Abstract
Satellite cells refer to resident stem cells in muscle that are activated in response to damage or disease for the regeneration and repair of muscle fibers. The use of stem cell transplantation to treat muscular diseases has been limited by impaired donor cell survival attributed to rejection and an unavailable stem cell niche. We isolated a population of adult muscle mononuclear cells (AMMCs) from normal, strain-matched muscle and transplanted these cells into δ-sarcoglycan-null dystrophic mice. Distinct from other transplant studies, the recipient mice were immunocompetent with an intact endogenous satellite cell pool. We found that AMMCs were 35 times more efficient at restoring sarcoglycan compared with cultured myoblasts. Unlike cultured myoblasts, AMMC-derived muscle fibers expressed sarcoglycan protein throughout their entire length, consistent with enhanced migratory ability. We examined the capacity of single injections of AMMCs to provide long-term benefit for muscular dystrophy and found persistent regeneration after 6 months, consistent with augmentation of the endogenous stem cell pool. Interestingly, AMMCs were more effectively engrafted into aged dystrophic mice for the regeneration of large clusters of sarcoglycan-positive muscle fibers, which were protected from damage, suggesting that the stem cell niche in older muscle remains permissive.
Skeletal muscle is a dynamic tissue that regenerates after damage from exercise or disease. Muscle regeneration is mediated by satellite cells, which are defined by their position between the basal lamina and the sarcolemmal membrane.1,2 Satellite cells are maintained throughout the life of the organism and are thought to asymmetrically divide to simultaneously replenish the satellite cell pool and produce myogenic precursor cells known as myoblasts. Myoblasts can be cultured and expanded and have been tested for their ability to treat degenerative diseases of muscle.3,4,5,6,7,8,9,10,11,12,13,14,15,16 Under specific culture conditions, myoblasts withdraw from the cell cycle and undergo terminal differentiation. In vivo, during myoblast transplantation, a similar cell cycle withdrawal is thought to occur followed by terminal differentiation and the fusion of myoblasts to existing myofibers.17 In this setting, myoblast transfer has been hampered by limited engraftment thought to relate to poor migratory ability and lack of long-term survival from rejection and ongoing muscle degeneration. An additional complication is that myoblasts do not appear to replace the satellite cell niche.
Duchenne muscular dystrophy (DMD) and a subset of the limb girdle muscular dystrophies are caused by mutations in genes encoding components of the dystrophin glycoprotein complex.18,19 This complex consists of dystrophin, dystroglycan, sarcoglycans, dystrobrevins, and syntrophins.20 The transmembrane dystrophin glycoprotein complex links laminin-α2 in the basal lamina to the actin cytoskeleton. Disruption of this protein chain leaves muscle membranes fragile and with permeability defects. Clinically, the loss of dystrophin or sarcoglycans leads to progressive muscle weakness that initially targets muscle groups proximal to the trunk. In time, most musculature is affected by muscle wasting. Pathologically, the muscle is replaced ultimately by connective tissue and adipose infiltration indicating an overall failure of the satellite cell and regenerative system of muscle. During aging in normal muscle, there is also a reduction in regenerative capacity.21,22,23 Mouse models of DMD and limb girdle muscular dystrophy lacking dystrophin (mdx) and δ-sarcoglycan (Sgcd-null), respectively, exhibit identical pathology to what is seen in human patients that begins as focal necrosis within muscle and eventual fibrofatty infiltration.24,25,26 Although the underlying genetic defects and disease progression are understood in muscular dystrophy, therapy is not effective. Thus, cell-based approaches remain an attractive strategy for treating these disorders.
Cell-based therapies for muscular dystrophies have the combined benefits of boosting regenerative capacity and simultaneously delivering normal copies of genes to dystrophic muscle. For long-term efficacy, adding a self-renewing source of stem cells to the endogenous stem cell pool of muscle is advantageous. Early attempts at cell-based therapy for muscular dystrophy relied on transfer of immortalized myoblast cultures to mdx mice.27 These studies demonstrated that myoblast transfer could restore dystrophin to a small percentage of recipient myofibers and inspired clinical trials in human DMD patients.10,12,13,14 Subsequent studies have identified the limitations of myoblast cell lines and cultured myoblasts. Cultured myoblasts are hampered by their inability to migrate throughout myofibers, limiting their muscle contribution to 60 to 900 μm from the injection site.28,29,30 Another shortcoming is that myoblasts quickly die after injection,15,31,32 at least partially because of host immune responses.16,33
Recent studies suggested that culture conditions promote partial cell differentiation, thus diminishing stem cell capabilities and limiting effectiveness of cultured cells to contribute to multiple rounds of muscle regeneration.34 Montarras and colleagues34 showed that freshly isolated cells regenerated muscle three times more efficiently than cells exposed to culture conditions. Consequently, cultured myoblasts have the advantage of ex vivo expansion but through this process differentiate sufficiently so that they do not efficiently replace satellite cells.
To expand on these findings, we transplanted a population of freshly isolated, adult muscle mononuclear cells (AMMCs) into immunocompetent Sgcd-null mice. Compared to mdx mice, Sgcd-null mice have increased cardiac and skeletal muscle necrosis and do not have revertant fibers that may complicate interpreting the regenerative potential of donor cells.24,25 We found that AMMCs regenerated muscle 35× more effectively than cultured myoblasts. Transplantation of AMMCs resulted in robust expression of sarcoglycan throughout the length of the transplanted muscle, even when competing against endogenous satellite cells. Subsequent injections of AMMCs yielded additional donor-derived fibers in immunocompetent recipients, suggesting that AMMCs contain a population of immunoprivileged cells with myogenic potential. Muscle fibers regenerated from AMMCs were resistant to further degeneration in vivo under sedentary conditions and when stressed by exercise. Importantly, AMMCs were detected in the sublaminal compartment, consistent with satellite cell localization. Moreover, AMMCs demonstrated long-term survival 6 months after transplantation into diseased muscle with ongoing degeneration and regeneration with no decrease in their population. Unexpectedly, transplantation into aged Sgcd-null recipients was associated with enhanced regeneration.
Materials and Methods
Animals
Sgcd-null mice were previously reported and were generated by deleting exon 2 that encodes the initiator methionine, the cytoplasmic and transmembrane domains.24,35 The Sgcd allele was backcrossed heterozygously 10 generations with C57BL6/J mice and then intercrossed to generate recipient animals. Donors were 6- to 10-week-old, sex-mismatched, C57BL/6J littermates. In experiments that used GFP transgenic mice, the donors were 6- to 10-week-old, sex-mismatched C57BL/6-Tg(ACTB-EGFP)1Osb/J mice (The Jackson Laboratory, Bar Harbor, ME) with an enhanced GFP transgene under the control of a chicken β-actin promoter and cytomegalovirus enhancer. Animals were housed, treated, and handled according to guidelines of the University of Chicago Institutional Animal Care and Use Committee, the Animal Welfare Act, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Isolation of AMMCs
Mononuclear cells were isolated from hindlimb muscles of 6- to 10-week-old syngeneic wild-type donors. Muscles were finely minced with razor blades. Collagenase/dispase solution (2.4 U/ml dispase II, 2.5 mmol/L CaCl2, 1% collagenase D; Roche Diagnostics, Indianapolis, IN) was added at 2 ml/g tissue followed by a 45-minute incubation at 37°C. Every 15 minutes during digestion, tissue slurries were triturated 15 times through a 5-ml pipette. The digestion was stopped by adding F-10 Ham’s media supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin. Digested muscle was passed through filters with pore sizes of 100 μm, 70 μm, and 40 μm before centrifugation at 200 × g for 5 minutes. Pellets were resuspended in supplemented F-10 Ham’s media and Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) was layered beneath the cell suspensions followed by centrifugation at 800 × g for 15 minutes with the brake off. AMMCs were collected at the media/Ficoll interface and pellet cells were collected after removing the supernatant. After washing, cells were resuspended in phosphate-buffered saline (PBS)/0.5% bovine serum albumin at 107 cells/ml and kept on ice until transplanted.
Primary Myoblast Cultures
Primary myoblasts were isolated from five C57BL/6J newborn mouse pups as described.36 Cells were preplated on uncoated plastic dishes (Techno Plastic Products, Trasadingen, Switzerland) three times before plating on dishes coated with ECL (entactin-collagen-laminin) attachment matrix (Upstate Biotechnology, Lake Placid, NY). Cells were passaged 2 days later after two additional preplatings on uncoated dishes. Three days later, cultures were at least 50% myoblasts. After 5 days in culture, the three cell preparations with the highest percentage of myoblasts were each injected into four recipient mice.
Cell Transplants
Recipient mice were nulliparous Sgcd-null mice at 8 to 10 weeks of age or 7 to 10 months of age. Mice were injected with 105 AMMCs for each injection. When using cultured myoblasts, 2 × 105 cells were injected to compensate for the presence of fibroblasts in the culture and ensure that at least 105 myoblasts were delivered. Recipients of AMMCs were irradiated with 5 Gy whole body irradiation immediately before transplantation. After anesthetizing the mice with isoflurane, a longitudinal incision was made to expose the tibialis anterior or biceps brachii. A 10-μl cell suspension was injected directly into the midbelly of the muscle with a 25-μl syringe (Hamilton Co., Reno, NV). Mice receiving two injections were given a second transplant 14 days later. Recipients of three doses of cells received their second and third injections on days 10 and 20 after the first injection. Recipients were sacrificed 1, 3, or 6 months after transplantation and transplanted muscles were removed and snap-frozen in liquid nitrogen-cooled isopentane.
Immunostaining
Every third 10-μm frozen cross section throughout the length of the recipient muscle was collected on a slide, and the two serial sections were collected on additional slides. An anti-γ-sarcoglycan rabbit polyclonal antibody37 was used at 1:500 in combination with Cy3- or fluorescein-conjugated goat anti-rabbit immunoglobulin antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to identify donor-derived muscle fibers.
For localization of GFP-AMMCs, the anti-GFP rabbit polyclonal antibody A11122 (Invitrogen, Carlsbad, CA) was used at 1:100, anti-dystrophin mouse monoclonal antibody NCL-DYS2 (Novacastra, Newcastle upon Type, UK) was used at 1:100, and anti-Pax7 mouse monoclonal (Developmental Studies Hybridoma Bank, Iowa City, IA) was used at 1:50 in combination with species-appropriate fluorophore-conjugated secondary antibodies. Background staining attributable to endogenous mouse immunoglobulins was reduced using the Mouse On Mouse kit according to the manufacturer’s protocol (Vector Laboratories, Burlingame, CA). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) and coverslips were mounted with Vectashield antifade medium (Vector Laboratories). Sarcoglycan-positive fibers were scored on a minimum of 144 sections per muscle, representing a minimum of 4.3 mm of the length of the tibialis anterior muscle. Comparison of transplant treatments was done by averaging the single section with the most sarcoglycan fibers from each animal within the treatment group. Scoring and image acquisition were done using an Axioskop, AxioCam, and AxioVision (Carl Zeiss, Inc., Thornwood, NY).
Exercise
Exercised mice received two injections of cells into their biceps brachii muscles as described above. One month after the first injection, mice were exercised on a Treadmill Simplex II (Columbus Instruments, Columbus, OH) at a 10 degree decline at 10 m/minute for 10 minutes each day for 3 consecutive days. After the exercise session on the second day, mice were injected intraperitoneally with 10 mg/ml of Evans blue dye (EBD) at 0.1 ml per 10 g of body weight. Immediately after the third exercise session, biceps muscles were removed, snap-frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until sectioning.
The biceps muscle was sectioned and stained as described above and sarcoglycan-positive fibers were counted throughout the length of the muscle. The percentage of EBD-positive area was determined by measuring the area of at least two muscle cross sections per animal stained with dye and dividing by the total muscle area using Image J software (National Institutes of Health, Bethesda, MD). For untransplanted animals, EBD was measured on the middle sections of the biceps brachii. For transplanted muscles, the sections containing the most sarcoglycan-positive fibers was used for analysis.
EBD Analysis
The serial section after the section with the most sarcoglycan-positive fibers was chosen for EBD analysis. Slides were incubated in 1 μg/ml of EBD in PBS for 30 minutes followed by three 10-minute washes in PBS. Sections were fixed in ice-cold acetone for 5 minutes, washed three times for 5 minutes in PBS, and immunostained for γ-sarcoglycan as described above.
Cytometry
For cytometric analysis by immunofluorescence, Ficoll upper layer and pellet AMMCs were isolated as described above and resuspended at 105 cells/ml. A 200-μl volume of cells was immediately centrifuged to polylysine-coated slides (Wescor, Inc., Logan, UT) using a Cytopro 7620 (Wescor, Inc.) set to 1000 rpm for 5 minutes with medium acceleration. Slides were fixed with ice-cold methanol for 2 minutes, rehydrated in PBS, and blocked with blocking buffer (5% fetal bovine serum, 0.1% Triton X-100 in PBS) for 30 minutes before antibody staining. Primary antibodies and dilutions used were as follows: rabbit polyclonal anti-MyoD (sc-760; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:500; mouse monoclonal anti-Pax7 (Developmental Studies Hybridoma Bank) at 1:50; mouse monoclonal anti-Pax3 (Developmental Studies Hybridoma Bank) at 1:50; rabbit polyclonal anti-Myf5 (sc-302; Santa Cruz Biotechnology, Inc.) at 1:100; mouse monoclonal anti-myogenin (556358; BD Pharmingen, Franklin Lakes, NJ) at 1:100. Cells were incubated with primary antibodies diluted in blocking buffer overnight at 4°C before being washed in PBS and incubated with species-appropriate secondary antibodies (Jackson ImmunoResearch Laboratories). After additional PBS washes, nuclei were stained with DAPI and coverslips were mounted with Vectashield medium. Antigen-positive cells were scored on a minimum of 20 microscope fields under the ×63 oil objective of an Axioskop microscope (Carl Zeiss, Inc.).
For analysis by fluorescence-activated cell sorting (FACS), AMMCs were resuspended at 5 × 107 cells/ml in FACS staining buffer (1% bovine serum albumin, 0.1% sodium azide in PBS) containing primary antibodies and incubated on ice for 30 minutes. Primary antibodies and dilutions used were as follows: phycoerythrin-conjugated anti-CD34 (RAM34, BD Pharmingen) at 1:20; fluorescein-conjugated anti-CD45 (30-F11, BD Pharmingen) at 1:20; rat monoclonal anti-Sca-1 (Ly-6AE; Cedarlane Laboratories, Hornby, Canada) at 1:100; rat monoclonal anti-C-kit (ack2; eBioscience, San Diego, CA) at 1:50; rabbit polyclonal anti-M-cadherin (sc-10734; Santa Cruz Biotechnology, Inc.) at 1:100; mouse monoclonal anti-CD31/PECAM (550274. BD Pharmingen) at 1:50; rat monoclonal anti-CD44 (558739. BD Pharmingen) and 1:50; fluorescein isothiocyanate-conjugated anti-CD13 (558744. BD Pharmingen) at 1:100; rabbit polyclonal anti-laminin α238 at 1:200, rabbit polyclonal anti-α5 integrin (AB1928; Chemicon, Temecula, CA) at 1:500. Cells were washed with FACS staining buffer and incubated with species-specific, fluorophore-conjugated secondary antibodies on ice for 30 minutes. Cells were washed again and kept on ice until analysis. After gating to remove dead cells, debris, and cell aggregates, at least 10,000 events were collected for analysis on a BD FACSCanto flow cytometer (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Statistical Analysis
Data are presented as the mean ± SEM. Nonparametric statistics were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). Differences were considered significant at two-tailed P < 0.05.
Results
Freshly Isolated AMMCs Regenerate Muscle More Effectively than Cultured Myoblasts
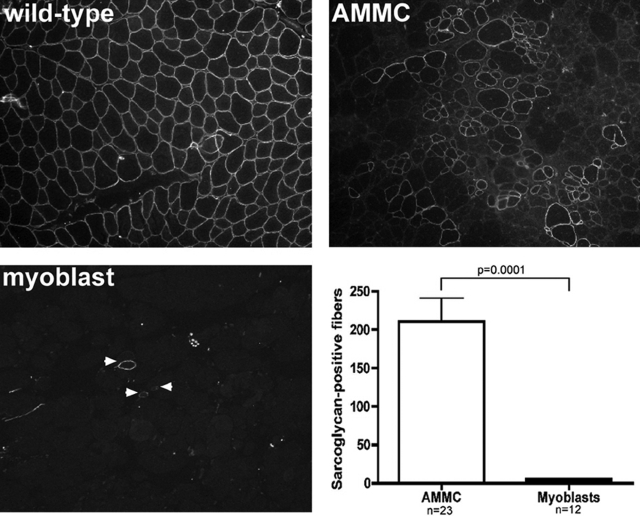
To examine the benefits of transplanting freshly isolated muscle cells into dystrophic muscle, we first examined whether a population of AMMCs could regenerate muscle after injection into Sgcd-null mice. Deletion of δ-sarcoglycan destabilizes the entire sarcoglycan protein complex in Sgcd-null mice.24 AMMCs were extracted by mincing hindlimb muscles from 6- to 10-week-old male wild-type mice, followed by gentle treatment with collagenase and dispase, trituration, and filtering to remove debris. These cells were transplanted into 8- to 10-week-old Sgcd-null recipients within 2 hours of isolation. Successful engraftment was confirmed by restored γ-sarcoglycan expression.35 A single injection of 105 AMMCs resulted in an average of 210 sarcoglycan-positive muscle fibers, or ∼10% of the total myofibers in the transplanted muscle (Figure 1). In contrast, 105 cultured primary myoblasts equally introduced into Sgcd-null recipients yielded an average of only six sarcoglycan-positive fibers. This low engraftment of myoblasts is comparable to what others have noted when normalized to injected cell number and taking into account the immunocompetent status of the recipients.34,39 Thus, AMMCs are a freshly isolated muscle cell population that is 35-fold more efficient at restoring sarcoglycan than cultured primary myoblasts.
Figure 1.
The myogenic ability of AMMCs is greater than primary myoblast cultures. Fluorescent staining for γ-sarcoglycan protein revealed large clusters of donor-derived myofibers 1 month after transplantation in Sgcd-null muscles injected once with 105 AMMCs. In contrast, injection of cultured myoblasts resulted in scattered, isolated sarcoglycan-positive muscle fibers. Muscles injected with AMMCs had 35-fold more sarcoglycan-positive myofibers than those injected with cultured myoblasts. Sarcoglycan-positive fibers were scored on at least 140 sections per recipient muscle. Shown are the average values from the section with the most sarcoglycan-positive fibers from each recipient. Original magnifications, ×100.
AMMCs Restore Sarcoglycan throughout the Length of Transplanted Muscles
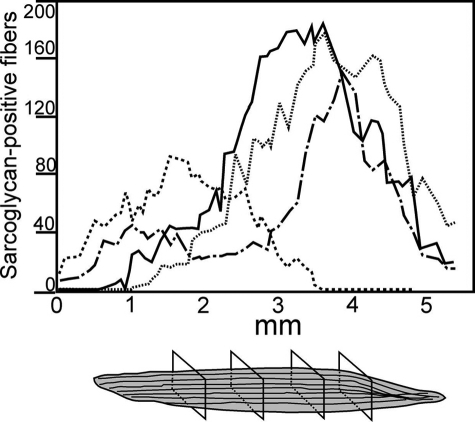
Because cultured myoblasts have very limited migration after transplant, we assessed the spread of sarcoglycan longitudinally from the injection site after AMMC transplantation. Sarcoglycan-positive fibers were scored in every third 10-μm section throughout the recipient muscle. In 10 mice analyzed, sarcoglycan-positive fibers were detected in an average of 144 of 180 sections of each muscle, representing more than 4.4 mm of muscle length (Figure 2). A peak of sarcoglycan-positive fibers was detected in the middle of the injected muscle, and presumably represented the injection site. When individual muscle fibers were tracked in each section, sarcoglycan protein could be detected in nearly the entire length of some fibers and only in segments of others (data not shown). Donor-derived fibers were not detected in the gastrocnemius or quadriceps muscles of recipient mice (data not shown). The extended tracts of sarcoglycan-positive fibers within injected muscles suggest that AMMCs contain potent myogenic precursor cells with the ability to migrate from the injection site to the ends of the myofiber.
Figure 2.
AMMCs restore sarcoglycan protein throughout the length of transplanted muscles. Sarcoglycan-positive myofibers were scored by immunofluorescence microscopy in at least 140 serial sections throughout 5 mm of recipient tibialis anterior muscles. Shown are data from four representative muscles from Sgcd-null mice injected once with 105 AMMCs and analyzed 1 month after transplantation.
AMMC-Derived Muscle Fibers Are Resistant to Degeneration
A hallmark of muscular dystrophy is the progressive muscle wasting that results from continued cycles of degeneration. To analyze whether donor-derived muscle fibers underwent degeneration, sections from transplanted tibialis anterior muscles were stained with EBD. EBD is a low-molecular weight dye that binds to serum albumin, which enters dystrophic fibers with damaged membranes.40 Clusters of EBD-positive fibers were detected in Sgcd-null muscle, consistent with the focal degeneration previously reported in these mice.24 EBD was not detected in any of 3711 sarcoglycan-positive fibers analyzed from 33 recipients, even when the donor-derived fiber was in the middle of an area of focal degeneration (Figure 3).
Figure 3.
AMMC-derived muscle fibers resist degeneration. For each recipient, the serial tibialis anterior section containing the most donor-derived myofibers was stained with anti-γ-sarcoglycan antibodies (green) and EBD (red), which binds albumin and enters damaged muscle fibers. Nuclei are stained with DAPI (blue). Damaged muscle fibers were detected throughout the muscle, but none of the 3711 donor-derived myofibers observed in 33 recipient muscles contained EBD. Original magnifications, ×100.
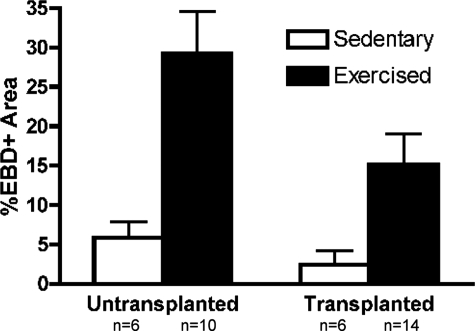
Downhill running produces muscle damage through eccentric contraction. In quadrupeds, this effect is most pronounced on the biceps brachii muscle. Therefore, we transplanted the biceps brachii muscles of Sgcd-null mice with two injections of 105 AMMCs 1 month before exercising them by three sessions of downhill running. Downhill running increased muscle damage fourfold in the biceps brachii in nontransplanted mice (Figure 4). Transplanted biceps muscles had an average of 20 sarcoglycan-positive fibers each, representing ∼4% of total muscle fibers in the biceps. Compared to untransplanted sedentary and exercised controls, AMMC recipients had reduced areas of EBD uptake (Figure 4). Biceps brachii muscles receiving AMMCs before running had nearly 50% less damage than nontransplanted muscles. Moreover, only 2 of the 189 donor-derived fibers observed in exercised mice were stained with EBD (data not shown). The lower occurrence of EBD-positive fibers demonstrates that transplant of AMMCs protects muscle from exercise-induced degeneration in vivo.
Figure 4.
Transplantation of AMMCs reduces exercise-induced damage in biceps brachii. The percentage of cross-sectional area that contained EBD (EBD)-positive fibers was determined in untransplanted biceps brachii and muscles injected twice with 105 AMMCs. Exercised mice were subjected to three sessions of downhill running to induce damage in transplanted and untransplanted biceps muscles. Muscle damage was almost 50% lower in mice that received injections of AMMCs. P = 0.05 for untransplanted, exercised animals compared to transplanted, exercised animals.
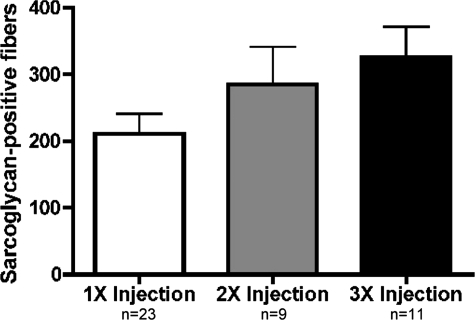
Enhanced Sarcoglycan Expression with Multiple Injections of AMMCs
Previous transplantation studies using cultured myoblasts have been hampered by the rapid clearance of injected cells, and much of this loss is attributed to immune responses.16,33 We examined the effect of additional injections of AMMCs on regeneration. Two weeks after a first injection, Sgcd-null recipients received a second injection of 105 AMMCs in one leg and saline in the other. Muscles receiving three injections were injected 10 and 20 days after the first transplant. Recipients were sacrificed 1 month after the initial injection. Subsequent analysis revealed a 40% increase in sarcoglycan-positive fibers in mice receiving two doses of cells compared to a single transplantation (Figure 5). A third dose of AMMCs resulted in an additional 15% increase in donor-derived fibers. The relatively modest increase from the third injection may be attributable to the shorter, 10-day period between delivery of the third dose of AMMCs and analysis. The increased restoration of sarcoglycan with repeated transplants indicates that multiple injections of AMMCs are more beneficial to dystrophic muscle than single injections and subsequent transplants of cells are not rapidly cleared by a recipient immune response.
Figure 5.
Multiple injections of AMMCs increase sarcoglycan expression. Sarcoglycan-positive fibers were quantified in tibialis anterior muscles 1 month after receiving the first of one, two, or three injections of 105 AMMCs. Three injections increased the number of sarcoglycan-positive fibers (P = 0.04, one injection compared to three injections) indicating that increased exposure to donor-derived cells and sarcoglycan expression did not adversely affect engraftment and survival.
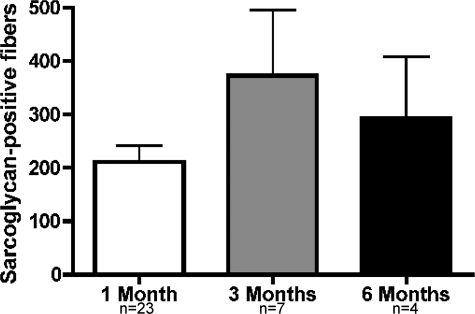
AMMCs Have Long-Term Regenerative Capacity in Dystrophic Mice
One characteristic of stem cells is their ability to self-renew, producing daughter cells for tissue regeneration as well as cells that retain stemness. To determine whether AMMCs have self-renewing properties, engrafted muscle was examined 3 and 6 months after a single injection of 105 AMMCs. Three months after injection of AMMCs, sarcoglycan-positive myofibers increased by 80% compared to recipients analyzed 1 month after injection (Figure 6). Recipients analyzed 6 months after AMMC transplants had 40% more sarcoglycan restoration than recipients at the 1-month time point. The increase in donor-derived muscle between 1 and 3 months is strong evidence that a subpopulation of AMMCs has stem cell activity and can augment the stem cell pool of dystrophic muscle to serve as a lasting source of regenerative cells.
Figure 6.
Long-term survival of donor-derived fibers in AMMC-transplanted muscles. Tibialis anterior muscles of mice given single injections of AMMCs were examined by immunofluorescence microscopy 1, 3, or 6 months after injection. Sarcoglycan-positive fibers were maintained 3 and 6 months after the single injection of donor cells. Because multiple rounds of regeneration occur during this period, this finding is consistent with successful replacement of the satellite cell pool and protection against the disease process for those fibers that express sarcoglycan.
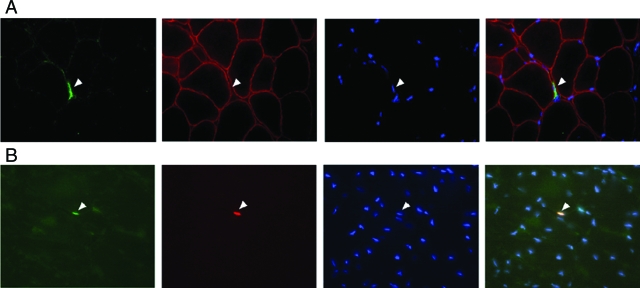
AMMCs Are Localized in the Satellite Cell Niche
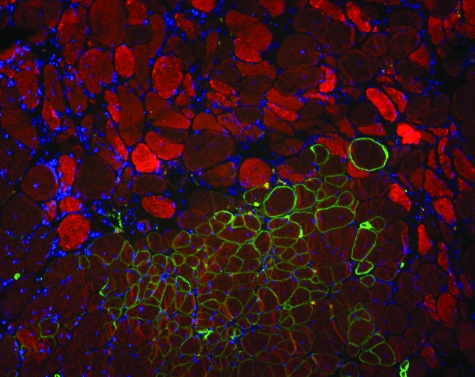
Satellite cells were initially identified based on their anatomical position between the plasma membrane and the basal lamina.1 To determine whether AMMCs contributed to the satellite cell pool of transplanted mice, Sgcd-null muscles were injected with AMMCs from transgenic mice expressing green fluorescent protein (GFP) under control of the β-actin promoter. Six weeks after transplantation, donor-derived fibers were detected expressing both GFP and sarcoglycan (Figure 7). Many recipient fibers expressed GFP, indicating donor cell fusion to recipient myofibers. GFP/Pax7-positive cells were detected outside of muscle fibers in recipients, closely apposed to the sarcolemma (Figure 7). The presence of GFP-positive cells expressing satellite cell markers in recipients demonstrates that AMMCs can supplement the pool of satellite cells in dystrophic muscle.
Figure 7.
Pax7-positive donor-derived AMMCs localize in a satellite cell position. Sgcd-null mice were injected with GFP-expressing donor cells. A: AMMCs from a transgenic GFP mouse were injected into a dystrophic recipient Sgcd-null mouse and were detected 1 month later closely associated with the extracellular sarcolemma (arrowhead), consistent with the localization of satellite cells. GFP-AMMCs were identified by GFP (green) and nuclear staining (DAPI, blue) and the muscle plasma membrane was labeled with anti-dystrophin antibodies (red). B: Pax7 (red) was also detected in GFP-AMMCs residing near the sarcolemma (arrowhead). Original magnifications, ×630.
AMMCs Are a Heterogeneous Cell Population
Because AMMCs are isolated without cell marker selection, we sought to characterize expression of marker proteins and determine what cell types are present in the AMMC population. Cell surface proteins including CD45, c-kit, Sca-1, CD34, M-cadherin, CD13, CD44, CD31, laminin α2, and α5 integrin were analyzed by incubating freshly isolated cells with antibodies followed by FACS. Transcription factor expression including Pax7, Myf5, MyoD, myogenin, and Pax3 was studied by centrifuging cells to slides and immediately staining them with antibodies for immunofluorescence microscopy.
Abundant detection of the myoregulatory factors Myf5, MyoD, and myogenin demonstrated that many AMMCs were committed muscle cells. Approximately 10% of AMMCs expressed the satellite cell markers M-Cadherin, Pax7, and Pax3 (Table 1). Likewise, hematopoietic stem cell markers including CD45, Sca-1, CD13, and c-kit were detected. Protein markers for endothelial cells (PE-CAM) and pericytes (CD44, CD13) were detected at low levels, indicating that these cell types represented a minor fraction of AMMCs. Detection of laminin α2 on cells suggests that the collagenase/dispase digestion of cells was gentle enough to leave the extracellular matrix partially intact and adherent to cells. Together, these data demonstrate that the major cell types in the AMMC population are myoblasts, satellite cells, and hematopoietic cells.
Table 1.
Ficoll Fractionation Results in Cell Populations with the Average Percentage of Protein-Positive Cells as Shown
| Protein | AMMCs | Pellet |
|---|---|---|
| FACS analysis | ||
| CD45 | 10.6% | 2.8% |
| c-kit | 1.0% | 1.3% |
| Sca-1 | 54.7% | 10.8% |
| CD34 | 2.5% | 3.2% |
| M-cadherin | 8.7% | 1.9% |
| Laminin α2 | 20.3% | 26.0% |
| CD31/PE-CAM | 2.9% | 3.2% |
| CD44 | 0.4% | 0.5% |
| CD13 | 5.9% | 6.0% |
| α5 integrin | 3.1% | 8.3% |
| Cytospin analysis | ||
| Pax7 | 11.6% | 20.8% |
| Pax3 | 10.5% | 14.8% |
| Myf5 | 68.3% | 67.2% |
| MyoD | 46.8% | 46.3% |
| Myogenin | 48.3% | 52.4% |
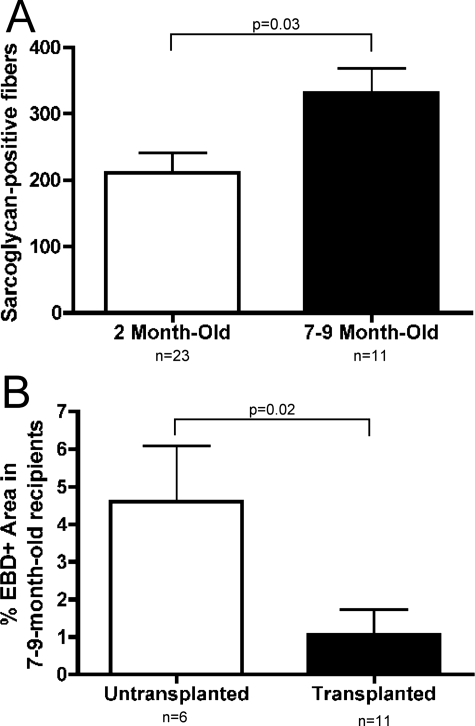
AMMCs Can Regenerate Muscle in Aged Dystrophic Mice
To determine whether AMMCs could effectively regenerate muscle in mice with advanced muscular dystrophy, 7- to 10-month-old mice were injected once with 105 AMMCs and analyzed 1 month later. By this age, Sgcd-null animals have advanced disease. Surprisingly, aged recipients had more donor-derived fibers than younger mice. Compared to 1.5- to 2.5-month-old recipients, sarcoglycan-positive fibers increased in 7- to 10-month-old recipients by 60% (Figure 8A). AMMC transplantation in aged mice led to an 80% decrease in damaged muscle fibers compared to nontransplanted controls as assayed by EBD uptake (Figure 8B). This demonstrates that AMMCs are effective even in advanced cases of muscle disease and that the environment of severely dystrophic muscle supports regeneration.
Figure 8.
Transplantation of AMMCs is more effective in aged Sgcd-null mice recipients. A: Sarcoglycan restoration was compared in 1.5- to 2.5-month-old recipients and 7- to 10-month-old recipients of AMMC transplants. Older mice receiving AMMCs had 60% more sarcoglycan-positive fibers than younger recipients (P = 0.03). B: Tibialis anterior sections of transplanted and untransplanted 7- to 10-month-old recipient Sgcd-null mice were stained with EBD to measure muscle damage. Aged mice receiving AMMC transplants had fewer dye-positive fibers than age-matched, untransplanted Sgcd-null mice (P = 0.02).
Density Gradient Centrifugation Yields Two Populations of Myogenic Cells
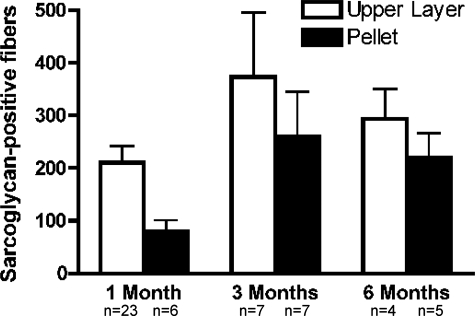
To eliminate red blood cells and debris, muscle cell preparations were subjected to density gradient centrifugation through Ficoll, resulting in the AMMC population at the Ficoll interface and a pellet cell population. Ficoll is a highly branched, high-mass, hydrophilic polysaccharide commonly used to separate blood into plasma mononuclear cells in the Ficoll upper layer and erythrocytes and granulocytes into the pellet. As a control, pellet cells were identically injected into contralateral tibialis anterior muscles of Sgcd-null mice. When analyzed 1 month after transplantation, muscles injected with pellet cells had 60% fewer donor-derived fibers than those injected with AMMCs (Figure 9). Interestingly, 3 months after injection with pellet cells the number of donor-derived fibers increased by more than threefold compared to the 1-month time point (Figure 9), and this trend continued at the 6-month time point. Moreover, when injected into aged Sgcd-null mice, pellet cells restored sarcoglycan to comparable levels as AMMCs (Figure 9).
Figure 9.
Fractionation of AMMCs through a Ficoll gradient. One month after transplantation, AMMCs resulted in 2.5× more donor-derived muscle fibers compared to Ficoll pellet transplants. At 3 and 6 months after transplantation, however, sarcoglycan-positive fibers were nearly equal in recipients of AMMCs and Ficoll pellet cells. The pellet fraction had increased expression of laminin α2, integrin α5, Pax7, and Pax3 suggesting that fragments of the stem cell niche may remain intact and promote long-term regeneration.
To determine how the pellet population differed from AMMCs, we examined the expression of cell markers by FACS and immunofluorescence microscopy. The expression profile of many proteins was similar between AMMCs and pellet cells. Notably, the percentage of cells expressing the hematopoietic cell lineage markers CD45 and Sca-1 was decreased in pellet cells (Table 1). Although the expression of muscle regulatory factors Myf5, MyoD, and myogenin was equal in pellet cells relative to AMMCs, expression of the satellite cell markers Pax3 and Pax7 was increased in pellet cells. Interestingly, the detection of laminin α2 on pellet cells was similarly increased in the pellet fraction (Table 1). The increase in donor-derived fibers from the 1-month to 3-month time point, increase in satellite cell markers, and increase in cells with laminin α2 and integrin α5 in the pellet suggests that fragments of the niche matrix may be sufficient to maintain the stem cell quality of the satellite cell.
Discussion
Although satellite cells have been extensively studied and are widely accepted as the primary regenerative cell in skeletal muscle, the ability to isolate these cells for effective use in stem cell therapies has been elusive. Recent experiments suggest that the difficulty in capturing satellite cells has primarily been attributable to the isolation and culturing techniques that result in their partial differentiation. In their quiescent state, most satellite cells express Pax7, CD34, and M-cadherin.41,42,43 Satellite cells in the diaphragm and, to a lesser extent in limb muscles, also express Pax3.44 On activation, satellite cells then enter a genetic program of MyoD and Myf5 expression.45 After subsequent division, myogenin expression and down-regulation of CD34 and Pax7 are thought to mark the differentiation of satellite cell progeny into committed myogenic cells.45 Because of variable expression patterns temporally and in different muscles, it has been difficult to identify a consensus protein marker for satellite cells that can be used for their unbiased isolation.17
Because satellite cells are defined by their position between the basal lamina and sarcolemma, Collins and Partridge,46 exploited the use of intact myofibers for transplantation. This strategy ensures the presence of satellite cells along with their niche. Intact myofibers contained between 7 and 22 Pax7-positive cells in the satellite cell position. Single myofibers were transplanted into immunodeficient nu/nu-mdx mice and where the endogenous satellite cell pool was depleted by high-dose irradiation. Three weeks after transplantation, successfully engrafted muscles had an average of 40 dystrophin-positive fibers. Some of the transplants also had hundreds of donor-derived nuclei in the satellite-cell position and demonstrated repair after further injury from notexin injection. Surprisingly, isolated fibers were unable to form myonuclei or satellite cells when transplanted into nonirradiated hosts suggesting that the niche was unavailable in these hosts.46
One of the earliest transplant studies into mdx mice also reported moderate results when using freshly isolated mononuclear cells.9 After injecting 5 × 105 to 4 × 106 muscle-derived cells, donor-derived muscle fibers and donor-host hybrid fibers were detected. In the best examples, dystrophin protein levels were restored to 10 to 40% of wild-type values. Successful transplantation, however, seemed dependent on immunosuppression because nu/nu-mdx were twice as likely to contain donor-derived fibers than immunocompetent mice, even though the immunocompetent mice were MHC-matched and pretolerized with neonatal injections of donor spleen cells.
Montarras and colleagues34 conducted transplants with donor cells isolated from mice expressing GFP under the control of Pax3 promoter. Donor cells were sorted from diaphragm muscle and transplanted into satellite cell ablated, immunodeficient nu/nu-mdx mice. Single grafts of 2 × 104 Pax3-GFP cells resulted in nearly 600 fibers in recipient muscles 3 weeks after transplantation. Injection of cells that were identically isolated but then cultured for 3 days before transplantation reduced donor-derived fibers by threefold.34 FACS analysis demonstrated that GFP-positive cells from the diaphragm were primarily Pax7+, CD34+, CD45−, and Sca1−. Injection of 2 × 104 Pax3-negative cells from hindlimb muscle with a similar FACS profile also resulted in significant dystrophin restoration. Together, these stem cell transplantation studies using freshly isolated cells suggest that uncultured cells may retain characteristics favorable to augment regenerative cell pools in muscle.
We now examined uncultured cells for the ability to engraft in immunocompetent recipients with an intact satellite cell pool and found that AMMCs are 35× more effective than cultured primary myoblasts. Unlike previous studies using freshly isolated cells, the recipients of these AMMC transplants did not have their satellite cells destroyed by high-level irradiation. In prior studies, without satellite cell ablation, transplants of single isolated fibers were unable to regenerate muscle or generate satellite cells.47 In our experiments, recipients of AMMCs were given a low dose (5 Gy) of whole body irradiation similar to our previous work35; this dose is insufficient to ablate endogenous satellite cells.48,49,50 Because these transplants were performed in a model that has ongoing degeneration and regeneration, the maintenance of donor-derived fibers at 3 and 6 months after injection is consistent with satellite cell contribution. Moreover, after transplant, AMMC-derived cells were found outside the sarcolemma and expressing Pax7, consistent with a satellite cell position.
AMMC-transplanted muscles exhibited functional protection against muscle degeneration under resting conditions and after exercise. Interestingly, the protective effect of AMMCs exceeded expectations given modest amounts of donor-derived fibers in transplanted biceps muscles before exercise. Previous studies demonstrated similar results when dystrophin was restored to a portion of mdx muscles using myoblast transfer51 or adenoviral vectors52 and protection against exercise-induced damage and ex vivo contractile properties were greatly improved. This suggests that successfully introducing even low numbers of donor-derived, gene-corrected fibers may confer protection on neighboring fibers to result in substantial functional benefits to transplanted muscles.
Another favorable characteristic of AMMCs is their ability to regenerate in immunocompetent mice. Previous transplant studies of freshly isolated cells have been done in nu/nu-mdx mice.34,46 Nu/nu mice are athymic and thus lack T-cell-mediated immunity and have secondary B-cell immunity defects.53 In contrast, our transplant recipients received 5 Gy whole body irradiation, a dose that transiently depletes lymphocytes.54,55,56 Transient suppression of CD4+ T cells is sufficient to suppress cellular and humoral immune responses and allow expression of neoantigens introduced by viral vectors.57 Despite delivering the δ-sarcoglycan neoantigen in Sgcd-null recipients, we detected no obvious evidence of increased immune cell infiltration or myofiber damage after transplantation, even in mice that received multiple injections of AMMCs without irradiation before subsequent injections. We cannot, however, exclude the possibility of a subtle immune response in recipients. Similar results were obtained in studies using mesoangioblast cells to restore α-sarcoglycan in mice. In that system, no overt immune response was detected in immunocompetent recipients, but use of immunodeficient hosts or immunosuppressants resulted in higher levels of sarcoglycan restoration.58,59 Coupled with their ability to survive for at least 6 months after transplantation, the ability of subsequent injections of AMMCs to regenerate more clusters of donor-derived fibers is strong evidence that AMMCs restore sarcoglycan without generating a host immune response sufficient to remove them.
Interestingly, AMMCs were able to restore more sarcoglycan-positive fibers in aged (7 to 9 months old) Sgcd-null recipients than younger (2 months old) recipients. Pathological signs of muscular dystrophy are evident by 2 months in Sgcd-null mice, but fibrofatty infiltration does not become widespread until after 4 months of age.24,25 It has been suggested that the extensive fibrosis in aged dystrophic muscle creates an unfavorable environment for transplanted cells. In contrast, we found donor-derived fibers engrafted readily in aged muscles where they effectively reduced muscle damage. In mdx mice and patients with DMD, myogenic cells have been shown to decrease or lose myogenic ability.60,61,62,63 Thus, one explanation for the enhanced regenerative ability of AMMCs in aged recipients is that reduced effectiveness of endogenous satellite cells creates a larger niche and less competitive environment for transplanted AMMCs. Further investigation of this phenomenon could yield valuable insight into the extent to which dystrophic muscle is permissive to cell transplantation.
In an attempt to remove debris and red blood cells from our AMMC populations, we used density gradient centrifugation through Ficoll. Although Ficoll pellet cells yielded 60% fewer donor-derived fibers than AMMCs 1 month after injection, the number of donor-derived fibers was nearly equal in recipients of AMMC and Ficoll pellet cell transplants 3 months after transplantation. We hypothesize that this is attributable to a population of satellite cells that is incompletely digested from the extracellular matrix during our cell isolation protocol. The increase in cells expressing the satellite cell markers Pax7 or Pax3 and cells bound to laminin α2 may define the myogenic population in the Ficoll pellet. In the study by Collins and colleagues,47 transplantation of whole muscle fibers with attached satellite cells was able to restore dystrophin, but enzymatic separation of the satellite cells from isolated fibers decreased their myogenicity by 1000-fold. If a cell was incompletely removed from its environment by collagenase/dispase digestion and retained a sizable piece of basal lamina or myofiber, that cell would likely be centrifuged as debris in the pellet. Preservation of the microenvironment around such a cell could be advantageous in maintaining stem cell properties. Our experiments suggest that a partially intact niche may be sufficient.
To advance toward a clinically feasible stem cell therapy for muscular dystrophies, stem cells need to be rapidly dissociated and isolated. Ideally, these cells would be delivered without extensive immunosuppression or lethal doses of irradiation. The ability for these cells to self-renew in vivo is central to successful long-term results. Our isolation of AMMCs demonstrates that such a population of stem cells can be easily and quickly isolated from adult skeletal muscle.
Footnotes
Address reprint requests to E.M. McNally, M.D., Ph.D., The University of Chicago, 5841 S. Maryland Ave., MC6088, Chicago, IL 60637. E-mail: emcnally@uchicago.edu.
Supported by the Muscular Dystrophy Association and the National Institutes of Health (grants HL61322, T32HL007381, and F32AR054700).
References
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Watt DJ, Sloper JC, Partridge TA. Partial correction of an inherited biochemical defect of skeletal muscle by grafts of normal muscle precursor cells. J Neurol Sci. 1988;86:137–147. doi: 10.1016/0022-510x(88)90093-7. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Practical aspects of myoblast implantation: investigations on two inherited myopathies in animals. Adv Exp Med Biol. 1990;280:86–95. doi: 10.1007/978-1-4684-5865-7_11. [DOI] [PubMed] [Google Scholar]
- Karpati G, Pouliot Y, Zubrzycka-Gaarn E, Carpenter S, Ray PN, Worton RG, Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- Huard J, Bouchard JP, Roy R, Labrecque C, Dansereau G, Lemieux B, Tremblay JP. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 1991;81:287–288. doi: 10.1042/cs0810287. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Huard J, Roy R, Bouchard JP, Malouin F, Richards CL, Tremblay JP. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992;24:3049–3051. [PubMed] [Google Scholar]
- Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, Walters GV, Abrahamowicz M, Duff C, Worton RG. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Nagaraja H, Stephens R, Lantry L, Morris GE, Burghes AHM. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Roy R, Tremblay JP, Huard J, Richards C, Malouin F, Bouchard JP. Antibody formation after myoblast transplantation in Duchenne-dystrophic patients, donor HLA compatible. Transplant Proc. 1993;25:995–997. [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Laval SH, Bushby KM. Limb-girdle muscular dystrophies—from genetics to molecular pathology. Neuropathol Appl Neurobiol. 2004;30:91–105. doi: 10.1111/j.1365-2990.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack AA, Lam MY, Cordier L, Shoturma DI, Ly CT, Hadhazy MA, Hadhazy MR, Sweeney HL, McNally EM. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J Cell Sci. 2000;113:2535–2544. doi: 10.1242/jcs.113.14.2535. [DOI] [PubMed] [Google Scholar]
- Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge TA. Stem cell route to neuromuscular therapies. Muscle Nerve. 2003;27:133–141. doi: 10.1002/mus.10243. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, Tremblay JP. Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle. Exp Neurol. 1999;155:22–30. doi: 10.1006/exnr.1998.6973. [DOI] [PubMed] [Google Scholar]
- Smythe GM, Hodgetts SI, Grounds MD. Problems and solutions in myoblast transfer therapy. J Cell Mol Med. 2001;5:33–47. doi: 10.1111/j.1582-4934.2001.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller GM, O'Day T, Watchko JF, Ontell M. Effect of injecting primary myoblasts versus putative muscle-derived stem cells on mass and force generation in mdx mice. Hum Gene Ther. 2002;13:1081–1090. doi: 10.1089/104303402753812485. [DOI] [PubMed] [Google Scholar]
- Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammels LM, Bosio E, Fragall CT, Grounds MD, van Rooijen N, Beilharz MW. Innate inflammatory cells are not responsible for early death of donor myoblasts after myoblast transfer therapy. Transplantation. 2004;77:1790–1797. doi: 10.1097/01.tp.0000131150.76841.75. [DOI] [PubMed] [Google Scholar]
- Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk11+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Chen YE, Earley JU, Heydemann A, Huber JM, Chien M, Ma A, McNally EM. Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle. J Clin Invest. 2004;114:1577–1585. doi: 10.1172/JCI23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally EM, Duggan D, Gorospe JR, Bonnemann CG, Fanin M, Pegoraro E, Lidov HG, Noguchi S, Ozawa E, Finkel RS, Cruse RP, Angelini C, Kunkel LM, Hoffman EP. Mutations that disrupt the carboxyl-terminus of gamma-sarcoglycan cause muscular dystrophy. Hum Mol Genet. 1996;5:1841–1847. doi: 10.1093/hmg/5.11.1841. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Tremblay JP. Pretreatment of myoblast cultures with basic fibroblast growth factor increases the efficacy of their transplantation in mdx mice. Muscle Nerve. 1995;18:834–841. doi: 10.1002/mus.880180806. [DOI] [PubMed] [Google Scholar]
- Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem (Tokyo) 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Partridge TA. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341. doi: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve. 1995;18:201–206. doi: 10.1002/mus.880180209. [DOI] [PubMed] [Google Scholar]
- Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Weller B, Karpati G, Lehnert S, Carpenter S, Ajdukovic B, Holland P. Inhibition of myosatellite cell proliferation by gamma irradiation does not prevent the age-related increase of the number of dystrophin-positive fibers in soleus muscles of mdx female heterozygote mice. Am J Pathol. 1991;138:1497–1502. [PMC free article] [PubMed] [Google Scholar]
- Brussee V, Merly F, Tardif F, Tremblay JP. Normal myoblast implantation in MDX mice prevents muscle damage by exercise. Biochem Biophys Res Commun. 1998;250:321–327. doi: 10.1006/bbrc.1998.9276. [DOI] [PubMed] [Google Scholar]
- Deconinck N, Ragot T, Marechal G, Perricaudet M, Gillis JM. Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc Natl Acad Sci USA. 1996;93:3570–3574. doi: 10.1073/pnas.93.8.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelouris EM. Athymic development in the mouse. Differentiation. 1973;1:437–450. doi: 10.1111/j.1432-0436.1973.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body gamma-irradiation: I. Lymphocytes and lymphoid organs. In Vivo. 2001;15:195–208. [PubMed] [Google Scholar]
- Lowenthal JW, Harris AW. Activation of mouse lymphocytes inhibits induction of rapid cell death by x-irradiation. J Immunol. 1985;135:1119–1125. [PubMed] [Google Scholar]
- Lubbe FH, Zaalberg OB. The effect of irradiation on the antibody enhancing helper T cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;41:1–13. doi: 10.1080/09553008214550011. [DOI] [PubMed] [Google Scholar]
- Yang Y, Haecker SE, Su Q, Wilson JM. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttinger M, Tafi E, Battaglia M, Coletta M, Cossu G. Allogeneic mesoangioblasts give rise to alpha-sarcoglycan expressing fibers when transplanted into dystrophic mice. Exp Cell Res. 2006;312:3872–3879. doi: 10.1016/j.yexcr.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L, Beauchamp JR, Tajbakhsh S, Buckingham ME, Partridge TA, Zammit PS. Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther. 2001;8:778–783. doi: 10.1038/sj.gt.3301463. [DOI] [PubMed] [Google Scholar]
- Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- Zacharias JM, Anderson JE. Muscle regeneration after imposed injury is better in younger than older mdx dystrophic mice. J Neurol Sci. 1991;104:190–196. doi: 10.1016/0022-510x(91)90309-u. [DOI] [PubMed] [Google Scholar]