleven (92%) of the 12 patients who received intramuscular midazolam were drowsy or asleep (defined as the presence of eye closure and lack of visible movement, although easily awakened with verbal or physical stimulus) at 10 minutes after drug administration, parental separation, papoose board restraint, nitrous oxide nasal hood placement, and venipuncture attempt. Interestingly, none of the patients from the intranasal group were rated as being asleep, and more participants were fully awake and alert compared with those of the intramuscular group. Table 1 also indicates that 9 (82%) of the 11 patients who received intranasal midazolam were drowsy at the 10 minutes after drug administration point and 2 (18%) were fully awake and alert. However, the number of drowsy sedation participants decreased to 7 (64%) and the fully awake and alert participants increased to 4 (36%) at parental separation, papoose board restraint, and nitrous oxide nasal hood placement periods. By the time of local anesthetic administration and venipuncture attempt, the number of intranasal midazolam recipients who remained drowsy decreased to 6 (55%) and 5 (45%), respectively. The number of fully awake intranasal midazolam recipients also increased at these 2 assessment times to 5 (45%) and 6 (54%) at local anesthetic administration and venipuncture, respectively.
Table 1.
Summary of Sedation Level†
Evaluation of Movement
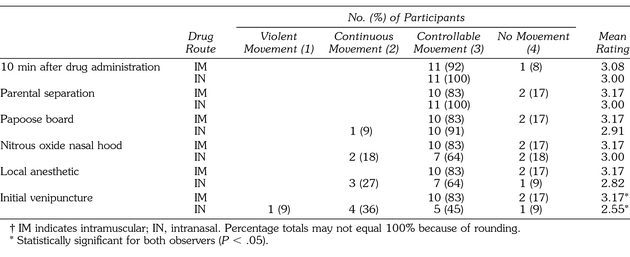
Table 2 provides a summary of movement ratings at each of the assessment time points. A statistically significant difference was found between patients receiving intramuscular versus intranasal midazolam for movement at the time of venipuncture, z = −2.181 (P < .029). Patients who received intramuscular injections displayed less (intermittent or no) movement compared with those receiving intranasal administration. All 12 patients from the intramuscular midazolam group displayed controllable to no movement. The 11 patients who received intranasal midazolam displayed controllable movement at the 10 minutes after drug administration and parental separation periods. However, this number decreased to 5 (45%) of 11 patients by the time of venipuncture assessment, with more patients judged to have continuous to violent movements at later assessment periods.
Table 2.
Summary of Degree of Movement†
Evaluation of Crying
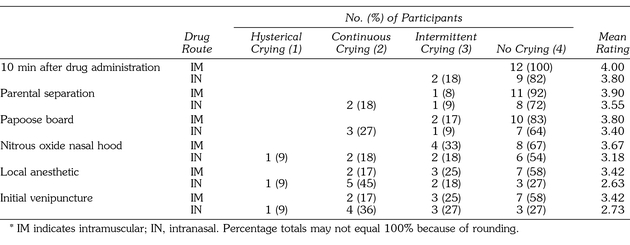
Table 3 displays a summary of the incidence of crying at each of the assessment time points. There was no statistical difference for crying between children receiving intramuscular versus intranasal midazolam. All 12 patients who received intramuscular midazolam displayed intermittent to no crying until local anesthetic administration and venipuncture, when 2 (16.7%) of the 12 participants cried continuously. A similar trend was seen with the 11 patients who received intranasal midazolam; however, twice as many patients, 4 (36.5%) of 11, demonstrated continuous and hysterical crying by the time of local anesthetic and venipuncture placements.
Table 3.
Summary of Degree of Crying*
Global Evaluation
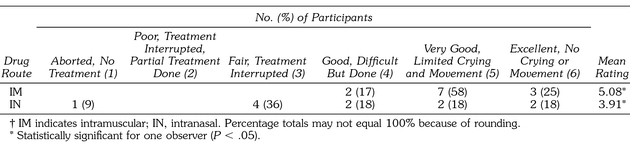
Table 4 summarizes the global evaluation assessments of both observers. Observer A found the intramuscular route to be significantly more effective than the intranasal route, z = −2.052 (P < .04). Observer B, however, did not find a statistically significant difference between the 2 groups, although z = −1.911 (P ≤ .056) approached statistical significance. All 12 of the patients from the intramuscular midazolam group were rated as achieving good to excellent sedation compared with 6 (64%) of those from the intranasal group. No statistical differences were found related to the patients' sex, age, or weight.
Table 4.
Summary of Global Rating of Overall Effectiveness†
Discussion
Unlike the previous study by de Santos et al,13 the present study found a tendency for intramuscular midazolam to be a more effective premedication than when given via the intranasal route. This tendency was found to generally become significant as the time following administration and the amount of stimulation to the patient both increased. When assessing the level of sedation, the difference between the 2 routes of administration reached significance for one examiner at the time of local anesthetic administration and for both examiners during venipuncture. Both examiners also noted significantly more movement at the time of venipuncture for patients receiving intranasal midazolam. The global rating of one examiner also indicated that the intramuscular route produced significantly better premedication.
Deterioration of sedation over time and with increased stimulation for patients receiving intranasal midazolam may have been affected by the method of drug administration and the amount of drug absorption. Unlike intramuscular administration, where a rapid and simple injection into the muscle tissue can be attained, too rapid an administration via the intranasal route could result in loss of the premedication into the oral cavity or incidental extrusion back out the nasal passage. The result is less drug absorption into the nasal mucosa and, therefore, a lower blood level of the drug and a decrease in sedation with time progression and increased stimulation.
Nitrous oxide and oxygen inhalation sedation was used in all study participants because of its routine use during intravenous sedation by the dental anesthesiologist. A uniform concentration of 50% nitrous oxide and 50% oxygen was used for each patient to provide constancy in concentration and consistency in treatment. However, its effectiveness was most likely diminished in more of the intranasal midazolam patients due to continuous and hysterical crying.
It was noted from review of the videotapes that tourniquet placement before local anesthetic administration may have acted as a stimulus to the patient when left on for too long. This may have affected the behavioral changes, particularly at the local anesthetic assessment time. Because it was applied to patients in both groups, this variable did not act to bias the results of the present study. Tourniquet placement should be used as another assessment point in future studies to determine whether it acts as a significant stimulus.
The dental anesthesiologist noted that the intranasal route of midazolam administration may be uncomfortable to the patients, because it could produce a burning sensation when the liquid is administered. Furthermore, the drug can have a noxious taste when administered via the intranasal route and more can be lost through the oronasal pathway, rendering less absorption into the tissue. An atomizer is suggested for future use of intranasal midazolam. However, based on a published abstract by Dabir et al,16 intranasal midazolam administered with a tuberculin syringe demonstrated a higher overall sedation effectiveness percentage (75% vs 50%) than when administered by an atomizer.
In both the previous study by de Santos et al13 and the present study, 0.2 mg/kg of midazolam was administered to each patient via either the intramuscular or intranasal route. In the previous study, the midazolam was combined with 0.015 mg/kg of atropine. It is doubtful that the addition of atropine would account for the difference in results found between the 2 studies. It is more likely that the small sample size would need to be considered as a contributing factor. The results of the present study must be interpreted in light of the small number of participants enrolled. Because of time limitations of the faculty and residents, additional patients could not be included, and thus the study is considered to be a pilot study. Further investigation with a greater number of patients might yield more meaningful information and/or confirm these pilot study results.
Conclusion
A trend that indicates that intramuscular midazolam could be more effective as a premedication than the intranasal route with time progression and increased stimulation was noted in the present study. When used before intravenous conscious sedation, the intramuscular route allowed for a better sedation level and less movement at the time of venipuncture than the intranasal route.
References
- Rita L, Seleny FL, Mazurek A, Rabins R. Intramuscular midazolam for pediatric preanethestic sedation: a double blind controlled study with morphine. Anesthesiology. 1985;63:528–531. doi: 10.1097/00000542-198511000-00009. [DOI] [PubMed] [Google Scholar]
- Taylor MB, Vine PR, Hatch DJ. Intramuscular midazolam premedication in small children. Anaesthesia. 1986;41:21–26. doi: 10.1111/j.1365-2044.1986.tb12698.x. [DOI] [PubMed] [Google Scholar]
- Abrams R, Morrison JE, Villasenor A, Hencmann D, Da Fonseca M, Mueller W. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog. 1993;40:63–66. [PMC free article] [PubMed] [Google Scholar]
- Wilton NCT, Leigh J, Rosen DR, Pandit UA. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–975. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- Raybould D, Bradshaw EG. Premedication for day case surgery. Anaesthesia. 1987;42:591–595. doi: 10.1111/j.1365-2044.1987.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Saint-Maurice C, Meistleman C, Rey E, Esteve C, De Lauture D, Olive G. The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology. 1986;65:536–538. doi: 10.1097/00000542-198611000-00019. [DOI] [PubMed] [Google Scholar]
- Tolksdorf W, Eick C. Rectal, oral and nasal premedication using midazolam in children aged 1–6 years: a comparative study. Anaesthesist. 1991;40:661–667. [PubMed] [Google Scholar]
- Lejus C, Renaudin M, Testa S, Malinovsky JM, Vigier T, Souron R. Midazolam for premedication in children: nasal vs. rectal administration. Eur J Anaesthesiol. 1997;14:244–249. doi: 10.1046/j.1365-2346.1997.00013.x. [DOI] [PubMed] [Google Scholar]
- Malamed SF. Sedation: A Guide to Patient Management. New York, NY: CV: Mosby-Year Book Inc; 1995. 3rd ed. [Google Scholar]
- Karl HW, Keifer AT, Rosenberger JL, Larach NG, Ruffle JM. Comparison of safety and efficacy of intranasal midazolam or sufentanil for preinduction of anesthesia in pediatric patients. Anesthesiology. 1992;76:209–215. doi: 10.1097/00000542-199202000-00009. [DOI] [PubMed] [Google Scholar]
- Malinovsky JM, Populaire C, Cozian A, Lepage JY, Lejus C, Pinaud M. Premedication with midazolam in children: effect of intranasal, rectal and oral routes on plasma midazolam concentration. Anaesthesia. 1995;50:351–354. doi: 10.1111/j.1365-2044.1995.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Karl HW, Rosenberger JL, Larach MG, Ruffle JM. Transmucosal administration of midazolam for premedication of pediatric patients. Anesthesiology. 1993;78:885–891. doi: 10.1097/00000542-199305000-00013. [DOI] [PubMed] [Google Scholar]
- de Santos P, Chabas E, Valero R, Nalda MA. Comparison of intramuscular and intranasal premedication with midazolam in children. Rev Espanola Anestesiol Reanimacion. 1991;38:12–15. [PubMed] [Google Scholar]
- Lu DP. Intramuscular sedation in dentistry. Compendium Contin Educ Dent. 1991;2:628–639. [PubMed] [Google Scholar]
- Houpt MI, Koenigsberg SR, Weiss NJ, Desjardins PJ. Comparison of chloral hydrate with and without promethazine in the sedation of young children. Pediatr Dentist. 1985;7:41–46. [PubMed] [Google Scholar]
- Dabir PA, Dummett CO, Musselman RJ, Schneider PE, Moerschbaecher JM, Gardiner D. Assessment of intranasal administration of midazolam for conscious sedation, using an atomizer. Pediatr Dentist. 2001;23:168–169. [Google Scholar]


