Abstract
Primary abnormalities in permeability barrier function appear to underlie atopic dermatitis and epidermal trauma; a concomitant barrier dysfunction could also drive other inflammatory dermatoses, including psoriasis. Central to this outside-inside view of disease pathogenesis is the epidermal generation of cytokines/growth factors, which in turn signal downstream epidermal repair mechanisms. Yet, this cascade, if sustained, signals downstream epidermal hyperplasia and inflammation. We found here that acute barrier disruption rapidly stimulates mRNA and protein expression of epidermal vascular endothelial growth factor-A (VEGF-A) in normal hairless mice, a specific response to permeability barrier requirements because up-regulation is blocked by application of a vapor-impermeable membrane. Moreover, epidermal vegf−/− mice display abnormal permeability barrier homeostasis, attributable to decreased VEGF signaling of epidermal lamellar body production; a paucity of dermal capillaries with reduced vascular permeability; and neither angiogenesis nor epidermal hyperplasia in response to repeated tape stripping (a model of psoriasiform hyperplasia). These results support a central role for epidermal VEGF in the maintenance of epidermal permeability barrier homeostasis and a link between epidermal VEGF production and both dermal angiogenesis and the development of epidermal hyperplasia. Because psoriasis is commonly induced by external trauma [isomorphic (Koebner) phenomenon] and is associated with a prominent permeability barrier abnormality, excess VEGF production, prominent angiogenesis, and epidermal hyperplasia, these results could provide a potential outside-inside mechanistic basis for the development of psoriasis.
In normal skin, external trauma, which is inevitably accompanied by permeability barrier disruption, initiates signaling sequences within the epidermis that up-regulate metabolic processes leading to barrier recovery.1,2,3 Acute barrier abrogation, even by methods that produce minimal trauma, such as solvent treatment, stimulates both the release of the preformed cytokine, interleukin-1α,4 and the rapid up-regulation of several epidermis-derived, primary cytokines and growth factors.5,6,7 These mediators then stimulate epidermal lipid and DNA synthesis,8,9,10 two metabolic responses that contribute to permeability barrier recovery.11,12 Although these cytokine/growth factors regulate metabolic responses that lead to barrier repair, with sustained barrier abrogation they stimulate the downstream generation of additional cytokines and chemokines, ultimately provoking epidermal hyperplasia and inflammation.13 With still-further prolongation of the barrier abnormality, angiogenesis and fibroplasia eventually can emerge.14 Together, this sustained cytokine cascade forms the theoretical basis for the proposed outside-inside pathogenesis of several inflammatory dermatoses that display prominent barrier abnormalities.15,16,17,18,19
Vascular endothelial growth factor-A (VEGF-A) is a potent regulator of tissue angiogenesis.20,21,22,23 VEGF-A levels increase in response to local reductions in epidermal oxygen content, which induce a key transcription factor, hypoxia-inducible factor-1α (HIF-1α), that potently induces transcription of VEGF-A and its receptors.24 Accordingly, constitutive expression of epidermal VEGF-A and HIF-1α is low under basal (unperturbed) conditions, but both up-regulate rapidly in response to hypoxic stress.24,25 We hypothesized here first, that the metabolic responses to acute barrier disruption could be limited by oxygen availability from the dermal vasculature; and accordingly, that barrier requirements could regulate epidermal VEGF-A production. The importance of vascular delivery of oxygen for permeability barrier homeostasis is suggested by experiments in which permeability barrier recovery kinetics proceed at normal rates, even in an oxygen-free, external environment.26 Accordingly, we show here that permeability barrier requirements, rather than mechanical injury, are the specific signal for VEGF-A mRNA and protein up-regulation, suggesting that barrier requirements regulate the vascular delivery of oxygen for normal repair processes, through increased expression of VEGF. Moreover, we show that epidermal VEGF is important for normal barrier function because epidermal-localized vegf-a−/− knockout (KO) mice demonstrate abnormal permeability barrier homeostasis because of decreased VEGF-induced stimulation of epidermal lamellar body production. Deletion of epidermal vegf-a in mice also results in a marked reduction in the density of the microvasculature in the papillary dermis; and vascular permeability is markedly impaired in epidermal vegf−/− mice. Furthermore, epidermal vegf−/− mice do not develop epidermal hyperplasia in response to repeated external trauma, demonstrating a likely additional role for epidermal VEGF in the autocrine signaling of epidermal proliferation. Because psoriasis is characterized by a persistent barrier abnormality, as well as elevated VEGF, HIF-1α, and VEGF receptor expression,22,24 our results suggest that this mechanism could account for both the provocation of psoriasis (Koebner phenomenon), as well as development of the characteristic, dilate, and tortuous microvasculature in the papillary dermis of psoriasis.27,28,29,30,31
Materials and Methods
Animals and Functional Studies
Male hairless mice (Charles River, Philadelphia, PA) were studied between 6 to 8 weeks of age. To prepare epidermal-vegf KO mice, male mice bearing the cre recombinase transgene, under the control of the keratin 5 (k5) promoter (k5-cre+), were mated to females homozygous for the floxed vegf-a allele (vegf-aloxP/loxP) (both mouse lines were in a C57B1/6 × 129 mixed background, prepared in the laboratory of Dr. Erwin Tschachler, University of Vienna, Vienna, Austria). vegf-aΔk5-cre/+ heterozygotes (one vegf-a allele deleted in K5-cre-expressing cells) were bred to mice homozygous for the vegf-aloxP/loxP allele.32 Offspring with deletions of the vegf-a allele in k5-cre- expressing cells (vegf-aΔk5-cre/Δk5-cre) were matched with same age and sex vegf-aloxP/loxP littermates, and studied at 6 to 8 weeks of age. Transepidermal water loss (TEWL) was assessed with an electrolytic water analyzer (MEECO, Warrington, PA).33 Acute barrier disruption was achieved in normal hairless mice by sequential applications of 22 mm D-Squame tapes (CuDerm, Fort Worth, TX) until TEWL rates exceeded 5 mg/cm2/hour (Nm: <0.2 mg/cm2/hour). Typically, four to five animals were included in each experimental group (see figure legends). TEWL was assessed immediately after disruption, as well as 3, 6, and 24 hours after acute barrier disruption. In parallel experiments, animals with disrupted sites were immediately placed in one finger of a Latex glove for 1 to 6 hours to restore barrier competence artificially.6,34 vegf mice and wild-type (WT) littermates were shaved 24 hours before acute barrier disruption, and assessed at the same time points after barrier disruption by tape stripping, as above. Sustained barrier disruption was achieved by tape-stripping twice daily for 6 days until TEWL rates achieved levels of ≥5 mg/cm2/hour (two to three strippings).13 Vascular permeability was assessed in repeatedly tape-stripped, epidermal VEGF−/− and WT mice after intravenous infusion of fluorescein isothiocyanate-albumen and Evans Blue, as described.35 Briefly, mice were shaved on both flanks; tape-stripped the next day and twice daily for 3 more days on one flank until TEWL ≥3 mg/cm2/hour. Mice then were anesthetized with intraperitoneal injections of 4.2% chloral hydrate solution, wrapped in a paper towel, and immersed in a water bath at 37°C for 20 minutes to maximize dilatation of their tail veins. Two percent Evans Blue (0.05 ml), containing fluorescein isothiocyanate-albumen in normal saline, was injected into the tail vein with a 30-gauge needle. After 15 to 20 minutes (when the skin of WT mice took on a bluish cast), the mice were sacrificed, and the full-thickness skin was excised from both flanks and immediately frozen in liquid nitrogen. Frozen sections (0.6 μm) were examined by phase contrast and fluorescence microscopy.
Stimulation of Lamellar Body Production in Cultured Keratinocytes with Exogenous VEGF
Normal human keratinocytes were isolated from neonatal foreskins by a modification of the method of Pittelkow and Scott36 under an institutional review board approval protocol (University of California, San Francisco, CA). Second-passage keratinocytes were grown in keratinocyte growth medium, supplemented with bovine epidermal growth factor, bovine pituitary extract, insulin, hydrocortisone, and 0.07 mmol/L calcium chloride (Cascade Biologics, Portland, OR). After reaching 90% confluence, cells were cultured in Dulbecco’s and Ham F-12 medium (2:1, v/v), containing 1.2 mmol/L calcium, supplemented with 1, 5, or 10% fetal bovine serum (FBS), insulin (10 μg/ml), hydrocortisone (0.4 μg/ml), and ascorbic acid (50 μg/ml) (differentiation medium)37 for 3 days and further incubating in differentiation medium containing 5% FBS for 2 days. Cells then were treated with or without a mixture of recombinant human VEGF-121 and VEGF-165 (final concentration, 50 ng/ml each) (Shenandoah Biotechnology, Inc., Warwick, PA) in the differentiation medium containing 1% FBS, or in the differentiation medium under serum-free conditions, supplemented with bovine serum albumin conjugates of palmitate (0.3 mmol/L), oleate (0.63 mmol/L), linoleate (0.38 mmol/L), arachidonate (0.18 mmol/L), and α-topopherol (0.03 mmol/L). After a further 2 days, cells were removed as intact sheets with a rubber policeman and processed for electron microscopy, as described below.
RNA Isolation and Northern Hybridization
Epidermal sheets were obtained from freshly-obtained mouse flank skin by incubation in 10 mmol/L ethylenediaminetetraacetic acid in Ca-free, Mg-free phosphate-buffered saline for 30 minutes at 37°C. After total RNA was obtained from frozen epidermal sheets, and poly(A)+ RNA was isolated,38 and electrophoresed before hybridization with a 32P-labeled cDNA consensus probe for VEGF-A.23 Membranes then were washed and the same mRNA samples were probed for the housekeeping gene, cyclophilin (ClonTech, Palo Alto, CA), which did not change significantly after either barrier disruption or occlusion.
Immunostaining for VEGF, DNA, and Vessel Markers
VEGF immunostaining was performed in biopsy samples from three each of WT and KO mice at various times points after acute barrier disruption, using a primary, chick polyclonal antibody from Abcam (Cambridge, MA). The secondary antibody was a biotinylated, goat anti-chicken antibody (Vector Laboratories, Burlingame, CA). Factor 8 immunostaining was performed in 5-μm paraffin-embedded sections, using an anti-human primary antibody (DAKO, Carpinteria, CA), a biotinylated anti-rabbit goat secondary antibody (Vector), and an avidin-biotin complex (ABC kit, Vector). Immunofluorescence of CD31 and NG2 was performed in 5-μm cryosections of WT and VEGF−/− mouse skin samples. Biopsies were fixed briefly in 2% paraformaldehyde, and then incubated with buffered bovine serum albumin to block nonspecific binding. Primary affinity-purified polyclonal antibodies against CD31 (BD Biosciences, San Jose, CA) and NG2 (Chemicon, Temecula, CA) were applied to 5-μm cryosections at concentrations of 0.5 μg/ml and of 0.625 μg/ml, respectively. Both immunoreagents were diluted in 10 mmol/L Tris buffer, pH 7.6, containing 4% bovine serum albumin, 1% teleost skin gelatin, 0.1% Tween 20, and 500 mmol/L NaCl. The binding of CD31 was detected by affinity-purified, Alex Fluor 488 donkey anti-rat IgG (Invitrogen, Carlsbad, CA), whereas NG2 antibody was detected by affinity-purified, Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen). Propidium iodide was used as a counterstain. Omission of the primary antibody resulted in either low levels of nonspecific staining or absence of staining.
For quantitation of vessel density in the upper dermis, micrographs were taken of 10 to 15 fields each from the superficial dermis from each sample, coded, randomized, and the density of dermal capillaries per 10 μm2 was quantitated by a blinded observer. All immunostained vessels in the papillary dermis, except for those encircling hair follicles, were counted, extending downward to the level of the bulb of the hair follicle. Vessel densities were calculated as mean ± SEM for each sample type. For PCNA staining, 5-μm sections of paraffin-embedded tissue samples were labeled first with antiKi67 antibody, followed by detection with ABC peroxidase reagents (both from Caltag Laboratories, Burlingame, CA). All statistical differences were determined by the Student’s t-test.
Electron Microscopy
Biopsy samples were taken from all experimental groups (n = 3 to 5 animals each) and processed for electron microscopy. Samples were minced to <0.5 mm3, fixed in modified Karnovsky’s fixative overnight, and postfixed in either 0.2% ruthenium tetroxide (RuO4), or 1% aqueous osmium tetroxide (OsO4), containing 1.5% potassium ferrocyanide. After fixation, all samples were dehydrated in graded ethanol solutions, and embedded in an Epon-epoxy mixture. Ultrathin sections were examined, with our without further contrasting with lead citrate, in an electron microscope (Zeiss 10A; Carl Zeiss, Thornwood, NY) operated at 60 kV.
For quantitative analysis of lamellar body densities, 10 to 15 micrographs of the outer epidermis (×22,500 magnification) from three blocks each from three vegf−/− mice were coded, randomized, and assessed by stereological methods, as follows. To quantitate differences in lamellar body density, micrographs were taken from the same blocks at various points along the stratum granulosum-SC junction, and the frequency of protrusions/μm length of interface was measured in coded, randomized micrographs, as described previously.39 Lamellar body and lamellar body secretory densities were calculated as mean ± SEM and compared statistically by the Student’s t-test.
Results
Epidermal VEGF Expression Is Regulated by Permeability Barrier Requirements
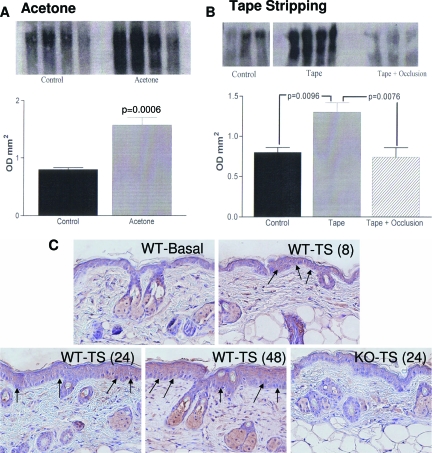
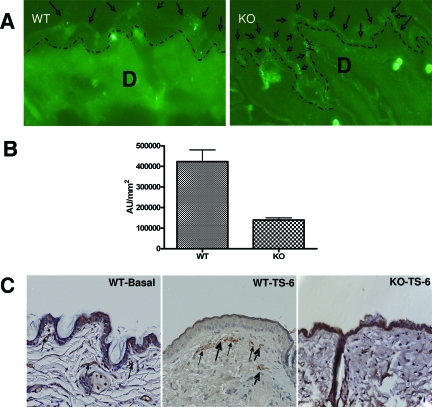
To assess whether vegf-a expression is regulated by acute barrier perturbations, we abrogated the permeability barrier in hairless mice by two unrelated methods (sequential cellophane tape stripping, or alternatively by a minimally-injurious method; ie, repeated acetone swabs). By using a consensus probe for the full-length vegf-a cDNA, we found epidermal mRNA levels for vegf increased by 1 hour after acute disruption, independent of the method of perturbation, peaking 3 hours after acute disruption (Figure 1, A and B; acetone and tape stripping, respectively). To assess whether the increase in vegf mRNA levels is barrier-related, or a nonspecific response to external injury, we next artificially restored normal barrier function by application of a vapor-impermeable (Latex) wrap, immediately after tape stripping of mouse skin. Artificial barrier restoration by occlusion primarily blocked the expected increase in vegf mRNA levels (Figure 1B; P < 0.01 for occluded versus nonoccluded). Finally, acute barrier disruption increased vegf immunostaining in epidermis for 4 hours, returning to normal by 48 hours (Figure 1C). With repeated tape stripping, epidermal hyperplasia develops in parallel with a further increase in vegf (Figure 1C). Notably, vegf was virtually undetectable in KO epidermis, even after repeated tape stripping. Together, these results demonstrate that epidermal VEGF-A expression up-regulates in response to acute abrogations of the permeability barrier, and that increased expression is linked to barrier requirements, rather than representing a nonspecific response to epidermal injury.
Figure 1.
Permeability barrier disruption specifically up-regulates VEGF-A expression. Male hairless mice (three groups of three to four animals each) were treated with either repeated acetone swabs (A) or repeated cellophane (Scotch-type) tape strippings (B) to both flanks. One additional group of tape-stripped animals was immediately occluded within fingers of a Latex glove (B). Untreated mice served as controls (n = 4 each). Experimental results were replicated in two subsequent experiments. Samples were taken 1, 3, 6, and 24 hours after acute barrier disruption. Changes at 3 hours are shown, when maximal changes occurred. Epidermis was separated from dermis; mRNA was isolated from epidermis, and Northern blots were prepared, as described in the Materials and Methods and in Harris and colleagues,38 using a full-length cDNA probe that recognizes VEGF-A and its isomeric products.23,82 Each lane represents VEGF-A mRNA isolated from a single mouse. Cyclophilin, which did not change with barrier disruption, was probed as the housekeeping gene in the same mRNA samples, and quantitative results were normalized to cyclophilin levels, and shown as mean ± SEM. C: Changes in VEGF immunostaining with time after tape stripping of epidermal VEGF−/− and WT mice (three biopsies were examined at each time point). Increased expression occurs by 4 hours (not shown), and increases with time in parallel with development of epidermal hyperplasia. Neither epidermal VEGF nor epidermal hyperplasia (arrows) appears in similarly tape-stripped KO mice. 200× magnification.
Epidermal VEGF Is Required for Normal Permeability Barrier Homeostasis
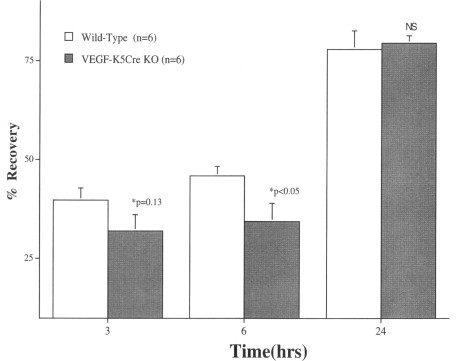
Although the previous studies show that changes in permeability requirements specifically regulate VEGF expression, they do not address the specific function(s) of epidermal VEGF. To address the potential roles of epidermal VEGF in normal skin, we first assessed barrier recovery kinetics in transgenic mice with epidermis-specific deletion of Vegf-a.32 Basal barrier function, assessed as TEWL levels (mg/cm2/hour), was comparable in KO and WT mice (not shown). However, barrier recovery kinetics appeared to be delayed by 3 hours after acute barrier disruption, and were significantly delayed at 6 hours, becoming comparable to recovery rates in WT mice by 24 hours (Figure 2). These results demonstrate that epidermal VEGF is required for normal permeability barrier homeostasis.
Figure 2.
Barrier recovery is delayed in transgenic mice with epidermal vegf-a−/− mice. For preparation of epidermis-localized VEGF KO mice, see Rossiter and colleagues.32 Acute barrier disruption was achieved with repeated D-Squame tape stripping until TEWL levels were ≥5 mg/cm2/hour, 24 hours after affected (vegf/f-k5Cre) and WT littermates (n = 6 each) were shaved. Barrier recovery was quantitated as percentage of normal (<0.2 mg/cm2/hour) 3, 6, and 24 hours after barrier disruption (±SEM).
Structural Basis for Abnormal Permeability Barrier Function in VEGF−/− Mice
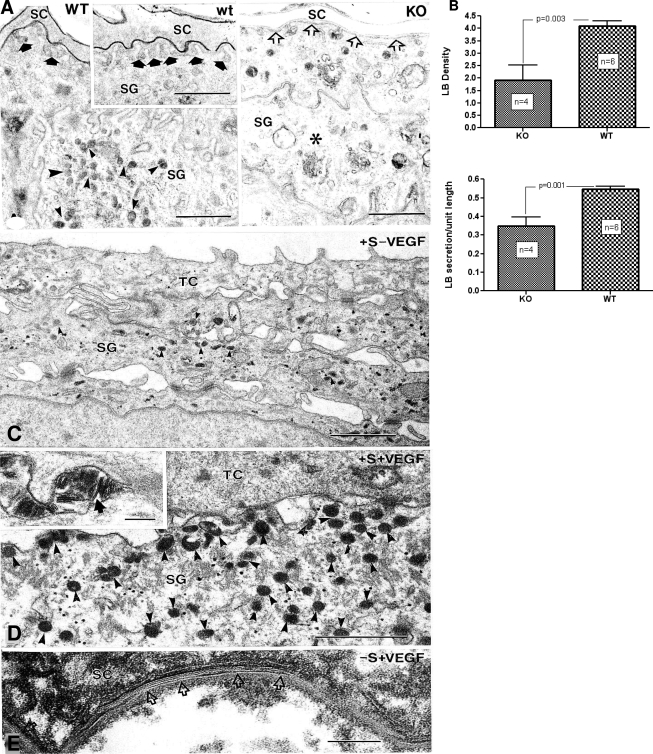
In normal skin, permeability barrier function recovers quickly from acute insults because of rapid up-regulation of epidermal lipid synthesis, and accelerated production and secretion of epidermal lamellar bodies.2,3 Therefore, we next assessed whether abnormal permeability barrier homeostasis in epidermal vegf−/− mice could be attributed to decreased production and/or secretion of epidermal lamellar bodies. Even under basal conditions; ie, before acute barrier perturbations, the density of epidermal lamellar bodies appeared much lower in vegf−/− than in WT mice (Figure 3, B versus A), an observation confirmed by quantitative measurements in randomized coded micrographs (Figure 3C). Decreased lamellar body production, in turn, correlated with a decreased amount of secreted lamellar body contents at the stratum granulosum-SC interface in KO mice (Figure 3, B versus A and inset in A; quantitative results are in Figure 3D).
Figure 3.
Abnormal permeability barrier correlates with decreased lamellar body production and secretion in vegf-a−/− mice. A: Abundant lamellar bodies (small arrowheads) are present in cytosol of stratum granulosum (SG) cells in WT epidermis. A: Inset: Abundant secreted lamellar body contents at SG-stratum corneum (SC) interface (solid arrows). Fewer lamellar bodies (asterisks) are in SG cytosol of vegf−/− (KO) mice, and there is less secreted contents at the SG-SC interface (open arrows). B: Quantitative data for lamellar body density and secretion ± SEM, respectively, in KO and WT mice. (n = number of samples each of WT and KO skin.) A, C, and D: Osmium tetroxide postfixation. E: Ruthenium tetroxide postfixation. C and D: Transitional cell (TC) postconfluent, cultured human keratinocytes with added 1% FBS ± exogenous recombinant VEGF (see Materials and Methods). Density of cytosolic lamellar bodies (arrowheads) markedly increases in cultures supplemented with VEGF. E: Cultured keratinocytes grown in a serum-free medium (-s) to postconfluence, supplemented with free fatty acids ± exogenous rVEGF (see Materials and Methods). VEGF-supplemented cultures show multiple arrays of lamellar bilayers within extracellular spaces of SC (arrows). Scale bars: 1 μm (A, A inset); 0.1 μm (C, D, E, E inset).
To assess whether the depletion of lamellar bodies in KO mouse epidermis reflects the lack of autocrine stimulation by VEGF, we next exposed cultured human keratinocytes to exogenous recombinant VEGF. When grown in both 10% fetal bovine serum under reducing conditions (50 μm ascorbic acid), postconfluent keratinocytes generate large numbers of lamellar bodies.40 In the presence of lower concentrations of FBS, fewer lamellar bodies are generated, and in the absence of FBS, proliferation ceases and the cells differentiate and cornify. We first assessed whether VEGF would stimulate lamellar body production when FBS concentrations were reduced to 1%. Lamellar bodies were very sparse in VEGF-free cultures, but were present in abundance in VEGF-supplemented cultures (Figure 3, C versus D), and variably secreted into the extracellular spaces (Figure 3D, inset). In serum-free, postconfluent cultures, supplemented with bovine serum albumin-free fatty acid conjugates, most of the keratinocytes appeared to be in various stages of terminal differentiation, and neither cytosolic lamellar bodies nor secreted lamellar membranes were seen (not shown). In contrast, when rVEGF was added to these serum-free cultures, lamellar bodies were present in noncornified cells, and abundant secreted multiple arrays of lamellar body-derived bilayers were present between corneocytes (Figure 3E). These results show that VEGF directly stimulates lamellar body production by epidermal keratinocytes. Together, these results show that defective permeability barrier homeostasis in epidermal vegf−/− mice correlates with diminished production/secretion of epidermal lamellar bodies; and that VEGF stimulates epidermal lamellar body production.
Deletion of Epidermal VEGF Leads to Depletion of Microvasculature in the Outer Dermis
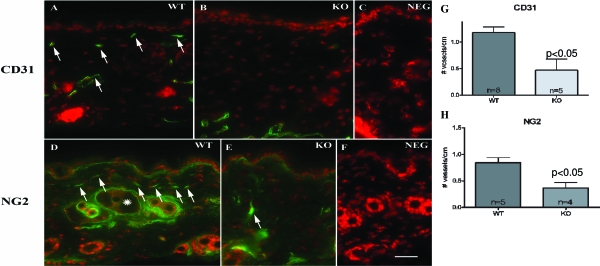
To assess further the role of epidermal VEGF in normal skin, we next assessed changes in the microvasculature in the upper dermis of epidermal vegf−/− versus WT mice. Three vessel markers were used for the immuno-identification of superficial capillaries; ie, the endothelial cell markers, CD31 and factor 8, and a pericyte marker, NG2. As previously reported by Rossiter and colleagues,32 deletion of epidermal VEGF resulted in a marked paucity of upper dermal microvasculature, assessed with all three markers (Figure 4, A–C: CD31; D–F: NG2; see below for factor 8), which was confirmed by quantitative studies in randomized, coded micrographs (Figure 4, G and H). In contrast, vessel densities did not differ in either the deeper levels of the dermis, or surrounding pilosebaceous follicles in epidermal vegf−/− mouse skin (not shown). These results demonstrate a specific requirement for epidermal VEGF for the formation of microvasculature in the upper dermis.
Figure 4.
Dermal microvasculature is reduced in epidermal vegf−/− mice. Frozen sections (5 μm) from epidermal vegf−/− versus WT biopsies (n = 3 each) were immunostained with a primary antibody against the endothelial cell marker, CD31 (A–C), or the pericyte marker, NG2 (D–F). C and F are negative controls from CD31 and NG2, respectively. Immunostained capillaries are indicated by arrows. NG2 also labels cells that encircle pilosebaceous structures (D, asterisk). The density of immunopositive vessels in the papillary dermis (-pilosebaceous structures) was quantitated in randomly-obtained, coded micrographs (G, CD31; H, NG2). A–F, Mag bar = 10 μm.
Repeated Barrier Disruption Stimulates Angiogenesis and Vascular Permeability in Wild-Type, but Not in Epidermal-VEGF−/− Skin
We next assessed whether repeated barrier disruption stimulates angiogenesis and/or vascular permeability in epidermal vegf−/− versus WT skin. Repeated barrier disruption increased the density of factor 8-positive capillaries in the upper dermis of WT, but not in epidermal vegf−/− mice, a change that could be detected as early as 6 hours after acute barrier disruption (Figure 5C). Because the diameter of dermal microvasculature also appeared to increase after barrier disruption in WT mice, suggestive of increased vascular permeability, we next assessed differences in vascular permeability in epidermal vegf−/− and WT mice after intravenous injections of 1% Evans blue with a fluorescent tracer (fluorescein isothiocyanate-albumen). The injected Evans blue turned WT mouse skin visibly blue, whereas no color change occurred in KO mice (not shown). Frozen sections examined 20 minutes after dye injection revealed diffuse fluorescence in the upper dermis of WT mice, but little or no fluorescence in the upper dermis of epidermal vegf−/− mice (Figure 5A), a difference confirmed by quantitative densitometry measurements (more than fourfold more fluorescence in the upper dermis of WT than in KO mice; Figure 5B). Together, these results show that the epidermal VEGF-induced changes in angiogenesis are required for basal vascular permeability and changes in angiogenesis that occur in response to barrier perturbations.
Figure 5.
Angiogenesis and vascular permeability are impaired in epidermal vegf−/− mice. A: Epidermal KO and WT mice (n = 3 each) were perfused (by tail vein injection) with 1% fluorescein isothiocyanate-albumen in normal saline 40 minutes before biopsy. WT dermis shows increased fluorescence throughout the dermis compared to the KO, suggesting reduced vascular permeability in the KO skin (dotted lines indicate dermo-epidermal junction; arrows indicate surface of SC). B: Fluorescence from samples in A was quantitated by scanning randomized, coded photomicrographs (controlled for equal exposure level) with a Fujifilm luminescent image analyzer (LAS-3000), using incident light and 1/4 second exposures, producing digitized images. The intensity of fluorescence of the papillary dermis from defined areas from four biopsies were measured at ×40 (arbitrary units), and compared statistically (±SEM). C: Six hours after tape stripping, WT mice demonstrate prominent vasodilatation and an increase in factor 8-positive immunostaining of capillaries in the papillary dermis (arrows), whereas KO mice demonstrate no change from basal immunostaining (see Figure 4, A–F).
Epidermal VEGF Regulates the Hyperplastic Response to Sustained Barrier Disruption
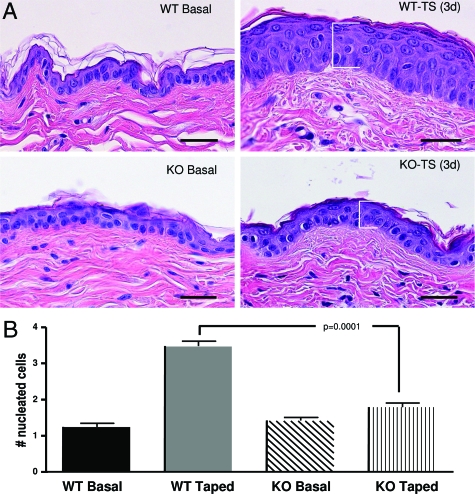
In addition to its effects on epidermal structure and function (as shown here), as well as its well-known effects on angiogenesis and vascular permeability, VEGF displays putative autocrine effects on keratinocyte function, including regulation of keratinocyte proliferation.41 Therefore, we next assessed whether epidermal VEGF is required for the development of epidermal hyperplasia; ie, the epidermal proliferative response to repeated barrier abrogation by tape stripping, a well-established mouse model of epidermal hyperplasia.13,42 Repeated tape stripping of WT skin for 3 to 6 days again resulted in marked epidermal hyperplasia (Figure 6A). In contrast, repeated tape stripping of epidermal vegf−/− mice did not stimulate epidermal hyperplasia (Figure 6A). Quantitative data for changes in the number of nucleated cell layers, obtained from coded and randomized micrographs, confirmed these observations (Figure 6B). Together, these results show that epidermal VEGF is required for the development of epidermal hyperplasia in response to sustained barrier disruption.
Figure 6.
Epidermal vegf−/− mice do not develop epidermal hyperplasia in response to repeated barrier disruption. A: Epidermal VEGF−/− and WT mice were tape-stripped twice daily for 5 days. Whereas WT mice develop epidermal hyperplasia, epidermal VEGF−/− mice fail to develop hyperplasia. H&E staining. B: Hyperplasia was quantified as the number of epidermal nuclei hit by straight edge rule ruler dropped at random perpendicular to the epidermis in multiple sections (n = 20) from four animals in each group. Scale bars = 20 μm.
Discussion
Maintenance of epidermal permeability barrier homeostasis is critical for survival of terrestrial mammals, who are threatened continually with desiccation by exposure to a xeric external environment.2,3 Hence, perturbations in barrier function, regardless of their nature (eg, organic solvents, surfactants, or tape stripping), induce metabolic responses in the underlying epidermis that rapidly normalize barrier function.3,43 These barrier-specific responses include in sequence: rapid up-regulation of epidermal lipid synthesis (1 to 3 hours); accelerated production and secretion of the lipid/protein contents of epidermal lamellar bodies (3 to 6 hours); and increased DNA synthesis (16 to 24 hours), the last of which provides both additional lipid-generating keratinocytes, as well as a new generation of corneocytes.44 Whether nutrient/oxygen delivery from the circulation to the epidermis is modulated to sustain these multiple, energy-requiring processes is not known. Because permeability barrier homeostasis remains normal, even when externally-perturbed mouse skin is maintained under an inert, argon atmosphere,26 the increased metabolic demands imposed by acute barrier disruption likely rely on the underlying vasculature.
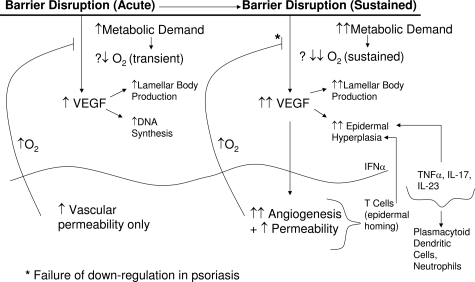
Here, we assessed first, whether epidermal VEGF-A production is regulated specifically by perturbations in permeability barrier function; and further, whether epidermal VEGF is required for normal permeability barrier homeostasis. The present studies clearly demonstrate a link between permeability barrier function and epidermal VEGF expression: epidermal VEGF-A mRNA and protein production rapidly up-regulates after acute barrier disruption, independent of the method of perturbation. Because the barrier disruption-induced increase in VEGF-A mRNA is blocked by artificial restoration of normal barrier function, increased VEGF-A expression is linked specifically to permeability barrier requirements, rather than representing a nonspecific response to epidermal injury (which should not occur with acetone-induced barrier disruption). It is likely that acute stress to the barrier increases epidermal metabolic demand, resulting in (transient) local hypoxia (Figure 7), which in turn, likely increases expression of HIF-1α,24 leading to increased VEGF-A mRNA production. But the effects of barrier disruption on either tissue oxygen levels or HIF-1α expression were not assessed here. The relatively-rapid increase in epidermal VEGF, in turn, stimulates angiogenesis in the superficial dermis, as well as allowing both basal vascular permeability and the increase in permeability that follows acute barrier disruption, which likely increases oxygen delivery to the epidermis. A recent article suggests that skin (epidermal) hypoxia stimulates vascular permeability by a rapid increase in nitric oxide.45 Thus, the changes in vascular permeability that we observed in vegf−/− mice may not reflect a failure in permeability barrier function, but likely rather a prior absence of dermal vasculature. With the return of permeability barrier function to normal, the transient demand for increased oxygen likely declines, down-regulating VEGF/HIF levels (Figure 7, left side). Because we used a consensus probe for VEGF-A mRNA, our study did not address whether the parental molecule alone, and/or one or more VEGF isoforms are generated in response to acute barrier disruption. Although barrier requirements also could regulate expression of one or more VEGF receptors, our previous studies showed that epidermal receptor levels for cytokines and growth factors rarely change after barrier disruption.46 Hence, this potential mechanism was not evaluated here.
Figure 7.
Potential role of epidermal VEGF in normal permeability barrier homeostasis and in psoriasis. Hypothetical scheme for regulation of epidermal VEGF in response to acute barrier disruption (left). Transient hypoxia (?↓O2) stimulates VEGF production, which stimulates vascular permeability. Re-oxygenation of epidermis then down-regulates VEGF production. In psoriasis, a sustained barrier abnormality results in even more intense metabolic activity, with a further reduction in tissue oxygen levels (shown as ?↓↓), leading to sustained production and increased levels of VEGF. Sustained VEGF overproduction, in turn, leads to epidermal hyperplasia, a superabundance of lamellar bodies, and the prominent capillary abnormality in psoriasis. According to this scheme, the TH1 infiltrate in psoriasis is recruited downstream in psoriasis, but it can further drive epidermal hyperplasia.
Our studies demonstrate not only the regulation of epidermal VEGF production in response to acute permeability barrier alterations, but also the importance of epidermal VEGF for permeability recovery after acute perturbation. Permeability barrier recovery is abnormally delayed in mice with epidermal localized deletion of vegf−/−. Epidermal vegf−/− mice display a paucity of lamellar bodies, providing a structural basis for the barrier abnormality. Because exogenous VEGF stimulates lamellar body production in cultured keratinocytes, this growth factor appears to regulate organelle production in an autocrine manner. Pertinently, the density of lamellar bodies is far greater in psoriatic than in normal epidermis,47 presumably because of hyperstimulation by excess VEGF. Epidermal vegf−/− mice display a diminished density of microvasculature in the upper dermis, demonstrating an additional, paracrine function of this growth factor. Dermal capillaries normally arborize immediately beneath the epidermis, exchanging nutrients, metabolites, and gases with surrounding/overlaying tissues.23,48 With depletion of these vessels in epidermal vegf−/− mice, it is not surprising that permeability barrier homeostasis would be impacted adversely, as shown here. Despite the likely ongoing generation of other cutaneous angiogenic factors, such as fibroblast growth factor-2,49 interleukin 8,50 epidermal parathyroid hormone-related protein,51 as well as extra-epidermal sources of VEGF, these results demonstrate a specific requirement for epidermal VEGF production in the maintenance of the microvasculature of the upper dermis. Thus, even if compensatory up-regulation of extra-epidermal VEGF or other potential signals of angiogenesis occurs, it does not compensate for loss of epidermal VEGF.
As the results of these studies emerged, we realized that they could be relevant for the provocation of psoriasis. Previous studies have shown characteristic dermal capillary abnormalities in psoriasis27,28,52 and a potential role for epidermal VEGF in the pathogenesis of psoriasis.22 Our observations provide several additional mechanistic insights that could be of direct relevance for the provocation of psoriasis, a disease that is often triggered by external injury.53,54,55 First, using analogous insults to the external skin surface, our results show that it is not epidermal injury, but rather permeability barrier disruption, an inevitable accompaniment of such trauma, which likely represents the specific trigger for provocation of psoriasis (Figure 7). Within minutes of barrier disruption, epidermal generation of several pro-inflammatory primary cytokines, such as interleukin-1α, increases,5,6,7,56 and one or more of these soluble factors could in turn up-regulate VEGF production,57,58,59,60 independent of, or in addition to, hypoxic stimulation. Whether barrier requirements up-regulate VEGF through transient hypoxia or indirectly via a previous increase in a stimulatory cytokine, such as interleukin-1α, was not addressed here. Second, occlusion alone can clear individual psoriatic lesions, and accordingly not only VEGF, but also cytokine/growth factor production declines after occlusion of chronically disrupted skin.34 Pertinently, occlusion of psoriatic skin likewise down-regulates expression of most of the cytokines that comprise the psoriasis cytokine network (compare Bonifati and Ameglio61 to Wood et al34).
Although immunological models have long dominated views on the pathogenesis of inflammatory skin disease, primary inherited abnormalities in epidermal barrier function are now strongly linked to atopic dermatitis.62,63,64,65 Evidence in support of an epidermal barrier-driven (outside-inside) pathomechanism of psoriasis is also gaining traction, based on well-established clinical observations that: i) new psoriatic lesions develop at sites of external trauma (isomorphic or Koebner phenomenon)53,54,55; ii) severity of the psoriatic phenotype correlates with the extent of the permeability barrier abnormality66; iii) barrier restoration by occlusion alone can clear psoriatic lesions67,68,69,70; as well as iv) studies in transgenic mice with deletions/overexpression of several different epidermal proteins that develop a psoriasiform skin phenotype.71,72 Pertinently, overexpression of vegf in the epidermis of transgenic mice provokes a psoriasiform dermatosis, including prominent angiogenesis, inflammation, and epidermal hyperplasia,73 whereas conversely, pharmacological inhibition of either VEGF expression (eg, with retinoids or cyclosporin A),74,75 or angiogenesis76 can improve psoriasis.
Of further potential relevance to the pathogenesis of psoriasis, we showed that epidermal VEGF production is required not only for the development of dermal angiogenesis, but also as an autocrine regulator of epidermal hyperplasia, two distinctive features of psoriasis. Although epidermal hyperplasia occurs with sustained barrier abnormalities in normal mice, it does not occur in similarly-treated epidermal vegf−/− mice subjected to comparable barrier insults. Because VEGF displays autocrine/paracrine effects that extend beyond lympho- and vascular-angiogenesis permeability,23,77,78 it could be important for permeability barrier homeostasis by mechanisms not duplicated by other growth factors/cytokines. In fact, the repeated tape-stripping studies strongly suggest a second autocrine function for epidermal VEGF as a key autocrine signal of the proliferative response to barrier disruption. Yet this study did not address whether epidermal VEGF stimulates epidermal hyperplasia directly, or through other downstream, paracrine mechanisms.
Together, these studies suggest that sustained permeability barrier-induced increases in epidermal VEGF expression could explain not only the distinctive angiogenesis, but also the epidermal hyperplasia that is a key feature of psoriasis (Figure 7, right side). Yet, although our results could potentially link epidermal barrier status with development of the characteristic capillary abnormalities and epidermal hyperplasia in psoriasis, it is important to emphasize that sustained barrier disruption alone does not suffice to produce psoriasis. Development of the full psoriatic phenotype requires the participation of additional abnormalities in the immune system.1,79,80,81 Thus, it must be emphasized that the mechanisms that lead from barrier disruption to development of the stable psoriatic phenotype are complex, and remain primarily unresolved.
Acknowledgments
We dedicate this article to Dr. Irwin M. Braverman (Yale University Medical School), who first characterized the capillary abnormality in psoriasis and recognized its potential importance for disease pathogenesis.
We thank Ms. Rachael Allen, Joan Wakefield, and Jerelyn Magnusson for providing valuable editorial assistance; and Sandra Chang, B.S., for providing valuable technical assistance.
Footnotes
Address reprint requests to Peter M. Elias, M.D., Dermatology Service (190), VA Medical Center, 4150 Clement St., San Francisco, CA 94121. E-mail: eliasp@derm.ucsf.edu.
Supported by the National Institutes of Health [grants AR19098, 39448 (PP), 49932, 50629], the Medical Research Service, the US Department of Veteran Affairs, and the CE.R.I.E.S. Foundation.
References
- Nickoloff BJ, Bonish BK, Marble DJ, Schriedel KA, DiPietro LA, Gordon KB, Lingen MW. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J Invest Dermatol Symp Proc. 2006;11:16–29. doi: 10.1038/sj.jidsymp.5650010. [DOI] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou A, Elias PM, Grunfeld C, Feingold KR, Wood LC. Amphiregulin and nerve growth factor expression are regulated by barrier status in murine epidermis. J Invest Dermatol. 1997;108:73–77. doi: 10.1111/1523-1747.ep12285638. [DOI] [PubMed] [Google Scholar]
- Ye J, Garg A, Calhoun C, Feingold KR, Elias PM, Ghadially R. Alterations in cytokine regulation in aged epidermis: implications for permeability barrier homeostasis and inflammation. I. IL-1 gene family. Exp Dermatol. 2002;11:209–216. doi: 10.1034/j.1600-0625.2002.110303.x. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Schutze S, Forl M, Kronke M, Proksch E. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999;104:1761–1770. doi: 10.1172/JCI5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow HJ. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be interleukin 1 or a related protein. Proc Natl Acad Sci USA. 1987;84:1940–1944. doi: 10.1073/pnas.84.7.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR. Barrier function regulates epidermal lipid and DNA synthesis. Br J Dermatol. 1993;128:473–482. doi: 10.1111/j.1365-2133.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Grubauer G, Feingold KR, Elias PM. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987;28:746–752. [PubMed] [Google Scholar]
- Denda M, Wood LC, Emami S, Calhoun C, Brown BE, Elias PM, Feingold KR. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Arch Dermatol Res. 1996;288:230–238. doi: 10.1007/BF02530090. [DOI] [PubMed] [Google Scholar]
- Tagami H, Kikuchi K. Feingold KR, Elias PM, editors. New York: Taylor & Francis; Diseases that Affect Barrier Function. 2006:pp 447–468. [Google Scholar]
- Elias PM. Stratum corneum architecture, metabolic activity and interactivity with subjacent cell layers. Exp Dermatol. 1996;5:191–201. doi: 10.1111/j.1600-0625.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–126. [PubMed] [Google Scholar]
- Taïeb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999;41:177–180. doi: 10.1111/j.1600-0536.1999.tb06125.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T. Molecular mechanisms of VEGF-A action during tissue repair. J Invest Dermatol Symp Proc. 2006;11:79–86. doi: 10.1038/sj.jidsymp.5650016. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122:xiv–xv. doi: 10.1046/j.0022-202X.2003.22140.x. [DOI] [PubMed] [Google Scholar]
- Perry BN, Arbiser JL. The duality of angiogenesis: implications for therapy of human disease. J Invest Dermatol. 2006;126:2160–2166. doi: 10.1038/sj.jid.5700462. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Solovan C, Rosenberger AD, Jinping L, Treudler R, Frei U, Eckardt KU, Brown LF. Up-regulation of hypoxia-inducible factors in normal and psoriatic skin. J Invest Dermatol. 2007;127:2445–2452. doi: 10.1038/sj.jid.5700874. [DOI] [PubMed] [Google Scholar]
- Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Sturzl M, Tschachler E. Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest. 1996;75:647–657. [PubMed] [Google Scholar]
- Jackson SM, Mao-Qiang M, Elias PM, Feingold KR. Role of exogenous oxygen in cutaneous barrier repair. Skin Pharmacol. 1994;7:316–319. doi: 10.1159/000211312. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Yen A. Microcirculation in psoriatic skin. J Invest Dermatol. 1974;62:493–502. doi: 10.1111/1523-1747.ep12681007. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Sibley J. Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol. 1982;78:12–17. doi: 10.1111/1523-1747.ep12497850. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Stenn KS, Fernandez LA, Braverman IM. Angiogenic properties of normal and psoriatic skin associate with epidermis, not dermis. Lab Invest. 1989;61:162–165. [PubMed] [Google Scholar]
- Petzelbauer P, Pober JS, Keh A, Braverman IM. Inducibility and expression of microvascular endothelial adhesion molecules in lesional, perilesional, and uninvolved skin of psoriatic patients. J Invest Dermatol. 1994;103:300–305. doi: 10.1111/1523-1747.ep12394720. [DOI] [PubMed] [Google Scholar]
- Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Pammer J, Rendl M, Haigh J, Wagner EF, Tschachler E. Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res. 2004;64:3508–3516. doi: 10.1158/0008-5472.CAN-03-2581. [DOI] [PubMed] [Google Scholar]
- Man MQ, Wood L, Elias PM, Feingold KR. Cutaneous barrier repair and pathophysiology following barrier disruption in IL-1 and TNF type I receptor deficient mice. Exp Dermatol. 1999;8:261–266. [PubMed] [Google Scholar]
- Wood LC, Elias PM, Sequeira-Martin SM, Grunfeld C, Feingold KR. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994;103:834–838. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Ghannadan M, Gruber F, Mildner M, Fodinger D, Tschachler E. Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 2007;21:3994–4004. doi: 10.1096/fj.07-8720com. [DOI] [PubMed] [Google Scholar]
- Pittelkow M, Scott R. New techniques for the in vitro culture of human skin keratinocytes and perspectives on their use for grafting of patients with extensive burns. Mayo Clin Proc. 1986;61:771–777. doi: 10.1016/s0025-6196(12)64815-0. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Harris IR, Farrell AM, Grunfeld C, Holleran WM, Elias PM, Feingold KR. Permeability barrier disruption coordinately regulates mRNA levels for key enzymes of cholesterol, fatty acid, and ceramide synthesis in the epidermis. J Invest Dermatol. 1997;109:783–787. doi: 10.1111/1523-1747.ep12340962. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, Jiang YJ, Fluhr JW, Feingold KR, Elias PM, Mauro TM. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847–2856. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Nardo AD, Collins V, Elias PM, Holleran WM. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J Invest Dermatol. 2003;120:662–669. doi: 10.1046/j.1523-1747.2003.12098.x. [DOI] [PubMed] [Google Scholar]
- Man XY, Yang XH, Cai SQ, Yao YG, Zheng M. Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med. 2006;12:127–136. doi: 10.2119/2006-00024.Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kömüves LG, Hanley K, Man MQ, Elias PM, Williams ML, Feingold KR. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPARalpha activators restores tissue homeostasis. J Invest Dermatol. 2000;115:361–367. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The regulation and role of epidermal lipid synthesis. Adv Lipid Res. 1991;24:57–82. doi: 10.1016/b978-0-12-024924-4.50007-9. [DOI] [PubMed] [Google Scholar]
- Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, Mauro T, Crumrine D, Roelandt T, Houben E, Elias PM, Feingold K. Acute modulations in permeability barrier functions regulate epidermal cornification: Role of caspase 14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, Feingold KR. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol. 1997;6:98–104. doi: 10.1111/j.1600-0625.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Mottaz JH, Zelickson AS. Keratinosomes in psoriatic skin. Acta Derm Venereol. 1975;55:81–85. [PubMed] [Google Scholar]
- Braverman IM. The cutaneous microcirculation. J Invest Dermatol Symp Proc. 2000;5:3–9. doi: 10.1046/j.1087-0024.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284. [PMC free article] [PubMed] [Google Scholar]
- Diamond AG, Gonterman RM, Anderson AL, Menon K, Offutt CD, Weaver CH, Philbrick WM, Foley J. Parathyroid hormone hormone-related protein and the PTH receptor regulate angiogenesis of the skin. J Invest Dermatol. 2006;126:2127–2134. doi: 10.1038/sj.jid.5700338. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Yen A. Ultrastructure of the capillary loops in the dermal papillae of psoriasis. J Invest Dermatol. 1977;68:53–60. doi: 10.1111/1523-1747.ep12485169. [DOI] [PubMed] [Google Scholar]
- Eddy DD, Aschheim E, Farber EM. Experimental analysis of the isomorphic (Koebner) response in psoriasis. Arch Dermatol. 1964;89:579–588. doi: 10.1001/archderm.1964.01590280079015. [DOI] [PubMed] [Google Scholar]
- Farber EM, Roth RJ, Aschheim E, Eddy DD, Epinette WW. Role of trauma in isomorphic response in psoriasis. Arch Dermatol. 1965;91:246–251. doi: 10.1001/archderm.1965.01600090054011. [DOI] [PubMed] [Google Scholar]
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401–410. doi: 10.1111/j.1365-4362.1990.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Wood LC, Feingold KR, Sequeira-Martin SM, Elias PM, Grunfeld C. Barrier function coordinately regulates epidermal IL-1 and IL-1 receptor antagonist mRNA levels. Exp Dermatol. 1994;3:56–60. doi: 10.1111/j.1600-0625.1994.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Valter MM, Wiestler OD, Pietsche T. Differential control of VEGF synthesis and secretion in human glioma cells by IL-1 and EGF. Int J Dev Neurosci. 1999;17:565–577. doi: 10.1016/s0736-5748(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Kwon YW, Kwon KS, Moon HE, Park JA, Choi KS, Kim YS, Jang HS, Oh CK, Lee YM, Kwon YG, Lee YS, Kim KW. Insulin-like growth factor-II regulates the expression of vascular endothelial growth factor by the human keratinocyte cell line HaCaT. J Invest Dermatol. 2004;123:152–158. doi: 10.1111/j.0022-202X.2004.22735.x. [DOI] [PubMed] [Google Scholar]
- Malaguarnera L, Imbesi R, Di Rosa M, Scuto A, Castrogiovanni P, Messina A, Sanfilippo S. Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages. Int Immunopharmacol. 2005;5:1458–1469. doi: 10.1016/j.intimp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Bonifati C, Ameglio F. Cytokines in psoriasis. Int J Dermatol. 1999;38:241–251. doi: 10.1046/j.1365-4362.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006;126:1200–1202. doi: 10.1038/sj.jid.5700365. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, Klopp N, Wagenpfeil S, Zhao Y, Liao H, Lee SP, Palmer CN, Jenneck C, Maintz L, Hagemann T, Behrendt H, Ring J, Nothen MM, McLean WH, Novak N. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin’s fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–1284. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996;107:558–564. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- Petzoldt DG, Braun-Falco O, Wenig KH. Effects of plastic-foil-occlusion on psoriatic lesions. Arch Klin Exp Dermatol. 1970;238:160–168. doi: 10.1007/BF00519901. [DOI] [PubMed] [Google Scholar]
- David M, Lowe NJ. Psoriasis therapy: comparative studies with a hydrocolloid dressing, plastic film occlusion, and triamcinolone acetonide cream. J Am Acad Dermatol. 1989;21:511–514. doi: 10.1016/s0190-9622(89)70217-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Staiano-Coico L, Cohen SR, Varghese M, Carter DM. Occlusive hydrocolloid dressings decrease keratinocyte population growth fraction and clinical scale and skin thickness in active psoriatic plaques. J Dermatol Sci. 1990;1:93–96. doi: 10.1016/0923-1811(90)90221-x. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Tranfaglia MG, Kang S. Prolonged occlusion in the treatment of psoriasis: a clinical and immunohistologic study. J Am Acad Dermatol. 1995;32:618–622. doi: 10.1016/0190-9622(95)90347-x. [DOI] [PubMed] [Google Scholar]
- van de Kerkhof PC. The evolution of the psoriatic lesion. Br J Dermatol. 2007;157:4–15. doi: 10.1111/j.1365-2133.2007.07907.x. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Diaz BV, Lenoir MC, Ladoux A, Frelin C, Demarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human keratinocytes by retinoids. J Biol Chem. 2000;275:642–650. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- Hernández GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder DN, Dekoven J, Champagne P, Croteau D, Dupont E. Neovastat (AE-941), an inhibitor of angiogenesis: randomized phase I/II clinical trial results in patients with plaque psoriasis. J Am Acad Dermatol. 2002;47:535–541. doi: 10.1067/mjd.2002.124702. [DOI] [PubMed] [Google Scholar]
- Lachgar S, Moukadiri H, Jonca F, Charveron M, Bouhaddioui N, Gall Y, Bonafe JL, Plouet J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- Le Gouill S, Podar K, Amiot M, Hideshima T, Chauhan D, Ishitsuka K, Kumar S, Raje N, Richardson PG, Harousseau JL, Anderson KC. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood. 2004;104:2886–2892. doi: 10.1182/blood-2004-05-1760. [DOI] [PubMed] [Google Scholar]
- Bachelez H. Immunopathogenesis of psoriasis: recent insights on the role of adaptive and innate immunity. J Autoimmun. 2005;25(Suppl):69–73. doi: 10.1016/j.jaut.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:779–798. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Lew W, Krueger JG. Current concepts in the immunopathogenesis of psoriasis. Dermatol Clin. 2004;22:349–369. doi: 10.1016/j.det.2004.03.010. vii. [DOI] [PubMed] [Google Scholar]
- Gira AK, Brown LF, Washington CV, Cohen C, Arbiser JL. Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J Am Acad Dermatol. 2004;50:850–853. doi: 10.1016/j.jaad.2003.11.061. [DOI] [PubMed] [Google Scholar]