Abstract
A long-recognized, pathognomonic feature of human papillomavirus (HPV) infection is the appearance of halo or koilocytotic cells in the differentiated layers of the squamous epithelium. These koilocytes are squamous epithelial cells that contain an acentric, hyperchromatic nucleus that is displaced by a large perinuclear vacuole. However, the genesis of the cytoplasmic vacuole has remained unclear, particularly because both HPV DNA replication and virion assembly occur exclusively in the nucleus. In clinical biopsies, koilocytosis is observed in both low- and high-risk HPV infections; therefore, in this study, we demonstrated that the E5 and E6 proteins from both low- and high-risk HPVs cooperate to induce koilocyte formation in human cervical cells in vitro, using both stable and transient assays. Both E5 and E6 also induce koilocytosis in human foreskin keratinocytes but not in primate COS cells. Deletion of the 20 C-terminal amino acids of E5 completely abrogates koilocytosis, whereas a 10-amino acid-deletion mutant retains ∼50% of its activity. Because the E6 protein from both the low- and high-risk HPVs is capable of potentiating koilocytosis with E5, it is apparent that the targeting of both p53 and PDZ proteins by E6 is not involved. Our data suggest new, cooperative functions for both the E5 and E6 proteins, hinting at additional targets and roles for these oncoproteins in the viral life cycle.
Epidemiological studies have shown that human papillomavirus (HPV) infection is the most common sexually transmitted disease, with nearly 80% of the female population being infected during their lifetime. More than 120 different HPV types have been isolated, of which more than 40 infect the genital mucosa.1 Some of these genital HPVs are low risk (eg, HPV-6 and -11) and are associated mainly with nonmalignant lesions. However, there are 12 to 15 high-risk HPVs that play a causative role in the development of cervical cancer and represent a major health threat worldwide.1,2,3,4 HPV-16 alone accounts for approximately half of all cervical cancers.3,5,6
Three HPV-16 genes possess transforming activity: E6, E7, and E5. The HPV-16 E6 gene encodes a 151-amino acid protein (16E6) that binds to the tumor suppressor protein, p53, and facilitates its degradation via ubiquitin-dependent pathways. This, together with inhibition of the pro-apoptotic protein, BAK, mediates resistance to apoptosis and chromosomal instability. 16E6 also transactivates the hTERT promoter, thereby increasing cellular telomerase activity and, in cooperation with the HPV-16 E7 protein (16E7), induces cellular immortalization.4,7,8 One of the primary functions of 16E7 is to inactivate the retinoblastoma tumor suppressor protein.7,8
In contrast to 16E6 and 16E7, biological functions of the HPV-16 E5 protein (16E5) are rather poorly understood.7 16E5 is a small (83 amino acids), hydrophobic protein that localizes to membranes of the endoplasmic reticulum.9,10 It exhibits weak transforming activity in vitro,7,11 but induces epithelial tumors in transgenic mice.12 Documented intracellular binding targets for 16E5 include the 16-kDa subunit of the vacuolar H+-ATPase,9,13 the heavy chain of HLA type I,14 and ErbB4, a member of the epidermal growth factor receptor family.15
A common feature of both high- and low-risk papillomavirus infection is the appearance of koilocytes in the differentiated layers of squamous epithelium. Koilocytes are epithelial cells that contain an acentric, hyperchromatic, moderately enlarged nucleus that is displaced by a large perinuclear vacuole.16 Although molecular methods are available for detecting papillomavirus infections of the female genital tract, the presence of koilocytes in Pap smears and cervical biopsies remains fundamental to pathological diagnosis.17 The viral genesis of koilocytes is somewhat enigmatic because most HPV proteins are localized in the nucleus where viral replication and assembly occur. In this article, we demonstrate that E5 from high-risk and low-risk HPV types cooperates with high- and low-risk E6 to induce koilocytosis in vitro.
Materials and Methods
Cells and Viruses
Retroviruses encoding 16E6 and 16E7 in the vector pBabePuro,18 or encoding codon-optimized 16E5 or a C-terminal deletion mutant of codon-optimized 16E5 lacking the last 10 amino acids [16E5 (−10)],19 16E6, HPV-6b E5 (6bE5), and HPV-6b E6 (6bE6) in the vector pLXSN, were generated using the Phoenix cell system.20 Both 16E5 and 6bE5 proteins were N-terminally tagged with the AU1 epitope, DTYRYI.21 AU1 epitope-tagged or untagged wild-type E6 constructs have been described before.22 To construct E6 mutants, overlapping polymerase chain reaction was used to replace cysteine 136 of both 6bE6 and 16E6 with glycine; primers containing the mutant codon are as follows: 6bE6C136G mutant, 5′-TGTACGTGGAAGGGTCGCGGCCTACACTGCTGGACAAC-3′ and 5′-GTTGTCCAGCAGTGTAGGCCGCGACCCTTCCACGTACA-3′; 16E6C136G mutant, 5′-GGTCGGTGGACCGGTCGAGGTATGTCTTGTTGCAGATC-3′ and 5′-GATCTGCAACAAGACATACCTCGACCGGTCCACCGACC-3′. All constructs were confirmed by sequencing.
Primary human ectocervical cells (HECs) were derived from cervical tissue after hysterectomy for benign uterine disease as described,23 and were immortalized by infection with a 16E6/16E7-encoding retrovirus and selection in the presence of puromycin (0.5 μg/ml). Stable 16E5-expressing cell lines were generated from immortalized HECs by infection with retroviruses encoding 16E5, 16E5 (−10) or the empty pLXSN expression vector, and selection in the presence of geneticin G418 (100 μg/ml). Nonimmortalized HECs expressing 16E6 or 16E7 alone were generated by infection with E6- or E7-encoding retroviruses and selection.
HEC lines and HECs were grown at 37°C and 5% CO2 in keratinocyte growth medium (Invitrogen, Carlsbad, CA), supplemented with gentamicin sulfate (10 μg/ml). To maintain constant levels of 16E5 and 16E5 (−10) expression, G418 was administered to cell cultures at alternating passages. Human foreskin keratinocytes (HFKs) were isolated from neonatal foreskins as described previously and cultured in keratinocyte growth medium.24 HFK cell strains were generated by infection with retroviruses encoding 16E6 and/or 16E7, or the empty pLXSN expression vector. COS cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate (Invitrogen).
Retroviral Infection
HECs and HEC lines, HFKs, and COS cells were grown as described in 25-cm2 or 75-cm2 tissue culture flasks. Before infection, the growth medium was aspirated and retroviruses mixed with polybrene (1 μl/ml) were added to the cells for 1.5 hours at 37°C while shaking. After infection, this mixture was removed and growth medium added to the cells.
Immunoprecipitation and Immunoblotting
AU1 epitope-tagged E5 proteins were detected in RIPA lysates by immunoprecipitation and Western blotting,25 using 4 μg of rabbit anti-AU1 polyclonal antibody for immunoprecipitation, and 1:5000-diluted rabbit anti-AU1 for immunoblotting (Covance, Princeton, NJ). Additional Western blots were labeled with 1:10,000-diluted anti-β-actin mouse monoclonal antibody (Sigma, St. Louis, MO), anti-p53 (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-pRb (1:1000 dilution; Cell Signaling, Beverly, MA), as described.26,27
Histological Staining
HECs and HFKs were grown on four-chamber microscope slides in keratinocyte growth medium, fixed in 4% (w/v) paraformaldehyde, and stained with hematoxylin and eosin (H&E) according to standard histological procedures. Alternatively, HECs were fixed, permeabilized in 0.1% (w/v) saponin, and blocked with 10% normal goat serum before staining with hematoxylin and labeling with Covance anti-AU1 mouse monoclonal antibody (1:1500 dilution of ascites) and goat anti-mouse IgG (peroxidase conjugate; DakoCytomation, Glostrup, Denmark). A BH-2 microscope (Olympus, Center Valley, PA) was used for visualization and microphotography, and an Axioskop microscope (Zeiss, Thornwood, NY) was used for counting cells.
Results
E5 Induces Koilocytosis
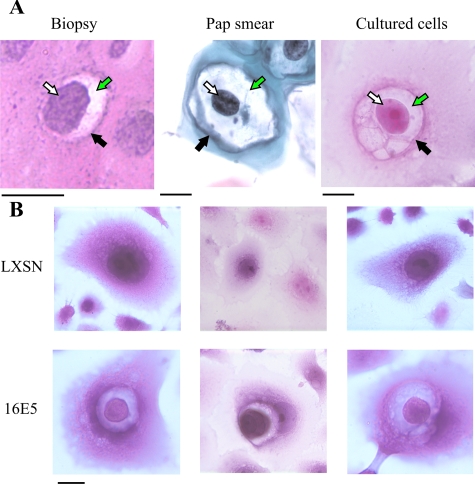
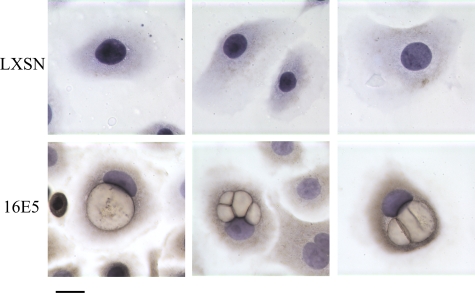
While investigating the effect of 16E5 on plasma membrane ganglioside content,28 we observed that a subpopulation of E5-expressing, immortalized HECs displayed the unique features of koilocytosis. As mentioned previously, koilocytosis is a diagnostic marker for papillomavirus infection and can be observed morphologically in both histological sections of HPV-infected cervix and in Pap smears. For example, in cervical biopsies, koilocytes can be detected in the differentiated layers of HPV-infected squamous epithelium (Figure 1A, left). In Pap smears, cytoplasmic vacuolization (Figure 1A, middle) is even more prominently displayed. We found morphologically similar, perinuclear vacuolization in cultured monolayers of 16E6/16E7-immortalized HECs expressing 16E5 (Figure 1A, right). This suggested that the phenomenon of koilocytosis might be associated with the expression of a specific subset of HPV-16 oncoproteins. We therefore fixed and stained 16E6/16E7-immortalized HEC lines (with and without the 16E5 gene) with H&E. Koilocytes were readily detected in the 16E5-expressing HEC lines compared to cells harboring the empty pLXSN expression vector (Figure 1B). Importantly, immunohistochemistry of these HEC lines (using an anti-AU1 antibody to detect the epitope-tagged E5 protein) revealed that 16E5 was present in the koilocytotic vacuolar membranes (Figure 2, bottom row). 16E5 was also noted in the cytoplasm, which we believe reflects its endoplasmic reticulum localization that is visualized more precisely by immunofluorescence microscopy.28
Figure 1.
Koilocytotic cells. A: The HPV cytopathic effect (koilocytosis) is shown in a cervical biopsy specimen (left, H&E staining), a cervical cytology preparation (middle, Pap stain), and a cultured monolayer of 16E6/16E7-immortalized HECs expressing 16E5 (right, H&E staining). Arrows show typical koilocyte features: an acentric, hyperchromatic, moderately enlarged nucleus (white arrow) displaced by a large perinuclear vacuole (green arrow), surrounded by a thickened cytoplasm (black arrow). B: H&E staining of 16E6/16E7-immortalized HEC lines demonstrates koilocytes in 16E5-expressing cells, but not in HECs containing the empty expression vector (LXSN). Scale bars = 10 μm.
Figure 2.
16E5 localizes to perinuclear vesicles in koilocytotic cells. HECs were fixed, stained with hematoxylin, and reacted with peroxidase-conjugated anti-AU1 antibody (brown). Scale bar = 10 μm.
E6 Cooperates with E5 to Induce Koilocytosis
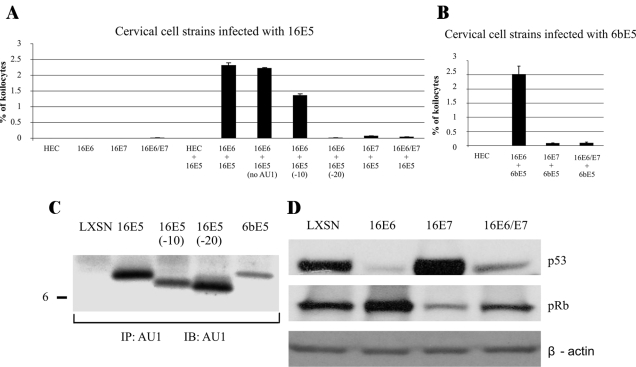
The above studies demonstrated that the stable expression of 16E5 induces koilocytosis in HECs immortalized with 16E6 and 16E7. Although E5 clearly is essential for this phenomenon, it was unclear whether 16E5 alone is sufficient to induce koilocytosis or whether there might be collaborative interactions with 16E6 and 16E7. To test this possibility, we developed an acute assay for koilocytosis in which various cervical cells are infected with retroviruses that encode wild-type (wt) or mutant 16E5 oncoproteins. As shown in Figure 3A, expression of HPV-16 E6, E7, or E5 alone was insufficient to generate koilocytes in HECs. In addition, the combined expression of 16E6 and 16E7 was ineffective; similar to the results obtained with immortalized pLXSN-HECs. However, co-expression of 16E5 and 16E6 resulted in levels of koilocytes that were nearly 10-fold higher than the 16E5/E6/E7 HEC line. It is also important to note that the AU1 epitope tag on 16E5 had no effect on its frequency of koilocyte formation. 16E5 and 16E5 (no AU1) induced very similar levels of koilocytosis (Figure 3A). This cooperative effect was not observed with 16E5 and 16E7. In fact, 16E7 dramatically suppressed koilocytosis when co-expressed with 16E5 and 16E6 (Figure 3A).
Figure 3.
Induction of koilocytosis by HPV E5 and E6 oncoproteins. Indicated HECs were transduced with HPV E5-encoding retroviruses and stained 4 days later (H&E) to determine the frequency of koilocytosis. Error bars represent SD. A: HECs transduced with 16E5 genes. B: HECs transduced with 6bE5 genes. C: E5 expression in 16E6-HECs 4 days after infection with E5-encoding retroviruses. Molecular mass marker (in kDa) is indicated on the left. IP, immunoprecipitation; IB, immunoblotting. D: Western blot showing 16E6 and 16E7 functionality in HECs. An identical immunoblot labeled with anti-β-actin confirms equal loading of protein in each lane.
Koilocytes were reduced by ∼50% in 16E6-HECs expressing a mutant 16E5 lacking the C-terminal 10 amino acids, 16E5 (−10). This mutant is also defective for endosome alkalinization19 and epidermal growth factor receptor activation.13 Importantly, deletion of the 20 C-terminal amino acids produced mutant 16E5 (−20) that was completely defective for koilocyte formation (Figure 3A). This suggests the existence of additional E5 targets that are separate from those regulating V-ATPase function and epidermal growth factor receptor signaling.
Because low-risk HPVs also induce koilocytosis in vivo,29 we used the acute transduction assay (with various HECs) to screen the biological activity of E5 from low-risk HPV-6b (6bE5). As with high-risk 16E5, we observed koilocytes when 6bE5 was co-expressed with 16E6 (Figure 3B). It should be noted that wild-type and mutant E5 proteins were expressed at similar levels in 16E6-HECs after acute retrovirus infection (Figure 3C). Thus, the decreased koilocyte-inducing activity of 16E5 (−10) and the complete defectiveness of 16E5 (−20) are not because of lower expression of these proteins but rather to altered activity. In addition, 16E6 and 16E7 are functional in these cells, because 16E6 degrades p53 and 16E7 triggers the loss of pRb (Figure 3D).
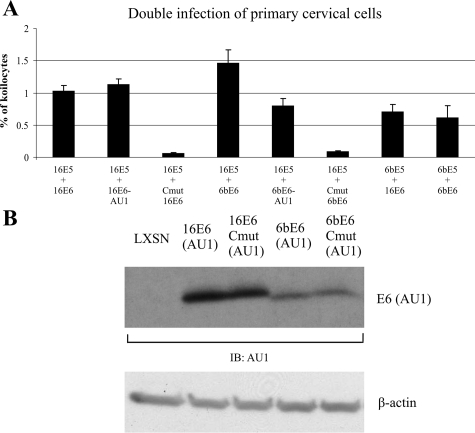
To evaluate the ability of various combinations of low- and high-risk oncoproteins to induce koilocytosis, we used a modified acute assay. Rather than infecting 16E6-HECs with different E5 proteins, we co-infected primary HECs with two different retroviruses (encoding either E5 or E6) and screened for koilocytes (Figure 4A). This modified assay generated koilocytosis with approximately half the efficiency of the previous assay but now permitted us to assay both E6 as well as E5 mutant proteins. Interestingly, all combinations of low- and high-risk E5 and E6 proteins collaborated to induce koilocytes, suggesting the retention of conserved functions in these variant and biologically distinct proteins. In addition, we showed that addition of the AU1 epitope to the HPV-16 E6 protein did not alter its ability to induce koilocytes (Figure 4A, compare lanes 1 and 2). Addition of the AU1 epitope to the HPV-6b E6 protein induced a slight (50%) decrease in its activity. We also demonstrated the requirement for E6 in this assay by measuring the activity of a mutant in which a conserved C-terminal cysteine residue (amino acid 136) was changed to glycine. Mutations at this site have been shown to abrogate many E6 activities, presumably because of gross alterations in protein conformation.30 In both the 6b and 16 E6 proteins, mutation of the terminal cysteine residue abrogated the induction of koilocytosis. To verify that the defective nature of the E6 cysteine mutants was not because of decreased protein expression, we examined the levels wild-type and mutant E6 proteins by Western blotting (Figure 4B). The cysteine mutants of 16E6 and 6bE6 were present at levels similar to the respective wild-type proteins (Figure 4B, compare lanes 2 with 3 and lanes 4 with 5).
Figure 4.
Acute induction of koilocytosis by HPV E5 and E6 oncoproteins in primary HECs. A: Primary HECs transduced with combinations of retroviruses encoding HPV-16 and HPV-6b E5 and E6. Error bars represent SD. B: E6 expression in primary HECs 4 days after infection with E6-encoding retroviruses. An identical immunoblot labeled with anti-β-actin confirms equal loading of protein in each lane.
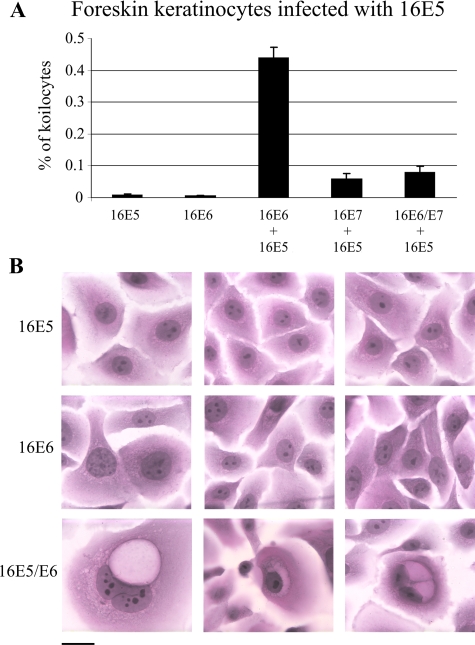
Although koilocytes are present in cervical HPV infections, they also are observed at other anatomical sites in different epithelial cell types. To investigate whether koilocytosis can be induced in another HPV target cell, the HFK, we transduced these cells with combinations of 16E6, 16E7 and empty pLXSN expression vector followed by 16E5 (Figure 5A). Koilocytosis was induced in the HFKs, but only when both 16E5 and 16E6 were present (Figure 5, A and B). As in HECs, 16E7 greatly suppressed the frequency of koilocytosis induced by E5 and E6. In contrast to HECs and HFKs, koilocytes did not form in COS cells (a primate kidney cell line) when 16E5 and 16E6 were co-expressed, indicating that not all cell lines provide suitable environments for koilocytosis.
Figure 5.
Induction of koilocytosis by HPV-16 E5 and E6 proteins in HFKs. Indicated HFKs were transduced with HPV-16 E5 and stained 4 days later (H&E) to determine the frequency of koilocytosis. A: HFKs transduced with HPV-16 E5. Error bars represent SD. B: H&E staining of HFKs demonstrates koilocytes in16E5- and 16E6-expressing cells, but not in HFKs expressing 16E5 or 16E6 alone. Scale bar = 10 μm.
Discussion
In contrast to the well-characterized E6 and E7 oncoproteins of HPV-16, biological activities of the 16E5 oncoprotein and its role in the HPV replication cycle are not well understood. The biological phenotypes of E5 are myriad and vary with the cell types used for assays. Briefly, E5 has been shown to alkalinize endosome pH,19 augment epidermal growth factor signaling pathways,31 alter membrane lipid composition,28 stimulate anchorage-independent growth,25 and interfere with gap junction communication.32 Similarly, there have been several cell proteins identified as targets for 16E5, including the V-ATPase,9,13 MHC complexes,14 and ErbB4.15 However, there is no single target that seems to explain the many properties ascribed to 16E5 and it is possible that E5 may have several cell targets for inducing its effects.
In the present study, we demonstrate that HPV E5 and E6 proteins cooperate to induce koilocytosis. Koilocytes are epithelial cells containing a hyperchromatic nucleus that is acentrically displaced by perinuclear vacuole(s),16 and these morphological alterations are used by pathologists to help identify HPV-infected epithelial cells in Pap smears. In clinical biopsies, koilocytosis is observed in both low- and high-risk HPV infections. Correspondingly, we found that the E5 and E6 proteins from low- and high-risk HPVs induce koilocytosis in vitro, using both transient and stable expression conditions. The koilocytes observed in our experiments are very similar to those observed in biopsies and Pap smears, although they exhibit some qualitative differences (Figure 1A). For example, although koilocytes consisting of a single vacuole surrounding the nucleus can be observed, there is a greater proportion of koilocytes with multivesiculation in vitro. This is most evident with the 16 E5 (−10) mutant that is partially defective for koilocytosis. We presume that this might reflect incomplete vesicle fusion, but we have not yet performed a thorough investigation of the time course of vesicle formation/fusion with the in vitro system. The difference between in vivo and in vitro koilocytosis might have several origins: i) the expression of E5 in our cells may not be as high as during a productive infection; ii) the cells that we study are not differentiated; and iii) our experiments use only two viral oncogenes rather than the complete viral genome and might not be expected to mimic precisely the morphological changes occurring during productive viral infection.
In natural infections, koilocytosis is only observed in the uppermost, well-differentiated layers of stratified squamous epithelium and it is in these layers (and specifically in koilocytes) that the viral capsid proteins are expressed and assembled into infectious virions. In contrast, we have been studying koilocytosis under conditions that do not favor terminal differentiation (ie, low calcium medium) and this might explain the low level (<3%) of koilocyte formation. However, even when the complete HPV genome is expressed in organotypic (raft) culture systems that favor keratinocyte differentiation, koilocytosis is rarely observed.33,34 Additionally, we found that the frequency of koilocytosis in cultured HEC lines does not increase under conditions that acutely induce differentiation (Ca2+ and 10% fetal bovine serum for 24, 48, or 72 hours) (data not shown). This might reflect the differentiation insensitivity of retroviral promoters controlling E5 and E6 expression or might also be the consequence of a need for more complete differentiation conditions. Regardless, the selective presence of koilocytes in the differentiated layers of the stratified squamous epithelium may reflect increased expression of E5 and/or E6 in these cells. Compatible with this suggestion is the hypothesis that HPVs use a leaky ribosome-scanning mechanism to translate proteins from polycistronic mRNAs in vivo and, as a consequence, the E5 protein is primarily synthesized in differentiating cells because of a shift of E5 from the fourth to second open reading frame of HPV transcripts.8 More directly, the BPV-1 E5 protein has been shown to be expressed highly in the uppermost layers of stratified squamous epithelium in papillomas productively replicating BPV-1.35
It is unclear why the E5 and E6 proteins fail to induce koilocytes in COS cells, but there are obviously many possible reasons for this, including differences in the species of origin, cell type, and the presence of SV40-T antigen (which has functional similarities to the HPV E7 protein that inhibits koilocytosis).36 It is also unclear why the E7 oncoprotein decreases the incidence of koilocytes in our study. In an infected cell, HPV-16 E6 and E7 are produced from polycistronic mRNAs8 and hence both proteins should be expressed. However, changes in cell differentiation could certainly affect the efficiency of splicing the E6/E7 regions and therefore alter the ratio of E6 and E7. In addition, it is possible that the translation of E7 mRNA or stability of E7 protein might be altered as a consequence of keratinocyte differentiation.
The koilocytes formed in our experiments do not appear to be apoptotic or senescent cells. The E5-expressing, immortalized HECs do not exhibit the pyknotic and shrunken nuclei usually observed in apoptotic cells.37 Additionally, we have found that koilocytosis is promoted by the E6 oncoprotein, which is known to inhibit apoptosis.38,39,40 E7, in contrast, strongly inhibits koilocyte formation in our study but promotes apoptosis in keratinocytes.38,39 Senescence-associated β-galactosidase, a diagnostic marker for cellular senescence, was detectable in only a very small number (<0.02%) of nonimmortalized HECs, and we found that koilocytes were no more likely to be senescent than nonkoilocytotic cells (data not shown).
The role of perinuclear vacuolization in viral replication is unclear. Cytoplasmic vacuolization could contribute to keratinocyte fragility and the release of viral-laden nuclei from HPV lesions. In fact, it has been suggested that papillomavirus egress in the upper epithelial layer is facilitated by disturbing keratin integrity and assembly of the cornified envelope.41 However, although we believe that E5 might have a significant effect on the viral life cycle in vivo, it has already been shown to have a modest effect on viral replication in raft keratinocyte cultures.33,34
Finally, we have also identified E5 and E6 mutants that are defective for inducing koilocytosis in vitro and anticipate that they may be useful in identifying specific pathways and cell targets for these viral oncoproteins. It will also be important to define the common properties of the low- and high-risk E6 proteins and how they contribute to the pathological changes in the cell cytoplasm.
Acknowledgments
We thank Drs. Elke A. Jarboe and Christopher P. Crum, Brigham and Women’s Hospital, Boston, MA, for the photo of koilocytosis in a Pap smear; and Tigest Seyoum for excellent technical assistance with histological staining.
Footnotes
Address reprint requests to Richard Schlegel, M.D., Ph.D., Department of Pathology, Georgetown University Medical School, 3900 Reservoir Rd. NW, Washington, DC 20057. E-mail: schleger@georgetown.edu.
Supported by the National Cancer Institute, National Institutes of Health (grant R01-CA053371 to R.S.).
References
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):4–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, de Villiers E-M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- Bosch FX, de Sanjosé S. Chapter 1: human papillomavirus and cervical cancer—burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;31:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(Suppl 1):16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Bubb VJ, Schlegel R. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J Virol. 1993;67:6170–6178. doi: 10.1128/jvi.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Sunitha I, Baker CC, Hanover J, Schlegel R. Codon optimization of the HPV-16 E5 gene enhances protein expression. Virology. 2003;311:105–114. doi: 10.1016/s0042-6822(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Campo MS, Schlegel R. Biological Activities of Papillomavirus E5 Protein. Campo MS, editor. Norfolk, Caister Academic Press,; 2006:pp 97–113. [Google Scholar]
- Genther Williams SM, Disbrow GL, Schlegel R, Lee D, Threadgill DW, Lambert PF. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 2005;65:6534–6542. doi: 10.1158/0008-5472.CAN-05-0083. [DOI] [PubMed] [Google Scholar]
- Rodríguez MI, Finbow ME, Alonso A. Binding of human papillomavirus 16 E5 to the 16kDa subunit c (proteolipid) of the vacuolar H+-ATPase can be dissociated from the E5-mediated epidermal growth factor receptor overactivation. Oncogene. 2000;19:3727–3732. doi: 10.1038/sj.onc.1203718. [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Haghshenas M, Marchetti B, Campo MS. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int J Cancer. 2006;119:2105–2112. doi: 10.1002/ijc.22089. [DOI] [PubMed] [Google Scholar]
- Chen SL, Lin ST, Tsai TC, Hsiao WC, Tsao YP. ErbB4 (JM-b/CYT-1)-induced expression and phosphorylation of c-Jun is abrogated by human papillomavirus type 16 E5 protein. Oncogene. 2006;26:42–53. doi: 10.1038/sj.onc.1209768. [DOI] [PubMed] [Google Scholar]
- Hajdu SI. A note from history: the link between koilocytes and human papillomaviruses. Ann Clin Lab Sci. 2006;36:485–487. [PubMed] [Google Scholar]
- Yamamoto LS, Alves VA, Maeda MY, Longatto-Filho A, Utagawa ML, Eluf Neto J. A morphological protocol and guide-list on uterine cervix cytology associated to papillomavirus infection. Rev Inst Med Trop Sao Paulo. 2004;46:189–193. doi: 10.1590/s0036-46652004000400003. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Hanover JA, Schlegel R. Endoplasmic reticulum-localized human papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH. J Virol. 2005;79:5839–5846. doi: 10.1128/JVI.79.9.5839-5846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PS, Jenson AB, Cowsert L, Nakai Y, Lim LY, Jin XW, Sundberg JP. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J Infect Dis. 1990;162:1263–1269. doi: 10.1093/infdis/162.6.1263. [DOI] [PubMed] [Google Scholar]
- Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280:10807–10816. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- Baege AC, Berger A, Schlegel R, Veldman T, Schlegel R. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am J Pathol. 2002;160:1251–1257. doi: 10.1016/S0002-9440(10)62552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988;7:3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz FA, Disbrow GL, Simic V, Schlegel R. Are transforming properties of the bovine papillomavirus E5 protein shared by E5 from high-risk human papillomavirus type 16? Virology. 2005;332:102–113. doi: 10.1016/j.virol.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Sparkowski J, Baege A, Schlegel R. E5 oncoprotein mutants activate phosphoinositide 3-kinase independently of platelet-derived growth factor receptor activation. J Biol Chem. 2000;275:5111–5119. doi: 10.1074/jbc.275.7.5111. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Baege A, Sunitha I, Schlegel R. c-Src activation by the E5 oncoprotein enables transformation independently of PDGF receptor activation. Oncogene. 2002;21:1695–1706. doi: 10.1038/sj.onc.1205223. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Disbrow GL, Krawczyk E, Simic V, Lantzky K, Schlegel R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene. 2008;27:1071–1078. doi: 10.1038/sj.onc.1210725. [DOI] [PubMed] [Google Scholar]
- Sjö NC, Heegaard S, Prause JU, von Buchwald C, Lindeberg H. Human papillomavirus in conjunctival papilloma. Br J Ophthalmol. 2001;85:785–787. doi: 10.1136/bjo.85.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Watanabe S, Zanma S, Sato H, Furuno A, Yoshiike K. Human papillomavirus type 16 E6 proteins with glycine substitution for cysteine in the metal-binding motif. Virology. 1991;185:536–543. doi: 10.1016/0042-6822(91)90523-e. [DOI] [PubMed] [Google Scholar]
- Crusius K, Auvinen E, Steuer B, Gaissert H, Alonso A. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp Cell Res. 1998;241:76–84. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- Tomakidi P, Cheng H, Kohl A, Komposch G, Alonso A. Connexin 43 expression is downregulated in raft cultures of human keratinocytes expressing the human papillomavirus type 16 E5 protein. Cell Tissue Res. 2000;301:323–327. doi: 10.1007/s004410000231. [DOI] [PubMed] [Google Scholar]
- Genther SM, Sterling S, Duensing S, Munger K, Sattler C, Lambert PF. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J Virol. 2003;77:2832–2842. doi: 10.1128/JVI.77.5.2832-2842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen LM, Wahlström T, Rihkanen H, Vaheri A. A novel method to culture laryngeal human papillomavirus-positive epithelial cells produces papilloma-type cytology on collagen rafts. Eur J Cancer. 1998;34:1111–1116. doi: 10.1016/s0959-8049(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Burnett S, Jareborg N, DiMaio D. Localization of bovine papillomavirus type 1 E5 protein to transformed basal keratinocytes and permissive differentiated cells in fibropapilloma tissue. Proc Natl Acad Sci USA. 1992;89:5665–5669. doi: 10.1073/pnas.89.12.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K, Heselmeyer K. Henson DE, Gazdar A, editors. Amsterdam: IOS Press; The Molecular Pathogenesis of Cervical CancerThe Role of Human Papillomaviruses. 1999:pp 97–111. [Google Scholar]
- Zamzami N, Kroemer G. Condensed matter in cell death. Nature. 1999;401:127–128. doi: 10.1038/43591. [DOI] [PubMed] [Google Scholar]
- Finzer P, Aguilar-Lemarroy A, Rösl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002;188:15–24. doi: 10.1016/s0304-3835(02)00431-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarti O, Krishna S. Molecular interactions of “high risk” human papillomaviruses E6 and E7 oncoproteins: implications for tumour progression. J Biosci. 2003;28:337–348. doi: 10.1007/BF02970152. [DOI] [PubMed] [Google Scholar]
- Li TT, Zhao LN, Liu ZG, Han Y, Fan DM. Regulation of apoptosis by the papillomavirus E6 oncogene. World J Gastroenterol. 2005;11:931–937. doi: 10.3748/wjg.v11.i7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32S:7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]