Abstract
During pursuit of moving targets that temporarily disappear, residual smooth eye movements represent the internal (extra-retinal) component of pursuit. However, this response is dependent on expectation of target reappearance. By comparing responses with and without such expectation during early random-onset pursuit, we examined the temporal development of the extra-retinal component and compared it with anticipatory pursuit, another form of internally driven response. In an initial task (mid-ramp extinction), a moving, random-velocity target was initially visible for 100 or 150 ms but then extinguished for 600 ms before reappearing and continuing to move. Responses comprised an initial visually driven rapid rise in eye velocity, followed by a secondary slower increase during extinction. In a second task (short ramp), with identical initial target presentation but no expectation of target reappearance, the initial rapid rise in eye velocity was followed by decay toward zero. The expectation-dependent difference between responses to these tasks increased in velocity during extinction much more slowly than the initial, visually driven component. In a third task (initial extinction), the moving target was extinguished at motion onset but reappeared 600 ms later. Repetition of identical stimuli evoked anticipatory pursuit triggered by initial target offset. Temporal development and scaling of this anticipatory response, which was based on remembered velocity from prior stimuli, was remarkably similar to and covaried with the difference between mid-ramp extinction and short ramp tasks. Results suggest a common mechanism is responsible for anticipatory pursuit and the extra-retinal component of random-onset pursuit, a finding that is consistent with a previously developed model of pursuit.
INTRODUCTION
When humans pursue a moving target from a standstill, there is initially a delay of 80–100 ms before smooth eye movement starts (Carl and Gellman 1987). Smooth movement then increases in speed toward target velocity, taking 200–300 ms to reach a steady state, which is then maintained partly by visual feedback and partly by internal (extra-retinal) drive mechanisms. Internal drive becomes evident if the moving target temporarily disappears, when smooth eye movements can continue for periods ≤4 s (Becker and Fuchs 1985; von Noorden and Mackensen 1962). The origin of the extra-retinal component is thought to lie in an internal feedback pathway, and although several representational models have been developed (Krauzlis and Lisberger 1994; Krauzlis and Miles 1996; Robinson et al. 1986), the dynamics of this pathway have not been investigated. Our objectives were to determine how the internal component develops over time and to compare it with another form of internal drive, anticipatory pursuit (Barnes and Asselman 1991; Barnes and Donelan 1999; Boman and Hotson 1992).
The starting point for this investigation was a recent experiment in which the effects of expectation were shown to influence the development of the extra-retinal component of random-onset pursuit (Barnes and Collins 2008). In one task (referred to as mid-ramp extinction), a moving target, randomized in speed, direction, and timing, was initially exposed for a brief period (50–200 ms) prior to extinction for ≤600 ms and subsequent reappearance. Two components of smooth eye velocity were identified in the response, an initial rapidly increasing component that appeared to be dependent on the visual input, followed by a secondary component that built up more slowly throughout the prolonged extinction. In a second, complementary task (referred to as short ramp), where only the initial stimulus presentation was given and there was no expectation of target reappearance, the secondary component did not develop; eye velocity simply decayed toward zero even though subjects attempted quite successfully to track the unseen target with a combination of smooth and saccadic eye movements. Given that the initial visually dependent response was very similar in both tasks, it was evident that the expectation of target reappearance in the first task allowed an internally driven component to develop during the period of extinction that was additional to the visually driven response. Moreover, because the magnitude of the secondary component was scaled to target velocity, it implied that target motion information had been sampled during the initial brief presentation and temporarily stored. The objective of the current experiments was to show whether the temporal development of this expectation-dependent component, which anticipates target reappearance, was similar to the anticipatory pursuit response elicited by repeated presentation of identical motion stimuli (Barnes and Donelan 1999; Boman and Hotson 1988). If so, it would imply that velocity information extracted in the initial presentation of a randomized pursuit stimulus can be used to control the smooth eye velocity prior to target reappearance in the same way that stored information derived from prior motion stimuli can be used to control anticipatory pursuit that occurs before actual target motion onset.
To examine this, we have conducted a set of three experiments. We have repeated the mid-ramp extinction and short ramp tasks outlined in the preceding text but have blocked the presentation of initial presentation duration and extinction time, while retaining the randomization of target speed, direction, and timing. The aim was to minimize the error of initial motion detection because our main concern was to examine the temporal development of the internal component rather than to test the probability of correct detection. We have then compared the expectation-dependent difference in response between these two tasks with a newly devised task that we refer to as the initial extinction task. This task is similar to the mid-ramp extinction task but lacks the initial target presentation. Without this initial presentation, any scaling of the anticipatory response must be derived from stored motion information obtained from prior stimuli. We show that after one to two repetitions of stimuli with identical target velocity, anticipatory smooth pursuit movements are indeed initiated with a latency of ∼200 ms and gradually increase in velocity during the 600-ms extinction. Moreover, the trajectory of this anticipatory pursuit bears a close similarity to that of the internal drive component represented by the difference between the nonpredictable mid-ramp extinction and short ramp tasks.
METHODS
Subjects
Six human subjects, who had all participated in our earlier experiment (Barnes and Collins 2008), took part with voluntary consent. They had no known neurological or oculomotor problems and had normal or corrected-to-normal vision. Experiments were conducted in accordance with the declaration of Helsinki and with the approval of the local ethics committee. Two subjects had no previous experience of pursuit experiments.
Apparatus
The subjects were presented with a red target that could be moved across a flat screen by a mirror galvanometer system. The target was formed by the projection of a ring of light-emitting diodes (LEDs; 1° diam.) onto the screen (2.5 m wide × 1.5 m high), which formed a blank background in a totally darkened room. The screen was placed at a distance of 1.5 m from the subject's head and subtended ±60o horizontally by ±45o vertically. The head was immobilized by side-clamps and a chin-rest. Eye movements were recorded by a limbus tracking device (Skalar Iris) that was firmly attached to the head.
Procedure
Four different tasks were undertaken by each subject. The stimulus was a step-ramp target motion (Rashbass 1961), in which the target initially stepped to the left or right and then moved with a velocity (V) of 5, 10, 15, or 20°/s in the opposite direction.
In the mid-ramp extinction task, the speed, direction (left, right) and step size for each stimulus presentation were randomized. The target was initially stationary for a randomized period of 500–1,000 ms, then stepped in the direction opposite to ramp motion. Following the step, the target was visible for an initial presentation duration (PD) of 100 or 150 ms. The target was then switched off for an extinction duration of 600 ms before reappearing and continuing to move at the same speed for a further 400 ms. In contrast to our previous study (Barnes and Collins 2008), in which PD and extinction duration were randomized, in this experiment, they were both held constant within a block of presentations to maximize the predictability of timing and facilitate the development of the internally generated response. In total there were eight repetitions of each target speed and direction for each PD.
In the short ramp task, the target was presented for the same brief initial exposure durations as in the mid-ramp extinction task but did not reappear after the extinction. The subjects were made aware of this but were instructed to try to continue eye movement during the extinction period as if the target would reappear.
In the initial extinction Task, each presentation started with fixation for a randomized period of 500–1,000 ms at the end of which the target was extinguished for 600 ms. Although the target could not be seen, motion of the mirror drive system was initiated at the time of extinction and continued to execute a step-ramp motion. Consequently, when the target appeared, it had already moved to an eccentric position and continued along its ramp trajectory from the time of reappearance for a further 200 ms. The target was presented in predictable blocks in which target speed and direction remained identical for six consecutive presentations, followed by a seventh in which the target was stationary; successive blocks had randomized speed and direction. Target speed and direction were the same as for the mid-ramp extinction task. Only the final four responses of each block were used for the analysis, but two repeats of each target speed and direction were given in separate runs, yielding a total of eight repetitions. Note that this procedure was identical to that described by Collins and Barnes (2006) except that subjects had to use the extinction of the fixation target as a “go” cue for the start of ramp motion rather than an auditory cue. The method was also similar to the “gap” technique used by Boman and Hotson (1988) but differed in the important detail that the target was in motion, not stationary, during the gap.
In the control task, the conditions were identical to those of the mid-ramp extinction task, except that the target remained visible throughout the whole of the ramp, for a period of 1,000 ms.
In total, the subjects performed eight separate runs, two for each task, presented in a balanced randomized design. Each run was preceded by a calibration of eye movement.
Data analysis
Analogue eye and target displacement signals were low-pass filtered at 80 Hz prior to digitization at 200 Hz and storage on disc. Eye and target velocity were derived from the digitized data by the two-point central difference method and saccades were removed from the eye velocity signal using techniques described in detail elsewhere (Bennett and Barnes 2003). Saccadic latencies were calculated in relation to target motion onset. Linear interpolation was used to fill the gaps left by the saccades removed (see Collins and Barnes 2006 for justification of using this technique), and the resultant data were then further filtered using a zero-phase autoregressive low-pass digital filter with a cut-off frequency of 30 Hz. The following variables were then examined in detail.
VPK AND TPK.
Responses to mid-ramp extinction tasks were typified by an initial rapid rise to a low-gain peak (Vpk), followed by a lower rate of rise during the remaining period of extinction (see Fig. 1, A and B). The time (Tpk) at which the initial peak velocity occurred was calculated by examining eye acceleration for each response and specifying Tpk as the time at which acceleration passed through zero (or a minimum if it did not fall to 0) prior to the start of the secondary, lower level of acceleration. Tpk was calculated in relation to the onset of target movement.
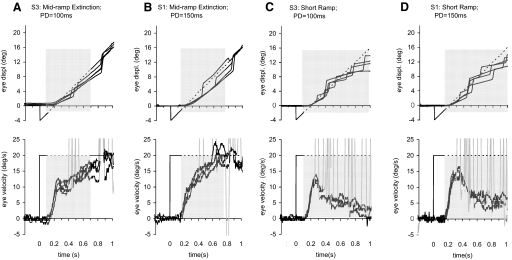
FIG. 1.
Examples of eye position (top) and velocity (bottom) elicited by the mid-ramp extinction and short ramp tasks for initial presentation durations (PD) of 100 and 150 ms. A and B: responses to the mid-ramp extinction task for subjects S3 and S1, respectively. C and D: responses of S3 and S1 in the short ramp task. Target velocity (V) was 20°/s in all examples. Four individual responses are plotted in each panel. Gray spikes on velocity traces indicate saccades. Gray shading indicates the period of target extinction.
LATENCY.
The latency of smooth eye movement initiation with respect to the start of target motion was calculated with a semi-automatic procedure. A linear regression was carried out from the time at which eye velocity reached 10% of Vpk until 100 ms thereafter, and latency was calculated by extrapolation back to zero velocity. On rare occasions (<2% of responses) the time window for the regression was inappropriately computed by automatic procedures, but an interactive procedure allowed this to be inspected, and the time window to be reset if necessary.
SED AND TED.
to compare measures of smooth eye displacement (SED) with total eye displacement (TED) during the extinction period, we first calculated the cumulative SED by integrating smooth eye velocity after removal of saccades. We then specifically compared TED and SED at the end of the extinction period, prior to any influence of visual feedback (see Orban de Xivry 2006).
Statistical analyses were carried out using SPSS software. All data were tested for departures from normality using the Shapiro-Wilk statistic (Shapiro and Wilk 1965); however, no transformation of the data was deemed necessary. The Mauchly test (Mauchly 1940) was used to test for sphericity within and between factors. If the assumption of sphericity was violated, a Greenhouse-Geisser correction (Greenhouse and Geisser 1959) was used to calculate adjusted P values. All comparisons were made using repeated-measures analysis of variance (ANOVA).
RESULTS
Mid-ramp extinction and short ramp tasks
GENERAL OBSERVATIONS.
In the mid-ramp extinction task, subjects were able to gain a clear perception of the motion and direction of the target during the initial exposure and generated a high proportion of smooth eye movements during target extinction, as shown by examples from two of the subjects in Fig. 1, A and B. As reported previously (Barnes and Collins 2008), the response was characterized by two components, a short initial phase of rapid velocity increase to an initial peak (Vpk), followed by a secondary phase in which eye velocity increased more gradually toward target velocity. The gradual increase in velocity in the secondary phase occurred even though there was no visual input during this stage. In 54% of responses, there were no saccades between the onset of motion and the reappearance of the target. When mid-extinction saccades occurred (Fig. 1, A and B), the majority were clustered around 400–450 ms after motion onset, regardless of PD. Overall, 76% of all responses to the mid-ramp extinction condition were made without any saccades occurring in the first 400 ms after target onset. When a saccade did occur in mid-extinction, it was evident that eye velocity after the saccade soon returned to follow along the same trajectory as those responses without a saccade. We examined for any evidence of postsaccadic enhancement of eye velocity (Lisberger 1998) by comparing the change in velocity across each saccade with the change in velocity occurring over the same period in responses without saccades. In only 1.3% of examples was the difference in velocity change greater than twice the SD for the responses without saccades, with 8.3% lying between 1 and 2 SD. There were thus very few examples of significant postsaccadic enhancement in the mid-ramp extinction task. Smooth eye velocity responses averaged across subjects and target direction are shown in Fig. 2, A and B, for the PD = 100 ms and PD = 150 ms conditions. Even when averaged, these responses still exhibited the same initial and secondary phases seen in individual responses. An example of the control response for a 20°/s stimulus is also shown for comparison in Fig. 2A.
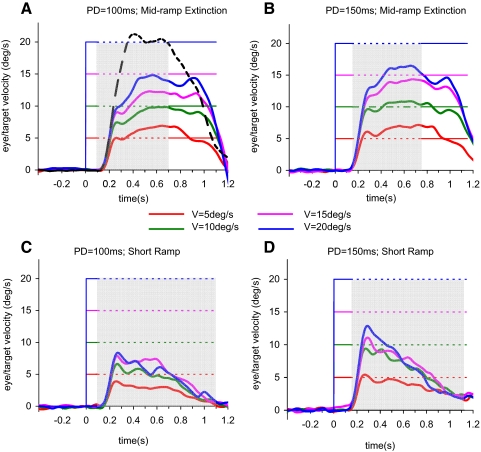
FIG. 2.
Smooth eye velocity, averaged across all 6 subjects, in the mid-ramp extinction task (A and B) and the short ramp task (C and D), for target velocities (V) of 5°/s (red), 10°/s (green), 15°/s (magenta), and 20°/s (blue) and 2 initial PDs (PD = 100 ms in A and C; PD = 150 ms in B and D). Broken black line in A denotes control response at 20°/s. The origin on the time axis represents the time of target motion onset; prior to this time subjects fixated the stationary target for a randomized duration of 500–1000 ms. Gray shaded areas indicate the period of target extinction.
In the short ramp task, where there was no expectancy of target reappearance, the initial rapid increase in smooth eye velocity was similar to that in the mid-ramp extinction task (Fig. 1, C and D), However, subjects were unable to sustain smooth eye movement in the same way that they could when reappearance was expected and after attainment of the initial peak (Vpk), smooth eye velocity decayed toward zero. The velocity decay occurred even though the eye displacement traces indicated that subjects had attempted to follow the path of the imagined target (top, Fig. 1, C and D). Figure 2, C and D, shows mean smooth eye velocity data from this task for the two initial PDs (PD = 100 ms and PD = 150 ms).
Characteristics of the initial phase: mid-ramp extinction task
As expected, randomization of the time of stimulus onset and velocity resulted in wholly reactive responses; the latency of smooth eye movement initiation in the mid-ramp extinction task averaged 142.8 ± 19.3 (SD) ms compared with 133.0 ± 29.3 in the control condition. Consequently, for PD = 100 ms, no eye movement response was elicited until after the target had disappeared and the extinction period had begun. In the initial phase of the smooth eye movement response (Fig. 2), eye velocity increased rapidly toward an initial peak (Vpk) that increased significantly with initial PD [F(1,5) = 61.15; P < 0.001] and target velocity [F(3,15) = 61.68; P < 0.001] as shown in Fig. 3A. Vpk values were not significantly different to those obtained previously (Barnes and Collins 2008). There was also a significant increase in the time (Tpk) to reach Vpk as target velocity increased [F(3,15) = 9.85; P = 0.010] but no significant effect of PD [F(1,5 = 1.69; P = 0.25]. Mean Tpk occurred 286.6 ± 26.1 ms after target motion onset for PD = 100 and 300.9 ± 26.4 ms after onset for PD = 150 ms.
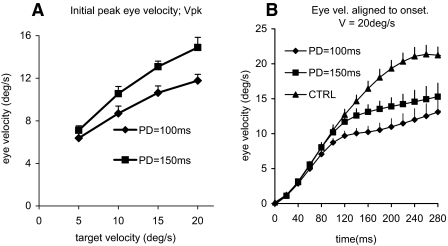
FIG. 3.
Characteristics of responses to mid-ramp extinction task. A: average (n = 6 + SE) peak smooth eye velocity (Vpk) of the initial phase of the response for PDs of 100 and 150 ms. B: average (n = 6 + SE) smooth eye velocity profiles, plotted at 20-ms intervals with respect to response onset, for PD = 100 ms, PD = 150 ms, and control (CTRL) conditions; target velocity = 20°/s.
After alignment of each response to its time of initiation, the initial phase of the mid-ramp extinction responses appeared similar to controls, as indicated in Fig. 3B. Eye velocity levels for PD = 100 ms and PD = 150 ms conditions were compared with controls at 20-ms intervals after initiation. No responses were significantly different from controls for the first 80 ms, but thereafter, the response to all conditions became progressively attenuated compared with the control condition. Responses to PD = 100 ms first became significantly different to the control 100 ms after response onset [F(1,51) = 9.12; P = 0.027], whereas the PD = 150 ms responses first showed a significant difference at 140 ms after initiation [F(1,5) = 8.43; P = 0.034].
Characteristics of the secondary phase: mid-ramp extinction task
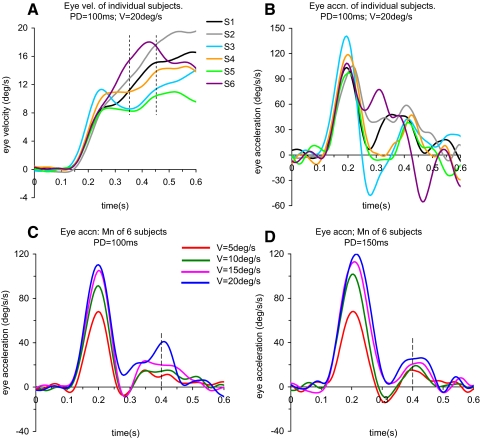
After reaching the initial peak velocity, eye velocity often exhibited a small dip or plateau before the secondary phase of increasing velocity began as shown in the averaged responses of Fig. 2, A and B. For both PD = 100 ms and PD = 150 ms, eye velocity continued to increase beyond the initial peak even in the absence of visual input. All subjects displayed this continuing increase in velocity as indicated in Fig. 4A, although there was considerable intersubject variation. Examination of eye acceleration (Fig. 4B) revealed a very similar bell-shaped profile in the initial phase followed by a much more variable secondary phase. Thus in subjects 3 and 5, eye acceleration in the secondary phase began after the initial phase had reached zero, whereas subjects 2 and 6 initiated the secondary phase well before the initial phase had reached zero. When averaged across all subjects, eye acceleration in the secondary phase reached a maximum between 350 and 450 ms after target motion onset for both initial PDs [PD = 100 ms (Fig. 4C) and PD = 150 ms (D)] and all target velocities. We calculated eye acceleration (A4001) at the center of this time window (i.e., 400 ms after target onset, indicated by vertical lines in Fig. 4, C and D) to assess its dependence on target velocity and PD. A4001 increased significantly with increasing target velocity [F(3,15) = 10.72; P = 0.001], but there was no significant effect of PD on A4001. Clearly acceleration was not at a maximum for all subjects at 400 ms, but analysis of average acceleration between 350 and 450 ms showed a similar dependence on target velocity. During extinction, eye velocity reached a peak ∼600 ms after target motion onset (and thus prior to any visual feedback), but it was clearly a less accurate estimate of true target velocity than obtained with the aid of visual feedback; on average, it overshot the lowest target velocity but undershot the highest (Fig. 2, A and B). Eye velocity at the end of extinction was obtained from the mean of the eight repetitions at each velocity and PD for each subject. ANOVA revealed no significant change in end-extinction velocity with repetition. However, end-extinction eye velocity was significantly modulated by target velocity for both levels of initial PD [F(3,15) = 42.07; P < 0.001], in contrast to our previous experiment (Barnes and Collins 2008), where this was not the case for PD = 100 ms. In fact, as anticipated, the greater predictability of PD and extinction duration resulted in end-extinction velocity averaged across all subjects and velocities being 23% higher than in the previous experiment.
FIG. 4.
A and B: mean eye velocity (A) and eye acceleration (B) of individual subjects (S1–S6) during the 1st 600 ms after target motion onset. - - - in A are time markers for 350 and 450 ms after onset. C and D: mean eye acceleration, averaged across all 6 subjects for each level of target velocity (V) for initial presentations of PD = 100 ms (C) and PD = 150 ms (D). - - - in C and D are time markers for 400 ms.
Characteristics of responses to the short ramp task
Latency of the smooth eye movement in the short ramp task (mean = 134.4.1 ± 16.1 ms) was not significantly different to that in the mid-ramp extinction task [F(1,5) = 3.85; P = 0.107]. Initial peak smooth eye velocity (Vpk) increased significantly with target velocity [F(3,15) = 78.98; P < 0.001] and initial exposure duration [F(3,15) = 38.03; P < 0.001] and was not significantly different to Vpk values for the mid-ramp extinction task. Despite the decaying eye velocity in this condition, subjects were able to achieve an overall displacement trajectory during extinction that was reasonably close to the desired target motion by executing a series of saccades (Fig. 1C) as found previously (Barnes and Collins 2008). We calculated the total eye displacement (TED) and the cumulative smooth eye displacement (SED) at the end of extinction and compared these measures for the mid-ramp extinction and short ramp tasks. Although SED was significantly less in the short ramp than the mid-ramp extinction task [F(1,5) = 118.87; P < 0.001] as a result of the decaying eye velocity profile, the TED was not significantly different between tasks. However, TED did increase significantly as target velocity increased [F(1,5) = 13.29; P < 0.001]. Thus the extrapolated eye displacement estimate appeared to be based on initial target velocity, but in both tasks, eye displacement was overestimated; mean gains of TED were 1.08 and 1.11 for the short ramp and mid-ramp extinction tasks, respectively.
Extraction of the internal drive component from mid-ramp extinction data
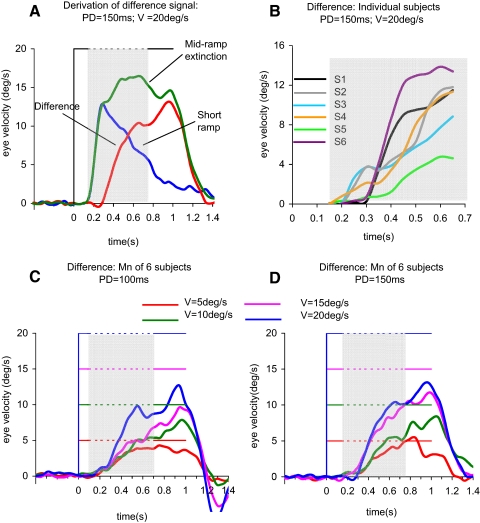
To determine the part of the mid-ramp extinction response associated specifically with expectation of target reappearance, we subtracted the responses in the short ramp condition (Fig. 2, C and D) from those in the mid-ramp extinction paradigms (Fig. 2, A and B). We will refer to the result as the difference signal. The process is represented in Fig. 5A for a 20°/s stimulus (PD = 150 ms). The mid-ramp extinction and short ramp responses shared a very similar initial phase but then diverged after reaching the initial peak. Consequently, in deriving the difference signal, the initial phases were mostly cancelled out and the difference signal exhibited a steady increase in velocity that started ∼200 ms after the onset of target motion. Small differences in initial peak velocity of the mid-ramp extinction and short ramp responses could lead to relatively large errors in determining the velocity of the difference signal around the time of initiation and this precluded obtaining accurate measures of latency for individual responses. Nevertheless, all subjects showed a characteristic pattern of increasing eye velocity from the time of initial peak velocity (i.e., ∼300 ms after target onset) until the end of the extinction period. This is evident in Fig. 5B, where responses to a 20°/s target motion for each subject are plotted between target onset and 650 ms after onset. In fact, the magnitude of the difference signal, as represented by its value at the end of extinction, increased significantly [F(3,15) = 10.61; P = 0.001] as target velocity increased, although there was no significant difference between the PD = 100 ms (Fig. 5C) and PD = 150 ms (D) conditions.
FIG. 5.
A: comparison of average eye velocity in the mid-ramp extinction (green trace), and short ramp (blue trace) conditions. The red trace represents the difference between these conditions. Target velocity (V) = 20°/s; PD = 150 ms. B: individual subject difference signals for a PD of 150 ms. Target velocity (V) = 20°/s. Color coding for subjects (S1–S6) is identical to Fig. 4. C and D: the difference signal averaged across all 6 subjects for PD = 100 ms (C) and PD = 150 ms (D) for target velocities (V) of 5°/s (red), 10°/s (green), 15°/s (magenta), and 20°/s (blue). Gray shading indicates period of target extinction.
Initial extinction task
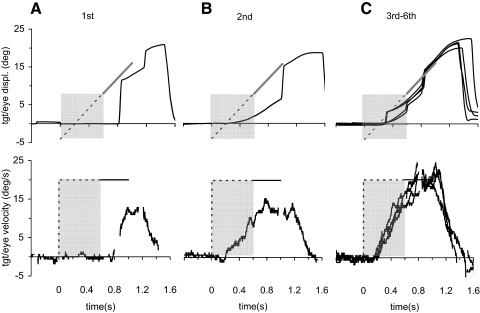
In the initial extinction task, subjects were able to use the extinction of the fixation target as the go cue to initiate an eye movement response in the blank interval before the target became visible. In the first presentation of the stimulus, subjects were not able to extract motion information prior to the first appearance of the target and thus made a large saccade to correct the positional error, followed by some smooth movement (Fig. 6A). However, in the second presentation (Fig. 6B), anticipatory smooth movement started to emerge in the extinction period. It tended to reach a slightly higher velocity in the third presentation, but its velocity trajectory remained similar thereafter (Fig. 6C). In fact, ANOVA revealed no significant change in end-extinction eye velocity with repetition (after excluding the 1st presentation); when averaged across all stimulus velocities and subjects, end-extinction eye velocity in the second presentation had already reached 93.5% of the mean level derived from the third to sixth presentations. Mean velocity profiles for each subject were obtained by averaging the responses to the final four presentations. Mean velocity profiles averaged across all subjects and both directions are illustrated in Fig. 7A. All responses were reactive to the offset of fixation, but once initiated, exhibited a relatively slow build up of eye velocity during the remaining extinction interval and increasing eye velocity as target velocity increased. There was no significant difference in latency of smooth eye movement initiation (relative to fixation offset) with speed or direction; overall mean latency was 196.8 ± 15.6 ms. Eye velocity at the time of target appearance (V600 –Fig. 7B) exhibited a significant increase with increasing target velocity [F(3,15) = 5.85; P = 0.007]. Thus in line with previous observations (Collins and Barnes 2006), these prolonged anticipatory eye movements were scaled in proportion to the expected target velocity.
FIG. 6.
Example of eye displacement (top) and velocity (bottom) of a single subject (S1) in response to the initial extinction paradigm. Saccades have been removed without replacement in the eye velocity traces, which are presented prior to digital low-pass filtering. A: 1st response; B: 2nd response; C: 3rd–6th responses overlaid. Subject fixated stationary target during a random period of 500–100 ms prior to time 0. At time 0, target was extinguished.  , period of extinction.
, period of extinction.
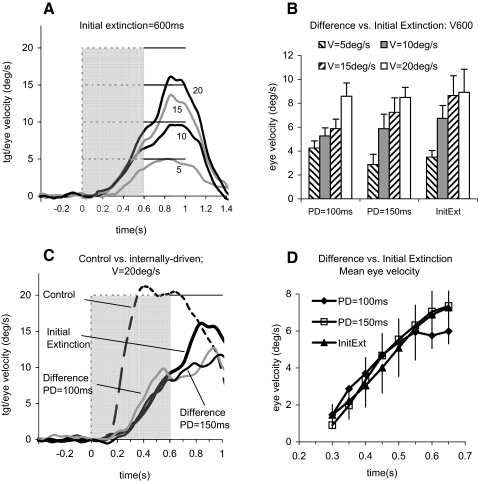
FIG. 7.
A: smooth eye velocity averaged across all 6 subjects in the initial extinction condition. Numbers beside eye traces indicate stimulus velocity (V = 5–20°/s). B: eye velocity at 600 ms after motion onset as a function of target velocity (V) and stimulus paradigm. Mean of 6 subjects +1 SE. C: comparison of mean responses to control, initial extinction, and difference signals (defined in Fig. 5) to 20°/s target velocity. D: initial extinction response and difference signals for PD = 100 and PD = 150 ms, averaged across all target velocities. Mean of 6 subjects +1 SE.
The anticipatory responses evoked in the initial extinction condition, like the difference signals shown in Fig. 5, clearly had a much slower acceleration than the visually driven control responses, as shown in Fig. 7C for a target velocity of 20°/s. To compare anticipatory and difference signals, we first examined eye velocity 600 ms after target motion onset (V600). This corresponded to the time of appearance in the initial extinction condition but occurred just before reappearance in the mid-ramp extinction condition. ANOVA was conducted on the V600 values with target speed, direction, and experimental condition (PD = 100 ms, PD = 150 ms, and initial extinction) as factors. There was a significant increase with target velocity [F(3,15) = 7.52; P = 0.003], but no significant difference between experimental conditions or directions and no significant interactions (Fig. 7B). Subsequent comparison of anticipatory and difference signals was made at 50-ms intervals from 300 ms after target motion onset until the end of extinction (Fig. 7D). This also revealed no significant difference between anticipatory and difference signals throughout this period. Thus the similarity between the anticipatory and difference signals was evident not only in the scaling of end-extinction velocity to target velocity but also in the temporal development of response velocity. This was assessed further by calculating eye acceleration 400 ms after onset (A4002) for anticipatory and difference signals. ANOVA revealed that there was no significant difference in A4002 between the anticipatory and difference signals nor was there any effect of target direction. However, there was a highly significant increase in A4002 with target velocity [F(3,15) = 24.22; P < 0.001], indicating that eye movements were scaled to expected target velocity.
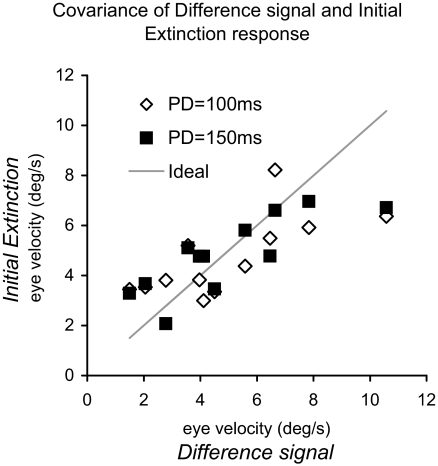
It is evident from the velocity traces of individual subjects shown in Fig. 5B that there was considerable intersubject variability in the difference signal. This variability was also present in the initial extinction condition and if these two signals are equivalent, significant covariance between them would be expected. We tested for covariance using two eye velocity measures. First, we examined mean eye velocity in the period between 200 ms after target motion onset (i.e., close to the time of response onset) and 600 ms after onset (the end of extinction) to give a more robust measure of the internally driven eye velocity during extinction than offered by end-extinction velocity alone. As expected, ANOVA revealed a significant effect of target velocity [F(3,18) = 16.71; P < 0.001], but no effect of direction and no difference between anticipatory and difference signals. To eliminate the dependence on target velocity within subjects, we averaged across target velocities but considered left and right-going responses separately for each subject. In this manner, we obtained 12 values of overall mean velocity for each PD, two for each subject as shown in Fig. 8. A significant correlation between the difference signals and the initial extinction responses was found for both the PD = 100 ms (r = 0.710; n = 12; P = 0.010) and PD = 150 ms (r = 0.812; n = 12; P = 0.001) conditions. We also examined the sensitivity of each subject's response by calculating the ratio of mean eye velocity to target velocity (gain), again, averaged across target velocity. This, too, revealed a significant correlation between the difference and anticipatory signals for both the PD = 100 ms (r = 0.58; n = 12; P = 0.046) and PD = 150 ms (r = 0.76; n = 12; P = 0.004) conditions. Thus subjects who produced a higher velocity in the initial extinction task also tended to generate a larger difference between the mid-ramp extinction and short ramp tasks and vice versa.
FIG. 8.
The relationship between anticipatory eye velocity in the initial extinction task and the velocity of the expectation-dependent difference signal derived from subtraction of the short ramp response from the mid-ramp extinction response. Both measures of velocity represent the average of the response between 200 and 600 ms after target motion onset. Each data point represents the response of a single subject, 1 for each direction of target motion and initial PD. “Ideal” line represents the line of exact equivalence for anticipatory and difference signals.
DISCUSSION
Temporal development of the internal drive component
The objective of the experiments described here was first to determine how the extra-retinal component of sustained pursuit might develop at the beginning of the response to a randomly presented step-ramp stimulus and second to show how this internal component might be related to anticipatory pursuit. The results suggest that the internal component is initiated soon after the onset of the visual response, that its temporal development bears a close resemblance to anticipatory pursuit, but that both develop much more slowly than the visually driven response.
To assess the development of the internal component of pursuit, we compared responses in the mid-ramp extinction task with those in the short ramp task. The results obtained in the current mid-ramp extinction task were qualitatively similar to those obtained in our previous experiment (Barnes and Collins 2008), which was carried out on the same group of subjects. There was no significant difference in initial peak velocity (Vpk) between experiments, and in both experiments, the initial phase of the response was similar to that of the control response for the first 80–100 ms, suggesting that this part is principally visually driven. However, the secondary phase of the response in the current experiment was somewhat greater than found previously. There was a sustained acceleration of the eye after the initial peak that did not simply represent an arbitrary, generalized anticipatory increase, but was scaled to initial target velocity (see Fig. 4, C and D) for both levels of initial PD. As a result, eye velocity at the end of the 600-ms extinction was 23% higher than found previously, probably as a result of blocking the duration of initial presentation and subsequent extinction and thus increasing the probability of correctly detecting target velocity. This accords with the known effects of predictability on anticipatory pursuit (Heinen et al. 2005; Kowler et al. 1984) and is in line with other experiments that have shown increased motion perception and improvement in pursuit with factors such as contrast (Spering et al. 2005). In the short ramp task, where there was no expectation of reappearance, eye velocity reached a peak within 150 ms of response initiation that was not significantly different to the peak of the initial phase of the mid-ramp extinction task but then decayed toward zero even though subjects attempted, with some success, to match total eye displacement to the expected trajectory. This decay of eye velocity on termination of the visual stimulus has been observed many times previously (Krauzlis and Miles 1996; Mitrani and Dimitrov 1978; Pola and Wyatt 1997). When the short ramp responses were subtracted from the mid-ramp extinction responses, the initial visually driven components were cancelled, leaving only the internal drive component that is dependent on expectation of target reappearance. The fact that this internal drive was scaled to target velocity, even though target velocity was randomized, indicates that it must have been obtained by sampling target motion during the initial exposure. The internal component represented by the difference signal was initiated soon after the start of the visual response, but developed much more slowly than the visually dependent response observed at the beginning of the control response (Fig. 7C).
To assess the relationship of the difference signal to another form of internal drive, anticipatory pursuit, we devised a new procedure, the initial extinction task, which was designed to show how the internal component would develop if there were no initial target exposure. This task was similar to the mid-ramp extinction task but lacked the initial visual stimulus. Without that initial stimulus, velocity information had to be provided by another source, in this case, velocity information stored from prior stimuli. The responses to the initial extinction task bore a remarkable similarity to the difference signal derived from the mid-ramp extinction and short ramp tasks. This similarity does not, in itself, prove that the two signals have the same origin. However, the fact that there was significant covariance across subjects in both mean eye velocity and the sensitivity of eye velocity to target velocity (gain) for both PD = 100 and PD = 150 ms conditions suggests that they are likely to have a common origin. The similarity between the difference signals for the PD = 100 and PD = 150 ms conditions also reinforces the notion that the mid-ramp extinction response is composed of a visually driven component that is dependent on the presence of visual input and an internal component that is sampled within the first 100 ms of target presentation and is thus similar irrespective of initial PD. Note that although the initial extinction task evokes a response that is anticipatory of target appearance, the timing of its release is motivated in a different way to the anticipatory pursuit evoked in more conventional cued response pursuit tasks (Barnes and Donelan 1999; Boman and Hotson 1988). In the conventional task, the cue can occur at any time (up to ∼2 s) before target motion onset, and the subject is able to determine, from the elapsed time between cue and motion onset, the best time at which to release anticipatory pursuit. In the initial extinction task, the subject knows that target offset marks the time at which motion starts and the incentive is to initiate eye movement as quickly as possible so that eye velocity can be closer to target velocity at appearance. The response is thus reactive to the cue but anticipatory of target appearance. In effect, it indicates the quickest time at which the anticipatory response can be evoked in response to a go signal.
The similarity between the anticipatory response in the initial extinction task and the difference signal derived from the other two tasks leads us to postulate that the same internal drive mechanism may be used for anticipatory pursuit and the extra-retinal component of sustained pursuit. We argue that this similarity is to be expected because in both contexts, this component has a common aim, namely to predict the future movement of the target and to take over control of eye motion on the assumption that target motion will continue as before. In fact, the difference signal itself represents an anticipatory response that is based on the brief initial presentation, whereas the anticipatory response evoked in the initial extinction task is based on motion information stored from prior presentations. Significantly, expectation has an important influence over response generation for both anticipatory pursuit (Barnes et al. 1997; Kowler 1989; Kowler and Steinman 1979) and the extra-retinal component of sustained pursuit (Becker and Fuchs 1985; Bennett and Barnes 2003) with some evidence indicating that learning improves the latter (Madelain and Krauzlis 2003). The major difference in the two manifestations of the response may simply lie in the time of initiation. In a normal randomized presentation, such as the control task used here, initiation of target motion itself probably acts as the go cue for subsequent triggering of the internally driven component ∼200 ms later, whereas, in a predictable paradigm, cues that occur at a regular time before target motion can be used to release the internal component in advance of target motion (Barnes and Donelan 1999; Boman and Hotson 1988).
Modeling the pursuit response
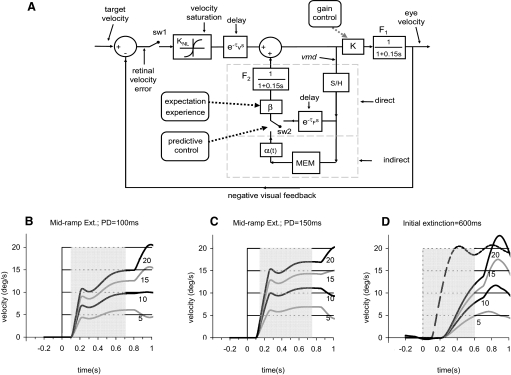
The existence of an internal (or extra-retinal) component of pursuit has long been acknowledged (Robinson et al. 1986; Yasui and Young 1975), and it has been customary to represent this component in the form of an internal efference copy feedback system (Churchland et al. 2003; Robinson et al. 1986; Yasui and Young 1975). The response of such models to extinction of the target is to maintain eye velocity at the preextinction level until after the target reappears (Krauzlis and Miles 1996). Consequently, our basic finding that eye velocity in the mid-ramp extinction condition can continue to rise after extinction (as indicated by the values of A4001) does not fit with this concept. Although a transient increase in gain of the efference copy loop could be used to increase eye velocity (Madelain and Krauzlis 2003), this type of model has difficulty in also explaining the occurrence of velocity-scaled anticipatory movements because the velocity information stored in the internal loop cannot be retained during fixation. This type of model cannot therefore explain the findings in the initial extinction task where subjects are required to fixate between stimulus presentations. Both of these findings can be explained, however, by an alternative model originally proposed by Barnes and Asselman (1991) and subsequently elaborated by Bennett and Barnes (2003, 2006). This model (Fig. 9A) also relies on internal positive feedback but with the major difference that the internal feedback is a system that samples the visuomotor drive signal (vmd in Fig. 9A) rather than relaying it continuously. The results of this and our previous experiment (Barnes and Collins 2008) now provide the evidence for this sample and hold process. In the simulations of responses to the mid-ramp extinction condition shown in Fig. 9, B and C, it is assumed that this pathway alone is used. The initial part of the response is driven by visual input, but after the target is extinguished (by opening switch sw1), eye velocity continues to increase as a result of activity within the direct loop, which starts after a short delay (τF = 50 ms). The direct pathway is supplemented by an indirect pathway that retains motion information from prior sampling in working memory (MEM). This allows motion information to be retained even during fixation and thus enables subsequent anticipatory pursuit responses to be generated. Simulations of the response to the initial extinction condition (Fig. 9D) are generated via this indirect pathway on the assumption that the stored information is released in response to the go signal provided by target extinction with a delay of 200 ms, in line with the latency of recorded responses. In explaining the results of the current experiments, only a single level of velocity needs to be retained in MEM, but it is known from other experiments (Barnes et al. 2002; Collins and Barnes 2005) that multiple levels of motion information may be retained in working memory; this is represented by the time-dependent gain α(t). Direct and indirect pathways cannot output simultaneously, otherwise internally generated estimations of required eye velocity would be inappropriate. Hence it is necessary to switch between direct and indirect pathways (via sw2) when external cues, such as the go signal provided by target extinction in the initial extinction condition, indicate that previously stored information may be released in anticipation of target appearance. An important feature of the model is that output from the direct and indirect pathways feeds out through a common low-pass filter (F2) that results in the more gradual development of the internally driven component when compared with the visually driven component. Critically, we also propose that, as in the earlier model (Bennett and Barnes 2003) and in line with the findings of the current experiments, the output of the internal pathway is regulated by expectancy, which controls the gain β of the internal feedback pathway. We propose that β is close to unity when past experience indicates a high probability of target reappearance but is close to zero when the probability is low, leading to the decay of eye velocity. The intersubject variability of the internally generated eye velocity (Fig. 8) may be associated with differences in the level of β between subjects.
FIG. 9.
A: model of ocular pursuit. The sample and hold system (S/H) is an integrator that averages its input over a period of 100 ms. MEM is a working memory that captures and holds the output of S/H and can be used to anticipate future target motion. β = 0.9; τV = 0.1 s; τF = 0.05 s; KNL = nonlinear velocity feedback gain; K = 2.5. B and C: simulated responses to mid-ramp extinction condition for target velocities of 5–20°/s and PD = 100 ms (B) or PD = 150 ms (C). D: simulated responses to initial extinction condition for target velocities of 5–20°/s plus control (broken line). Numbers beside eye velocity traces indicate stimulus velocity.
Evidence suggests that modulation of internal loop gain with changing expectation may be mediated by the supplementary eye fields (SEFs). SEF has often been associated with predictive pursuit (Fukushima et al. 2004; Heide et al. 1996; Schmid et al. 2001) but, in particular, Heinen and Liu (1997) established that prepursuit activity in SEF is dependent on stimulus predictability. More recently, Missal and Heinen (2004) have shown that stimulation of SEF changes the gain of anticipatory smooth pursuit in a way that would fit with an expectation-dependent modulation of the output of the internal loop (Fig. 9A). Moreover, de Hemptinne et al. (2008). have recently shown that SEF participates in direction discrimination for anticipatory pursuit. The motor output for anticipatory pursuit almost certainly emanates from frontal eye fields (FEFs) and, given the established interconnections with SEF (Huerta and Kaas 1990), is probably strongly influenced by SEF activity (de Hemptinne et al. 2008),. It is of particular interest that microstimulation of FEF (Gottlieb et al. 1993) has been shown to elicit smooth eye movements with a velocity that increases much more slowly than a visually driven response, in line with differences observed here between internally and visually driven eye movements.
Summary
Results presented here indicate a close similarity between the anticipatory responses evoked by repeated predictable stimuli and the internally driven (extra-retinal) component of random-onset pursuit. The mid-ramp extinction experiments provide evidence that velocity information for pursuit eye movements is sampled and stored within the first 100–150 ms of target motion. This stored information appears to form a goal for smooth eye movement, which continues to increase in velocity, even in the absence of the moving target. However, the ability to drive the eye toward this goal is dependent on expectation of target reappearance; without it, eye velocity simply decays toward zero. The expectation-dependent difference between these responses is internally driven and increases more slowly than the corresponding visually driven response. Moreover, the trajectory of this internally driven response bears a close similarity to anticipatory smooth pursuit movements observed when target motion is predictable, suggesting a common internal mechanism.
GRANTS
This work was supported by the Medical Research Council, UK.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Barnes and Asselman 1991.Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. J Physiol 439: 439–461, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes and Collins 2008.Barnes GR, Collins CJS. The influence of briefly presented randomised target motion on the extra-retinal component of ocular pursuit. J Neurophysiol 99: 831–842, 2008. [DOI] [PubMed] [Google Scholar]
- Barnes and Donelan 1999.Barnes GR, Donelan AS. The remembered pursuit task: evidence for segregation of timing and velocity storage in predictive oculomotor control. Exp Brain Res 129: 57–67, 1999. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 1997.Barnes GR, Grealy MA, Collins S. Volitional control of anticipatory ocular smooth pursuit after viewing, but not pursuing, a moving target: evidence for a re-afferent velocity store. Exp Brain Res 116: 445–455, 1997. [DOI] [PubMed] [Google Scholar]
- Barnes et al. 2002.Barnes GR, Schmid AM, Jarrett CB. The role of expectancy and volition in smooth pursuit eye movements. Prog Brain Res 140: 239–254, 2002. [DOI] [PubMed] [Google Scholar]
- Becker and Fuchs 1985.Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res 57: 562–575, 1985. [DOI] [PubMed] [Google Scholar]
- Bennett and Barnes 2003.Bennett SJ, Barnes GR. Human ocular pursuit during the transient disappearance of a moving target. J Neurophysiol 90: 2504–2520, 2003. [DOI] [PubMed] [Google Scholar]
- Bennett and Barnes 2006.Bennett SJ, Barnes GR. Smooth ocular pursuit during the transient disappearance of an accelerating visual target: the role of reflexive and voluntary control. Exp Brain Res 175: 1–10, 2006. [DOI] [PubMed] [Google Scholar]
- Boman and Hotson 1988.Boman DK, Hotson JR. Stimulus conditions that enhance anticipatory slow eye movements. Vis Res 28: 1157–1165, 1988. [DOI] [PubMed] [Google Scholar]
- Boman and Hotson 1992.Boman DK, Hotson JR. Predictive smooth pursuit eye movements near abrupt changes in motion direction. Vis Res 32: 675–689, 1992. [DOI] [PubMed] [Google Scholar]
- Carl and Gellman 1987.Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. JNeurophysiol 57: 1446–1463, 1987. [DOI] [PubMed] [Google Scholar]
- Churchland et al. 2003.Churchland MM, Chou I-H, Lisberger SG. Evidence for object permanence in the smooth-pursuit eye movements of monkeys. J Neurophysiol 90: 2205–2218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins and Barnes 2005.Collins CJS, Barnes GR. Scaling of anticipatory smooth eye velocity in response to sequences of discrete target movements in humans. Exp Brain Res 167: 404–413, 2005. [DOI] [PubMed] [Google Scholar]
- Collins and Barnes 2006.Collins CJS, Barnes GR. The occluded onset pursuit paradigm: prolonging anticipatory smooth pursuit in the absence of visual feedback. Exp Brain Res 175: 11–20, 2006. [DOI] [PubMed] [Google Scholar]
- de Hemptinne et al. 2008.de Hemptinne C, Lefevre P, Missal M. Neuronal bases of directional expectation and anticipatory pursuit. J Neurosci 28: 4298–4310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima et al. 2004.Fukushima J, Akao T, Takeichi N, Kurkin S, Kaneko CRS, Fukushima K. Pursuit-related neurons in the supplementary eye fields: discharge during pursuit and passive whole body rotation. J Neurophysiol 91: 2809–2825, 2004. [DOI] [PubMed] [Google Scholar]
- Gottlieb et al. 1993.Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol 69: 786–799, 1993. [DOI] [PubMed] [Google Scholar]
- Greenhouse and Geisser 1959.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 24: 95–111, 1959. [Google Scholar]
- Heide et al. 1996.Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain 119: 1951–1969, 1996. [DOI] [PubMed] [Google Scholar]
- Heinen et al. 2005.Heinen SJ, Badler JB, Ting W. Timing and velocity randomization similarly affect anticipatory pursuit. J Vision 5: 493–503, 2005. [DOI] [PubMed] [Google Scholar]
- Heinen and Liu 1997.Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis Neurosci 14: 853–865, 1997. [DOI] [PubMed] [Google Scholar]
- Huerta and Kaas 1990.Huerta M, Kaas J. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299–330, 1990. [DOI] [PubMed] [Google Scholar]
- Kowler 1989.Kowler E Cognitive expectations, not habits, control anticipatory smooth oculomotor pursuit. Vis Res 29: 1049–1057, 1989. [DOI] [PubMed] [Google Scholar]
- Kowler et al. 1984.Kowler E, Martins AJ, Pavel M. The effect of expectations on slow oculomotor control. IV. Anticipatory smooth eye movements depend on prior target motions. Vision Res 24: 197–210, 1984. [DOI] [PubMed] [Google Scholar]
- Kowler and Steinman 1979.Kowler E, Steinman RM. The effect of expectations on slow oculomotor control. II. Single target displacements. Vis Res 19: 633–646, 1979. [DOI] [PubMed] [Google Scholar]
- Krauzlis and Lisberger 1994.Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comput Neurosci 1: 265–283, 1994. [DOI] [PubMed] [Google Scholar]
- Krauzlis and Miles 1996.Krauzlis RJ, Miles FA. Transitions between pursuit eye movements and fixation in the monkey: dependence on context. J Neurophysiol 76: 1622–1638, 1996. [DOI] [PubMed] [Google Scholar]
- Lisberger 1998.Lisberger SG Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79: 1918–1930, 1998. [DOI] [PubMed] [Google Scholar]
- Madelain and Krauzlis 2003.Madelain L, Krauzlis RJ. Effects of learning on smooth pursuit during transient disappearance of a visual target. J Neurophysiol 90: 972–982, 2003. [DOI] [PubMed] [Google Scholar]
- Mauchly 1940.Mauchly JW Significance test for sphericity of a normal n-variate distribution. Ann Math Stat 11: 204–209, 1940. [Google Scholar]
- Missal and Heinen 2004.Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol 92: 1257–1262, 2004. [DOI] [PubMed] [Google Scholar]
- Mitrani and Dimitrov 1978.Mitrani L, Dimitrov G. Pursuit eye movements of a disappearing moving target. Vis Res 18: 537–539, 1978. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry et al. 2006.Orban de Xivry JJ, Bennett SJ, Lefevre PP, Barnes GR. Evidence for synergy between saccades and smooth pursuit during transient target disappearance. J Neurophysiol 95: 418–427, 2006. [DOI] [PubMed] [Google Scholar]
- Pola and Wyatt 1997.Pola J, Wyatt HJ. Offset dynamics of human smooth pursuit eye movements: effects of target presence and subject attention. Vision Res 39: 2767–2775, 1997. [DOI] [PubMed] [Google Scholar]
- Rashbass 1961.Rashbass C The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson et al. 1986.Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern 55: 43–57, 1986. [DOI] [PubMed] [Google Scholar]
- Schmid et al. 2001.Schmid AM, Rees G, Frith C, Barnes GR. A fMRI study of anticipation and learning of smooth pursuit eye movements in humans. Neuroreport 12: 1409–1414, 2001. [DOI] [PubMed] [Google Scholar]
- Shapiro and Wilk 1965.Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika 52: 591–599, 1965. [Google Scholar]
- Spering et al. 2005.Spering M, Kerzel D, Braun DI, Hawken MJ, Gegenfurtner KR. Effects of contrast on smooth pursuit eye movements. J Vision 5: 455–465, 2005. [DOI] [PubMed] [Google Scholar]
- von Noorden and Mackensen 1962.von Noorden GK, Mackensen G. Pursuit movements of normal and amblyopic eyes - an electro-ophthalmographic study. I. Physiology of pursuit movements. Am J Ophthalmol 53: 325–336, 1962. [DOI] [PubMed] [Google Scholar]
- Yasui and Young 1975.Yasui S, Young LR. Perceived visual motion as effective stimulus to pursuit eye movement system. Science 190: 906–908, 1975. [DOI] [PubMed] [Google Scholar]