Abstract
Silencing-induced homeostatic plasticity is usually expressed as a change in the amplitude or the frequency of miniature postsynaptic currents. Here we report that, prolonged (∼24 h) silencing of mature (20–22 days in vitro) cultured hippocampal neurons using the voltage-gated sodium channel blocker tetrodotoxin (TTX) produced no effects on the amplitude or frequency of the miniature excitatory postsynaptic currents (mEPSCs). However, the silencing changed the intrinsic membrane properties of the neurons, resulting in an increased excitability and rate of action potentials firing upon TTX washout. Allowing neurons to recover in TTX-free recording solution for a short period of time after the silencing resulted in potentiation of mEPSC amplitudes. This form of activity-dependent potentiation is different from classical long-term potentiation, as similar potentiation was not seen in nonsilenced neurons treated with bicuculline to raise their spiking activity to the same level displayed by the silenced neurons during TTX washout. Also, the potentiation of mEPSC amplitudes after the recovery period was not affected by the N-methyl-d-aspartate receptor blocker d-2-amino-5-phosponopentanoic acid or by the calcium/calmodulin-dependent kinase II (CaMKII) inhibitor KN-62 but was abolished by the L-type calcium channel blocker nifedipine. We thus conclude that the potentiation of mEPSC amplitudes following brief recovery of spiking activity in chronically silenced neurons represents a novel form of metaplasticity that differs from the conventional models of homeostatic synaptic plasticity.
INTRODUCTION
Neurons display homeostatic plasticity in response to changes in their environment, often resulting in altered spiking activity. This type of plasticity is aimed at maintaining a uniform level of synaptic activity in the network in the face of recurring changes in excitability or synaptic drive. To maintain a constant output in the face of an everchanging input, neurons will change their intrinsic membrane properties or the strength of their synaptic connections (Desai 2003).
Most frequently, neurons adjust their synaptic strength in response to experimentally induced changes of spiking activity by way of changing the amplitude or frequency of miniature excitatory postsynaptic currents (mEPSCs). Some of the earliest examples of homeostatic synaptic plasticity were found in peripheral neurons—in preparations of the neuromuscular junction and neuronal cultures from the spinal cord (Davis and Goodman 1998; Petersen et al. 1997; Sandrock et al. 1997). Recently, homeostatic synaptic plasticity was also demonstrated in neurons of the CNS. In developing neurons cultured from the sensory visual cortex, amplitudes of mEPSCs scaled up or down in response to chronic changes in neuronal firing (Turrigiano et al. 1998). This scaling of mEPSC amplitudes was linked to changes in glutamate receptor expression at the synapses (Turrigiano et al. 1998; Wierenga et al. 2005). In contrast to such postsynaptic origin of the homeostatic plasticity, in cultured hippocampal neurons, a block of spontaneous action potential firing with TTX produced a presynaptic change. In these neurons, silencing with TTX increased the readily releasable pool of neurotransmitter and the probability of quantal release (Murthy et al. 2001). Although not demonstrated by the study, these presynaptic changes should have been associated with an upregulation of neuronal synaptic strength. It was later established that presynaptic changes induced by silencing is a property of mature, rather than developing, neurons in culture. In mature visual cortical cultured neurons, in contrast to the developing neurons from the same preparation, silencing produced robust presynaptic effect, whereas the effect on mEPSC amplitudes was diminished (Wierenga et al. 2006). More recently, homeostatic plasticity induced by silencing action potentials has also been shown to exist in vivo (Echegoyen et al. 2007).
In an effort to study the mechanisms of homeostatic plasticity, we attempted to reproduce the effect of prolonged silencing in mature mouse embryonic hippocampal cultures. We report here that in dissociated mouse hippocampal cultures, prolonged (∼24 h) silencing with TTX produced no effect on mEPSC frequency or amplitude. However, silencing induced an enhancement of neuronal excitability that, when unmasked by washout of TTX, resulted in an increased level of neuronal spiking activity. Allowing for a short period of activity after a 1-day silencing resulted in the potentiation of mEPSC amplitudes without an effect on mEPSC frequency. This type of plasticity can be described as a form of metaplasticity, i.e., an altered potential form of plasticity, rather than homeostatic plasticity.
METHODS
Neuronal culture
Mouse hippocampal embryonic cultures were prepared as described (Li et al. 1998). Briefly, hippocampi isolated from C57 mouse embryos at days 17–18 of gestation (E17-18) were treated with papain (20 mM) for 15 min at 36°C and then dissociated by trituration and plated on glass coverslips precoated with poly-d-lysine (0.5 mg/ml). Cells for mEPSC were plated at densities of 400 cells/mm2. Cultures were maintained at 36°C in a 5% CO2 incubator and were grown in neurobasal cell culture medium (Gibco) supplemented with B27 (a complex of vitamins and neurotrophic factors; Gibco), 0.5 mM each glutamax cell culture medium supplement (Gibco) and glutamine, and 5% horse serum. All components of the culture medium were purchased from Invitrogen. As glia formed a monolayer 11 days after plating, an anti-mitotic agent 1-β-d-arabinofuranosylcytosine (Sigma) was added to the medium at 0.05% final concentration to inhibit further glial growth. Half of the medium in the dish was then substituted by fresh serum-free medium once every week. Cultures used for mEPSC recordings were held for 21–22 days in vitro (DIV) unless otherwise indicated.
Electrophysiology
A glass coverslip with cultured neurons was placed into a perfusion chamber with a solution volume of 0.2–0.5 ml. Perfusion rate was set at 0.5–1 ml/min. All experiments were done at room temperature (21–23°C). The following is a composition of the bathing solution used in experiments (in mM) 145 NaCl, 5 KCl, 1.5 MgCl, 2 CaCl2, 5 HEPES, and 10 glucose, pH 7.4. For mEPSC recordings, the bath was supplemented with 0.5 μM TTX. Cells were voltage clamped at –60 mV using an Axopatch 2B amplifier. The contribution of inhibitory currents during recordings of the TTX-resistant spontaneous activity was negligible because the –60-mV membrane potential (without liquid junction potential compensation) was close to the reversal potential for chloride. In addition, picrotoxin (40 μM) did not significantly change the frequency of TTX-resistant spontaneous currents. Pipette solution for the whole cell experiments contained (in mM) 150 potassium gluconate, 5 NaCl, 0.1 CaCl2, 2 MgATP, 0.5 Na2GTP, 5 EGTA, and 5 HEPES, pH 7.2. Access resistance in the whole cell experiments was in the range of 10–18 MΩ. Access resistance did not change >15% in during the recordings.
Recordings in mEPSC experiments were filtered at 2 kHz and digitized at 10 kHz. mEPSCs were analyzed using a custom-program written in LabView. Asymmetric events with the rise time shorter than the decay time and amplitudes >4 pA (the threshold for event detection) were chosen for the analysis. The frequency and amplitudes of mEPSC were analyzed in 40- to 90-s-long segments. Extending the analysis for longer periods in a number of experiments did not influence the results. In recordings of spontaneous activity, each recorded neuron was first current-clamped at 0 pA. Then a small positive or negative current was injected to maintain its membrane potential at –60 mV. In a number of silenced neurons, relatively long periods of low spiking activity during the recovery period were interrupted by brief periods of high-frequency spiking bursts. To avoid an error in evaluating firing rate in cultures recovering after silencing, only records >8 min were included in the analysis. The average recording period analyzed to quantify the level of spontaneous firing in neurons during the recovery period after silencing was 10 ± 0.5 min. Firing rate in each record was calculated by dividing the total number of action potentials fired by neuron during recording by the duration of the recording. Firing rate in nonsilenced neurons was calculated by analyzing records of 60- to 90-s duration. The input resistance of neurons voltage-clamped at −60 mV was assessed by measuring the steady-state current produced by 100-ms-long, 5-mV voltage steps. Cultures were silenced with 1–2 μM TTX for 22–26 h prior to the experiments. Silenced cultures were exposed (for 20–25 min at room temperature) to the external solution containing either 0.5 μM TTX (no recovery) or no TTX (recovery). Immediately after this period, recordings of mEPSCs were done in external solution containing 0.5 μM TTX. Drug effects on the mEPSC potentiation after recovery of silenced neurons were assessed by first exposing the cells to the drugs in the culture medium shortly before the experiment [d-2-amino-5-phosponopentanoic acid (d-AP5) and nifedipine –for 10 min, KN-62 –for 30 min].
Statistics
In each experiment, mEPSC amplitudes were compared within neurons in different dishes that belonged to the same batch of culture (neurons obtained from the same dissociation). Data are presented as means ± SE. Results were assessed using two-way ANOVA and paired or unpaired t-test (significance was set at P < 0.05 for each test). Each group included in the analysis contained at least four neurons.
RESULTS
Developmental changes of mEPSC amplitudes and frequencies
The outcome of experiments on homeostatic changes in cultured neurons depends on the level of neuronal development and their maturity (Wierenga et al. 2006). To characterize cultures with respect to their maturity, we followed developmental changes in mEPSC amplitudes and frequency as a function of DIV. The youngest cultures used for recordings were at 14 DIV, ∼3 days after the antimitotic drug was used to stop glial proliferation. Formation of a glial monolayer in neuronal cultures is a prerequisite for synapse formation and maturation because glial cells are known to release a number of growth and trophic factors critical for neuronal development (Freeman 2006).
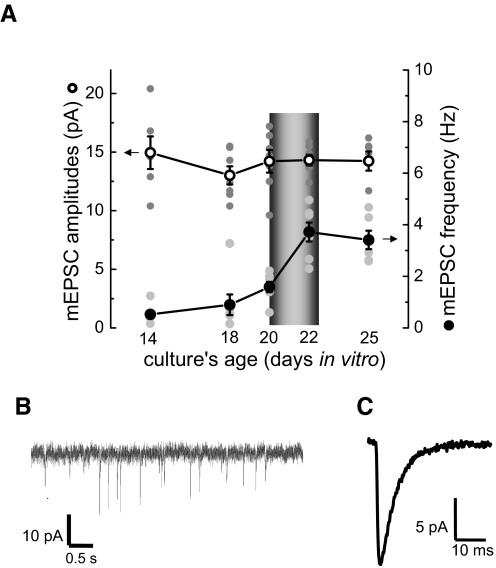
As shown in Fig. 1A, mEPSC amplitudes did not significantly change during neuronal development in culture. In contrast, mEPSC frequency increased from the low level of 0.53 ± 0.15 Hz at 14 DIV to 1.59 ± 0.20 Hz at 20 DIV. At about this time, when the increase of mEPSC frequency accelerated, neurons also started displaying moderate levels of spontaneous action potential firing during experiments (0.02 ± 0.008 Hz, 2 batches at 20 DIV, n = 7). All experiments demonstrating potentiation of mEPSC amplitudes were performed in cultures at 21–22 DIV, soon after they acquired spontaneous activity. An example of the membrane current recordings and an averaged mEPSC of a representative recording are in Fig. 1, B and C.
FIG. 1.
Changes in miniature excitatory postsynaptic current (mEPSC) amplitudes and frequency in neuronal cultures during development. A: mEPSC amplitudes did not change significantly during the period between 14 and 25 days in vitro (DIV). In contrast, mEPSC frequency steadily increased until ∼18 DIV. After 18 DIV, the increase in mEPSC frequency accelerated. Unless otherwise noted, all recordings in this study were obtained in cultures at 21–22 DIV ( ).
).  , raw data; ○, the average mEPSC amplitudes; •, the average mEPSC frequency. B: an example of membrane current recordings used for mEPSC analysis. C: averaged mEPSC (obtained from 97 individual mEPSCs) of a representative recording.
, raw data; ○, the average mEPSC amplitudes; •, the average mEPSC frequency. B: an example of membrane current recordings used for mEPSC analysis. C: averaged mEPSC (obtained from 97 individual mEPSCs) of a representative recording.
Prolonged silencing does not affect mEPSC amplitudes or frequency
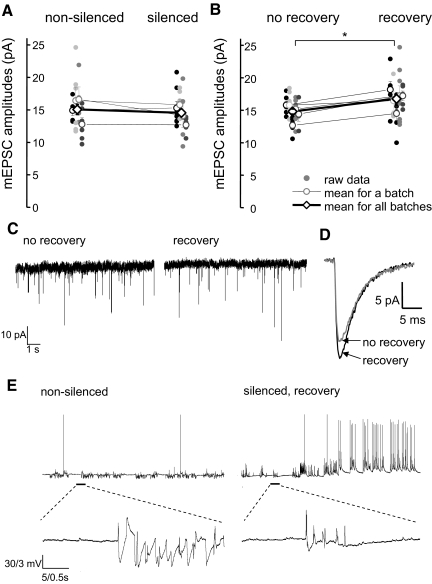
Cultures were silenced for 22–26 h by incubation in a culture medium supplemented with TTX (2 μM). After this period of silencing, just prior to recording of mEPSCs, cultures were washed with a recording solution (see methods) containing 0.5 μM TTX. mEPSC frequency and amplitudes in silenced cultures were compared with those recorded in control, nonsilenced, sister cultures. To ensure reproducibility, experiments were repeated in several batches of neurons. In four batches, recordings of mEPSCs 20–60 min after the 24 h silencing period did not reveal any change in either mEPSC frequency or amplitudes as a result of silencing. mEPSC frequency in silenced neurons was 97 ± 40% of that in nonsilenced neurons (P > 0.05, 2-way ANOVA, 4 batches, n = 4–8 neurons in each batch). mEPSC amplitudes, averaged over four batches in control and silenced cultures were 15.1 ± 0.8 and 14.5 ± 0.6 pA, respectively (P > 0.05, 2-way ANOVA, n = 4–8 neurons in each batch, Fig. 2A).
FIG. 2.
Effect of silencing and recovery after silencing on mEPSC amplitudes A: silencing cultures with TTX (2 μM) for 24 h did not affect the amplitudes of mEPSCs. mEPSC amplitudes in control nonsilenced cultures were not significantly different from those recorded in neurons of silenced cultures. B: mEPSC amplitudes in silenced cultures allowed to recover in a TTX-free solution just before recordings were significantly higher than in silenced cultures without recovery. *, significance in the statistical data analysis (2-way ANOVA, P < 0.05). In A and B, raw data are presented as small circles. The average values for each batch are presented as open circles. The average mEPSC amplitudes across all batches are presented as open diamonds. C: representative traces from experiments demonstrating mEPSC potentiation in silenced neurons after recovery in the TTX-free recording solution. D: averaged mEPSCs of representative records from silenced cultures with no recovery (gray) and after recovery (black) from 1 of the batches of neurons presented in B. The gray trace is an average of 78 individual mEPSCs recorded in a silenced neuron with no recovery. The black trace is an average of 76 individual mEPSCs recorded in a silenced neuron after recovery. E: representative traces of whole cell current-clamp recordings demonstrating neuronal spiking activity in nonsilenced neurons (left) and silenced neurons during the recovery period (right). Silenced neurons displayed increased level of spiking activity during the recovery period. On average, the frequency of action potential firing in silenced neurons during the recovery period was significantly higher than that of nonsilenced neurons (see text for details). The lower traces show on an expanded timescale the synaptic activity [excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs)] resulting from the overall activity of the neurons in the cultures. The flurry of postsynaptic potentials are interspersed with periods of steady membrane potential reflecting periods of relatively low neuronal inactivity.
Unlike mEPSCs, the average input resistance of silenced neurons was significantly higher than that of control, nonsilenced, neurons (208 ± 17 MΩ, n = 20, vs. 171 ± 10 MΩ, n = 16, in silenced and nonsilenced neurons, respectively, P < 0.05, t-test).
Recovery of activity potentiates mEPSC amplitudes in silenced neurons
In this set of experiments, we recorded mEPSCs in silenced neurons after washing them with a recording solution that did not contain any TTX. Such washing of culture dishes to eliminate components of the culture medium is a standard procedure commonly performed before electrophysiological recordings (MacDonald et al. 1989). Besides eliminating components of the culture medium, washing with the TTX-free bathing solution might allow neurons to recover their spiking activity. We tested whether washing and incubation of silenced cultures in the TTX-free bathing solution before the recordings affected mEPSC frequencies or amplitudes.
Cultures were incubated in the culture medium containing TTX (2 μM) for 24 ± 2 h. Following removal from the incubator, cultures were rinsed several times with a TTX-free bathing solution and then allowed to recover in the same solutions for 20–25 min at room temperature. Following this recovery from silencing, the TTX-free recording solution was changed to the one containing 0.5 μM TTX and the recordings of mEPSC commenced. mEPSC amplitudes in neurons recovered after silencing were significantly larger than those in “sister” neurons without recovery of activity, i.e., when a similar washing period was carried out in 0.5 μM TTX containing solution. On average, mEPSC amplitudes were potentiated by 16 ± 3% in neurons allowed to recover compared with nonrecovered neurons (17.1 ± 1 vs. 14.7 ± 1.2 pA, P < 0.05, 2-way ANOVA, 7 batches, n = 4–8 neurons in each group, Fig. 2B). mEPSC frequency was not changed during the recovery of activity. (mEPSC frequency in silenced neurons recovered after silencing was 99 ± 16% of that in nonrecovered silenced neurons). Representative traces of mEPSC recordings in silenced neurons before and after recovery of activity, and an averaged mEPSC of these recordings are presented in Fig. 2, C and D.
Potentiation of mEPSC amplitudes after recovery was not reproducible in silenced cultures <20 DIV. In one set of experiments on cultures at 18 DIV, mEPSC amplitudes in neurons with or without recovery were not significantly different. The average mEPSC amplitude in silenced neurons after incubation in a TTX-free recording solution was 99 ± 13% of the average amplitude recorded in neurons incubated in the TTX-containing solution (12.7 ± 1.0 and 12.8 ± 0.7 pA, respectively, P > 0.05, t-test, n = 4 neurons/group). mEPSC frequency in these cultures was 1 ± 0.13 Hz. In our experience, cultures with such low frequency of mEPSCs do not display spontaneous activity during the recovery part of the experiment.
Mature silenced neurons display increased spontaneous firing during recovery
It is possible that potentiation of mEPSC amplitudes in silenced cultures was activity-dependent and resulted from an increased firing rate of neurons during the recovery period. To address this possibility, we measured the firing rates of silenced and nonsilenced neurons in current-clamp recordings. Action potential firing rates of silenced neurons during the recovery period were significantly higher than the basal firing rates observed in control, nonsilenced, neurons (0.49 ± 0.1 Hz, n = 5, vs. 0.007 ± 0.005 Hz, n = 6, respectively, P < 0.05, t-test, 2 batches of neurons). Representative traces of spontaneous neuronal activity in control cultures and cultures recovering after silencing are shown in Fig. 2D.
Further enhancement of neuronal activity by bicuculline during recovery increases mEPSC potentiation
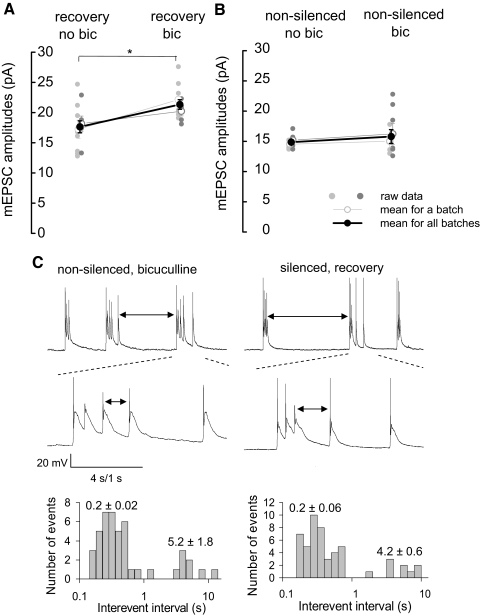
Because mEPSC amplitudes were not potentiated in younger cultures that normally lack spontaneous activity, we reasoned that enhancement of spontaneous activity during the recovery period would strengthen mEPSC potentiation. To facilitate neuronal spiking during the recovery period, cultures were disinhibited with a GABAA receptor blocker bicuculline (20 μM). mEPSC amplitudes in silenced cultures after the recovery period in the presence of bicuculline were compared with those in sister cultures recovered without the drug. mEPSC amplitudes in cultures recovered in bicuculline were significantly higher (21 ± 4%) than those recovered without the GABAA receptor blocker (21.3 ± 1 vs. 17.6 ± 0.8 pA, 2 batches, n = 5–8 neurons/batch, P < 0.05, 2-way ANOVA, Fig. 3A).
FIG. 3.
Similar bursting has different outcome on mEPSC potentiation in silenced and nonsilenced neurons. A: mEPSC potentiation in silenced cultures following a 20-min recovery of activity in 20 μM bicuculline-containing bathing solution was more pronounced than that in bicuculline-free solution. mEPSC amplitudes were significantly higher (21 ± 4%) in cultures recovering in the presence of bicuculline than those without. *, significance in the statistical data analysis (2-way ANOVA, P < 0.05). B: disinhibition of control (nonsilenced) neurons with bicuculline (20 μM) for 20- 25 min did not significantly change mEPSC amplitudes. In A and B, data from 2 batches of neurons are presented (small gray circles). The average for each batch is presented as open circles. The average mEPSC amplitudes for the 2 batches of cultures are presented as black circles (n = 4–8 neurons/batch). C, top: representative traces of membrane potential recordings in nonsilenced neurons disinhibited by bicuculline (left) and in silenced neurons during the recovery period (right). In both groups of neurons, firing of action potentials occurred in bursts with similar patterns. ↓, interburst intervals. Middle: expanded view of the burst of action potentials of nonsilenced neurons disinhibited by bicuculline (left) and in silenced neurons during the recovery period (right). ↓, intraburst intervals. Bottom: histograms of log-binned (10 bins/decade) action potential interspike intervals in 2 recordings. Values represent the average intraburst interspike intervals (0.2 ± 0.02 and 0.2 ± 0.06 s for nonsilenced and silenced neurons, respectively), and the average interburst intervals (5.2 ± 1.8 and 4.6 ± 0.6 s for nonsilenced neurons and silenced neurons, respectively).
Interestingly, control, nonsilenced cultures exposed to bicuculline for the same period of time as those silenced (20–25 min), did not display any potentiation of mEPSC amplitudes as a result of this treatment. mEPSC amplitudes in control cultures and those stimulated by bicuculline were 14.9 ± 0.4 and 15.8 ± 1.1 pA, respectively (2 batches, n = 4–7 neurons/batch, P > 0.05, 2-way ANOVA, Fig. 3B).
The reason for the lack of mEPSC potentiation in nonslicenced neurons stimulated by bicuculline might have been the lower rates of spiking in the presence of the drug than those observed in silenced cultures during the recovery period. However, analysis of the action potential firing rates during recovery in silenced cultures and control cultures disinhibited by bicuculline revealed no difference in frequencies of action potential firing (0.59 ± 0.07 vs. 0.72 ± 0.17 Hz, respectively, 2 batches, n = 5–7/group, P > 0.05, t-test, Fig. 3C).
Different patterns of stimulation used in LTP experiments might have differential effects on neuronal synaptic strength (Adesnik and Nicoll 2007;Plant et al. 2006). Similarly, differing patterns of action potential firing by silenced and nonsilenced neurons in our experiments, in spite of the same average firing frequency, might produce differential effect on their synaptic strength. Therefore in addition to firing frequencies, we also analyzed the firing pattern of the two groups of cells—neurons recovering after prolonged silencing and nonsilenced neurons stimulated by bicuculline. Analysis of firing patterns of the two groups of neurons demonstrated that, in both groups, neurons were firing in bursts with a similar average number of spikes per burst (5.6 ± 1.2 and 5.1 ± 0.8 in silenced neurons during recovery and in nonsilenced neurons in bicuculline, respectively, P > 0.05, t-test, n = 5 neurons/group) and a similar firing frequency during the burst (6.1 ± 0.7 and 7.3 ± 1.4 Hz in silenced neurons during recovery and in nonsilenced neurons in bicuculline, respectively, P > 0.05, t-test, n = 5 neurons/group). Thus similar action potential firing rates and patterns of firing have markedly different effects on mEPSC amplitudes in silenced and control cultures.
Quantitative analysis of the firing patterns in the two groups of neurons is shown in Fig. 3C, bottom, as histograms of log-binned action potential interspike intervals. The histograms reflect the presence of the bursts of action potential firing in the two groups of neurons with similar intraburst interspike intervals (0.2 ± 0.02 and 0.2 ± 0.06 s for nonsilenced and silenced neurons, respectively). The periodicity of the bursts of action potential firing was also similar in silenced neurons during recovery and in nonsilenced neurons disinhibited by bicuculline. The average interburst intervals in the two groups of neurons was not significantly different (5.2 ± 1.8 and 4.6 ± 0.6 s for nonsilenced neurons in bicuculline and silenced neurons during recovery, respectively, t-test, P > 0.05).
Potentiation after silencing does not require activation of NMDA receptors or CaMKII
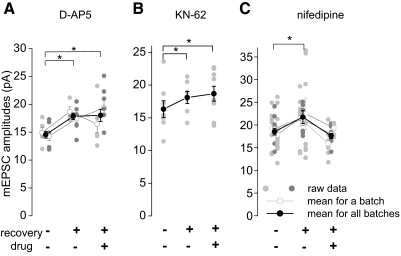
Activation of NMDA receptors, the subsequent Ca2+ entry, and the recruitment of CaMKII are a common triad of activity-induced plasticity (Bliss and Collingridge 1993). We therefore wanted to know whether these key factors of neuronal plasticity also participate in the potentiation of mEPSC amplitudes after recovery of silenced cultures. Block of NMDA receptors with d-AP5 (50 μM) during the recovery after silencing did not abolish mEPSC potentiation. mEPSC amplitudes in neurons recovering in the presence of d-AP5 were still significantly larger than those in nonsilenced neurons (18 ± 1.1 and 14.6 ± 0.6 pA, respectively, P < 0.05, 2-way ANOVA, 2 batches, n = 4–8 neurons/group, Fig. 4A). The amount of potentiation of mEPSC amplitudes after recovery with or without d-AP5 was similar in the two experiments: 22 ± 12 and 25 ± 9%, respectively.
FIG. 4.
Effect of drugs on mEPSC potentiation. A: the specific N-methyl-d-aspartate receptor blocker d-2-amino-5-phosponopentanoic acid (50 μM) applied during the recovery period did not abolish mEPSC potentiation in silenced cultures. mEPSC amplitudes recovered with or without the drug were significantly higher than those in nonrecovered neurons. B: the inhibitor of CaMKII KN-62 (3 μM) applied during the recovery period did not abolish mEPSC potentiation in silenced cultures. mEPSC amplitudes in neurons recovered with or without the drug were significantly higher than those in nonrecovered neurons. C: L-type calcium channel inhibitor nifedipine (10 μM) applied during the recovery period effectively blocked mEPSC potentiation in silenced cultures. mEPSC amplitudes in neurons recovered in the presence of nifedipine were not significantly different from those in nonrecovered neurons. In A–C, data are presented as  . The average for each batch is presented as ○. The average mEPSC amplitudes across all batches are presented as •. *, significance in the statistical data analysis (2-way ANOVA or t-test, P < 0.05).
. The average for each batch is presented as ○. The average mEPSC amplitudes across all batches are presented as •. *, significance in the statistical data analysis (2-way ANOVA or t-test, P < 0.05).
Similar to d-AP5, the CaMKII blocker KN-62 (3 μM) did not abolish mEPSC potentiation during the recovery period. mEPSC amplitudes in cultures recovered after silencing with KN-62 (3 μM) were significantly larger than those in nonrecovered neurons [18.7 ± 1.1 pA (n = 9) and 16.3 ± 1.3 pA (n = 8), respectively, P < 0.05, t-test, Fig. 4B]. Interestingly, KN-62 potentiated mEPSC amplitudes in silenced neurons even without recovery, when applied in the presence of TTX 25–50 min prior to recording. mEPSC amplitudes measured in silenced neurons incubated for 20 min in the bathing solution containing both TTX (0.5 μM) and KN-62 (3 μM) were 20.3 ± 1.2 pA. These mEPSC amplitudes were significantly higher than those in silenced cultures incubated for the same period of time in TTX without KN-62 (16.3 ± 1.3 pA, n = 8 neurons/group, P < 0.05, t-test).
Activation of L-type calcium channels during the recovery period is required for mEPSC potentiation
Action potentials are known to facilitate Ca2+ entry into neurons through voltage-gated calcium channels. We aimed to establish whether increased neuronal firing and the subsequent calcium influx through L-type calcium channels during recovery of silenced neurons contributed to potentiation of mEPSC amplitudes. The presence of the specific L-type Ca2+ channel blocker nifedipine (10 μM) during the recovery period completely abolished mEPSC potentiation in silenced neurons (21.8 ± 1.4 and 17.6 ± 0.6 pA after recovery without nifedipine and in nifedipine, respectively, P < 0.05, 2-way ANOVA, 3 batches, n = 6–8 neurons/group, Fig. 4C). mEPSC amplitudes in neurons recovered in nifedipine were not significantly different from those in nonrecovered neurons (17.6 ± 0.6 and 18.5 ± 0.8 pA, respectively, P > 0.05, 2-way ANOVA, 3 batches, n = 6–13 neurons/group). Unlike KN-62, incubation of silenced cultures with nifedipine (10 μM) in the presence of TTX for 20–25 min did not change mEPSC amplitudes. The average amplitudes of mEPSCs in silenced neurons incubated for 20 min in nifedipine and TTX were 101 ± 9% of the mEPSC amplitudes in silenced neurons incubated in TTX without nifedipine (n = 5–6 neurons/group).
The lack of potentiation of mEPSC amplitudes in silenced neurons recovering in the presence of nifedipine could be due to a nonspecific, inhibitory, effect of the drug on the rate of action potential firing during recovery rather than by its specific inhibitory action on the L-type Ca2+ channels. To address this potentially confounding issue, we measured the rate of neuronal firing during the recovery after silencing in the presence of nifedipine. Remarkably, in silenced neurons during the recovery period, the rate of action potential firing in the presence of nifedipine was actually higher than that without the drug (0.90 ± 0.15 Hz, n = 6, vs. 0.20 ± 0.05 Hz, n = 8, respectively, P < 0.05, t-test), indicating that the effect of nifedipine did not result from a reduced action potential firing during the recovery period.
The summary of the results of experiments using d-AP5, KN-62 and nifedipine are shown in Fig. 4. These results are consistent with a Ca2+-dependent, but NMDA receptor and CaMKII-independent process of mEPSC potentiation.
DISCUSSION
We report here that mature embryonic dissociated hippocampal cultures of mice lack homeostatic plasticity in the form reported earlier for other central neurons. Specifically, silencing of cultures with TTX (2 μM) for 24 h did not produce potentiation of mEPSC amplitudes or frequencies. mEPSC amplitudes potentiated, however, if after prolonged silencing, cultures were allowed to fire action potentials for a short period of time, in a TTX-free recording solution. This effect of recovery of silenced neurons in a TTX-free solution was reproducible in mature cultures that displayed spontaneous firing activity during the period of recovery. Spontaneous action potential firing and the associated influx of calcium through the L-type calcium channels were critical for mEPSC potentiation.
In none of the four sets of experiments presented in Fig. 2A did we see potentiation of mEPSC amplitudes or frequency as a result of prolonged silencing. These results are in contrast to several reports on the effect of silencing on mEPSCs. Enhancement of mEPSC amplitudes as a result of silencing has been previously reported in such preparations as cultures dissociated from rat primary visual cortex and hippocampus, acute hippocampal slices, and in in vivo hippocampus (Echegoyen et al. 2007; Huupponen et al. 2007; Turrigiano et al. 1998; Wierenga et al. 2006). In our preparation, the lack of a similar effect of silencing could be explained by the difference in the maturity of neurons in our preparation compared with those used by other groups. Indeed the effect of silencing on mEPSCs may depend on the age of the culture. For example, in primary visual cortical cultures, increase of mEPSC amplitudes was less pronounced at 18 DIV or older cultures than it was at 7–9 DIV cultures (Wierenga et al. 2005, 2006). In acute hippocampal slices, developing granule cells displayed an increase of mEPSC amplitudes after 15–17 h silencing with TTX at postnatal day 4 (P4) but not at P8 (Huupponen et al. 2007). The dependence on the age of cultures is not ubiquitous, however, because in rat hippocampal cultures, silencing produced the same level of potentiation of mEPSC amplitudes in young and more mature neurons (Wierenga et al. 2006).
The results presented here were specific to cultures at 21–22 DIV. Age in vitro, however, may not be an exact determinant of the culture's state of development. Neuronal development and maturation are controlled by growth and trophic factors released by glia (Freeman 2006). Glial cells in culture proliferate into a monolayer which forms a bed for neuronal network. Formation of this glial monolayer is a critical step for neuronal development. The day on which formation of the glial monolayer is complete is marked by the use of the antimitotic drug supplemented to the culture medium to stop further glial proliferation. Thus the information on the time elapsed from the day the antimitotic drug was used to the day of the experiment might be more informative about the maturity of neurons than the total number of days the neurons spent in vitro. Cultures used for experiments presented here were at 21–22 DIV, 10–11 days after the antimitotic drug was supplemented to the culture medium.
Although cultures at 21–22 DIV did not display mEPSC potentiation as a result of activity deprivation, they developed another form of homeostatic plasticity. Prolonged silencing with TTX in these cultures produced an increase in neuronal input resistance. This effect could have resulted from a decrease in persistent potassium currents previously reported for primary cortical cultures as a result of prolonged silencing (Desai et al. 1999). Increase in the membrane input resistance might contribute to increased neuronal excitability and a subsequent enhancement of the rate of action potential firing that neurons displayed upon the TTX washout. A similar increase of neuronal spiking activity after silencing was reported in early studies on the effect of prolonged silencing (Ramakers et al. 1990; van den Pol et al. 1996). Increased excitability and action potential firing rate might also result from an upregulation of the voltage-dependent sodium currents as demonstrated in silenced hippocampal slices and cortical neuronal cultures (Aptowicz et al. 2004; Desai et al. 1999).
Spiking activity in neurons following TTX washout is, most probably, a critical factor in mEPSC potentiation induced in silenced neurons by brief incubation in TTX-free bathing solution. The same treatment of younger cultures, which do not display spontaneous activity at room temperature, failed to produce mEPSC potentiation. On the other hand, when activity in mature silenced cultures during the recovery period was facilitated by disinhibition with the GABAA receptor blocker bicuculline, mEPSC potentiation was even more pronounced. These experimental results attest to the role of spiking activity in mEPSC potentiation in silenced cultures following recovery of activity.
It is not clear from other published studies whether spiking activity was allowed to take place in neuronal cultures after prolonged silencing prior to mEPSC recordings. In studies using AMPA receptor blockers to silence neurons, however, neuronal spiking activity during the washout of AMPA receptor blockers before mEPSC recordings cannot be excluded as a factor contributing to potentiation of mEPSCs (Bacci et al. 2001; Buckby et al. 2006; Galvan et al. 2003; Liao et al. 1999; O'Brien et al. 1998; Thiagarajan et al. 2002, 2005). Remarkably, one of the reports pointed out that incubation of cultures in the bathing solution did take place after silencing prior to mEPSC recordings (Sutton and Schuman 2006). In this report, upregulation of mEPSC amplitudes after prolonged silencing was demonstrated in mature rat hippocampal cultured neurons. Similar artifactual effect of neuronal stimulation after prolonged silencing could have taken place in studies using fluorescent styryl dyes. In a number of such studies using FM1-43, it was demonstrated that prolonged silencing caused an upregulation of presynaptic vesicle pools (Murthy et al. 2001; Wierenga et al. 2006). Visualization of presynaptic vesicle pools using styryl dyes involves stimulation of neuronal activity by depolarization. Depolarization and the spiking associated with it together with a massive calcium influx after silencing could all have contributed to the effects reported as resulting from prolonged activity deprivation.
Our experiments begin to shed light on the mechanisms of mEPSC potentiation during recovery of activity after prolonged silencing. We tested whether spiking activity during recovery after silencing induces mEPSC potentiation through the same mechanisms as classical forms of activity-dependent LTP. Using the NMDA receptor blocker d-AP5, we demonstrated that in contrast to activity-dependent LTP in hippocampal pyramidal neurons, potentiation in silenced neurons was NMDA receptor-independent. Similar to d-AP5, a CaMKII inhibitor KN-62 failed to abolish mEPSC potentiation after silencing. Remarkably, KN-62 by itself upregulated mEPSC amplitudes when applied in the presence of TTX for 50 min at the end of the silencing period. This effect of KN-62 is similar to the effect of NMDA receptor blocker reported by Sutton et al. (2006). In this study, APV facilitated potentiation mEPSC amplitudes in hippocampal cultured neurons if it was applied during the last hour of the 24-h silencing period.
Independence of mEPSC potentiation on NMDA receptor activation indicates that potentiation in silenced neurons takes place through mechanisms unlike those responsible for LTP in hippocampal neurons. We presently favor the idea that the potentiation of mEPSC amplitudes after prolonged silencing is a form of metaplasticity (Abraham and Bear 1996) because control, nonsilenced, neurons were not potentiated by increasing spiking and bursting activity through disinhibition with bicuculline. In the presence of bicuculline, nonsilenced neurons displayed the same level and pattern of bursting activity as silenced neurons during the recovery period yet their mEPSCs were not potentiated. The differential effects of a similarly elevated spiking activity on mEPSC potentiation in silenced versus nonsilenced neurons is consistent with the idea that silencing changes hippocampal cultures to make them more susceptible to potentiation produced by spiking activity.
Such a change induced in neurons by silencing could be the accumulation of GluR1, an AMPA receptor subunit highly expressed in the hippocampus. Upregulation of GluR1 protein during silencing has been demonstrated in numerous studies (Buckby et al. 2006; Galvan et al. 2003; O'Brien et al. 1998; Watt et al. 2000; Wierenga et al. 2005). However, synaptic expression of GluR1 might require spiking activity and calcium influx associated with the spiking. Indeed using a single-molecule tracking approach, it has recently been demonstrated that, in the absence of activity, the proportion of time spent by individual GluR1 receptors at synaptic sites was significantly lower than that at active synapses. Synaptic activity induced accumulation of GluR1 at active synapses by reducing diffusional exchange of GluR1 between synaptic and extrasynaptic domains (Ehlers et al. 2007). In another study, it was established that circuit reactivation after silencing with TTX induced colocalization of GluR1- and NR2B-specific staining at previously silent synapses (Nakayama et al. 2005).
Although the exact mechanism of mEPSC potentiation after prolonged silencing remains to be determined, we have demonstrated that the process induced by reactivation of silenced neurons was blocked by nifedipine applied during the recovery period. In view of the putative role of GluR1 in mEPSC potentiation, it is possible that calcium influx through the L-type calcium channels during spiking activity is required for synaptic targeting of GluR1 accumulated during silencing.
In summary, the results presented here demonstrate that prolonged silencing changed mature hippocampal cultured neurons in a way that makes them more susceptible to activity-induced potentiation than control (nonsilenced) neurons. The potentiation of mEPSC amplitudes is only observed if a short period of action potential firing is allowed to permit calcium entry through L-type calcium channels into the neurons.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant NS-35985 and the Coelho Endowment to I. Mody.
Acknowledgments
We are thankful to M. Lazaro for providing timed pregnant mice and to B. Huang for help in preparing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abraham and Bear 1996.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996. [DOI] [PubMed] [Google Scholar]
- Adesnik and Nicoll 2007.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptowicz et al. 2004.Aptowicz CO, Kunkler PE, Kraig RP. Homeostatic plasticity in hippocampal slice cultures involves changes in voltage-gated Na+ channel expression. Brain Res 998: 155–163, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci et al. 2001.Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated membrane protein. J Neurosci 21: 6588–6596, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss and Collingridge 1993.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. [DOI] [PubMed] [Google Scholar]
- Buckby et al. 2006.Buckby LE, Jensen TP, Smith PJ, Empson RM. Network stability through homeostatic scaling of excitatory and inhibitory synapses following inactivity in CA3 of rat organotypic hippocampal slice cultures. Mol Cell Neurosci 31: 805–816, 2006. [DOI] [PubMed] [Google Scholar]
- Davis and Goodman 1998.Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature 392: 82–86, 1998. [DOI] [PubMed] [Google Scholar]
- Desai 2003.Desai NS Homeostatic plasticity in the CNS: synaptic and intrinsic forms. J Physiol 97: 391–402, 2003. [DOI] [PubMed] [Google Scholar]
- Desai et al. 1999.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999. [DOI] [PubMed] [Google Scholar]
- Echegoyen et al. 2007.Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age dependence. PLoS ONE 2: e700, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers et al. 2007.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54: 447–460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman 2006.Freeman MR Sculpting the nervous system: glial control of neuronal development. Curr Opin Neurobiol 16: 119–125, 2006. [DOI] [PubMed] [Google Scholar]
- Galvan et al. 2003.Galvan CD, Wenzel JH, Dineley KT, Lam TT, Schwartzkroin PA, Sweatt JD, Swann JW. Postsynaptic contributions to hippocampal network hyperexcitability induced by chronic activity blockade in vivo. Eur J Neurosci 18: 1861–1872, 2003. [DOI] [PubMed] [Google Scholar]
- Huupponen et al. 2007.Huupponen J, Molchanova SM, Taira T, Lauri SE. Susceptibility for homeostatic plasticity is down-regulated in parallel with maturation of the rat hippocampal synaptic circuitry. J Physiol 581: 505–514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. 1999.Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci 2: 37–43, 1999. [DOI] [PubMed] [Google Scholar]
- MacDonald et al. 1989.MacDonald JF, Mody I, Salter MW. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol 414: 17–34, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy et al. 2001.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32: 673–682, 2001. [DOI] [PubMed] [Google Scholar]
- Nakayama et al. 2005.Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci 25: 4040–4051, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien et al. 1998.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21: 1067–1078, 1998. [DOI] [PubMed] [Google Scholar]
- Petersen et al. 1997.Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19: 1237–1248, 1997. [DOI] [PubMed] [Google Scholar]
- Plant et al. 2006.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9: 602–604, 2006. [DOI] [PubMed] [Google Scholar]
- Ramakers et al. 1990.Ramakers GJ, Corner MA, Habets AM. Development in the absence of spontaneous bioelectric activity results in increased stereotyped burst firing in cultures of dissociated cerebral cortex. Exp Brain Res 79: 157–166, 1990. [DOI] [PubMed] [Google Scholar]
- Sandrock et al. 1997.Sandrock AW, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science 276: 599–603, 1997. [DOI] [PubMed] [Google Scholar]
- Sutton et al. 2006.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006. [DOI] [PubMed] [Google Scholar]
- Sutton and Schuman 2006.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127: 49–58, 2006. [DOI] [PubMed] [Google Scholar]
- Thiagarajan et al. 2005.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47: 725–737, 2005. [DOI] [PubMed] [Google Scholar]
- Thiagarajan et al. 2002.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron 36: 1103–1114, 2002. [DOI] [PubMed] [Google Scholar]
- Turrigiano et al. 1998.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998. [DOI] [PubMed] [Google Scholar]
- van den Pol et al. 1996.van den Pol AN, Obrietan K, Belousov A. Glutamate hyperexcitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture. Neuroscience 74: 653–674, 1996. [DOI] [PubMed] [Google Scholar]
- Watt et al. 2000.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26: 659–670, 2000. [DOI] [PubMed] [Google Scholar]
- Wierenga et al. 2005.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci 25: 2895–2905, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga et al. 2006.Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol 96: 2127–2133, 2006. [DOI] [PubMed] [Google Scholar]