Abstract
Insulin-like growth factor-1 (IGF-1) stimulates proliferation, regulates tissue development, protects against apoptosis, and promotes the malignant phenotype in the breast and other organs. Some epidemiological studies have linked high circulating levels of IGF-1 with an increased risk of breast cancer. To study the role of IGF-1 in mammary tumorigenesis in vivo, we used transgenic mice in which overexpression of IGF-1 is under the control of the bovine keratin 5 (BK5) promoter and is directed to either the myoepithelial or basal cells in a variety of organs, including the mammary gland. This model closely recapitulates the paracrine exposure of breast epithelium to stromal IGF-1 seen in women. Histologically, mammary glands from transgenic mice were hyperplastic and highly vascularized. Mammary glands from prepubertal transgenic mice had significantly increased ductal proliferation compared with wild-type tissues, although this difference was not maintained after puberty. Transgenic mice also had increased susceptibility to mammary carcinogenesis, and 74% of the BK5.IGF-1 mice treated with 7,12-dimethylbenz[a]anthracene (20 μg/day) developed mammary tumors compared with 29% of the wild-type mice. Interestingly, 31% of the vehicle-treated BK5.IGF-1 animals, but none of the wild-type animals, spontaneously developed mammary cancer. The mammary tumors were moderately differentiated adenocarcinomas that expressed functional, nuclear estrogen receptor at both the protein and mRNA levels. These data support the hypothesis that tissue overexpression of IGF-1 stimulates mammary tumorigenesis.
Insulin-like growth factor-1 (IGF-1) is a single-chain 7-kd polypeptide that plays a critical role in development and stimulates growth and organogenesis via mitogenic, antiapoptotic, and chemotactic activity.1,2,3 IGF-1 is a primary mediator of the actions of growth hormone on postnatal growth and regulates fetal development of many tissues, including bone and reproductive organs.4,5,6 In the mammary gland, IGF-1 stimulates terminal end bud formation and ductal elongation.7,8
High serum levels of IGF-1 are associated with an increased risk of breast, prostate, and colon cancer.9,10,11,12 Circulating IGF-1 and most of the IGF binding proteins are produced in the liver, through stimulation by growth hormone.13 Circulating IGF-1 levels vary throughout life, increasing from birth to a pubertal peak and decreasing steadily after age 30.14 High circulating IGF-1 levels have been found in a number of epidemiological studies to be associated with increased risk of premenopausal breast cancer,15 while other studies have found an association with breast cancer risk only in older women16 or no association at all.17 These discrepancies may be due to differences in study designs, assays used to determine IGF-1 levels, and the large variations in “normal” IGF-1 serum levels.15 The expression of high levels of the IGF-1 receptor (IGF-1R) in breast cancers is strongly associated with breast cancer recurrence and decreased survival.18
In addition to circulating levels of IGF-1, local expression of IGF-1, which is associated with increased angiogenesis in colorectal cancers,19 may also be important in breast cancer development. In the breast, IGF-1 has been shown by in situ hybridization to be produced predominantly by stromal cells and very rarely by normal or malignant breast epithelia.8,20,21 On the other hand, IGF-1R is expressed by normal and malignant mammary epithelium, indicating that the effects of IGF-1 are achieved through a paracrine mechanism.20,21 Growth hormone, through interaction with its receptor on mammary stromal cells, induces IGF-1 production, which in turn activates IGF-1R, expressed by the ductal epithelium.20 IGF-1 mRNA has also been shown to be expressed in the epithelium of terminal end buds, but only in nonproliferating cells, during pubertal ductal development.20 Thus, whether expressed in the stroma or the terminal end buds, IGF-1 functions as a paracrine growth stimulator of adjacent mammary epithelial cells.20
Transgenic and knockout mouse models have become valuable experimental systems to study the role of IGF-1 in the regulation of mammary gland development, lactation, and tumorigenesis. A number of transgenic models use the whey acidic protein and the mouse mammary tumor virus (MMTV) promoters. Both of these promoters are hormonally regulated and generate increased transgene expression during pregnancy and lactation.22 Transgenic overexpression of human IGF-1 or des(1-3)IGF-1 (a potent IGF-1 analog with reduced affinity for IGF binding proteins) in the mammary glands, under the control of the whey acidic protein promoter, produces high levels of circulating growth factor.23,24 In these models, high levels of transgenic IGF-1 inhibit postlactation involution of the mammary gland predominantly through an antiapoptotic mechanism. In addition, whey acidic protein-des(1–3)IGF-1 overexpression leads to spontaneous mammary tumor formation in 53% of transgenic females by 23 months of age.24 In a model using the MMTV promoter, overexpression of ovine prepro-IGF-1 stimulates inappropriate alveolar bud development in peripubertal, virgin mice.25 Conversely, in female mice deficient in IGF-1, mammary development is grossly impaired.7 Treatment of deficient mice with IGF-1 plus estradiol (E2) restores pubertal mammary development, while treatment with growth hormone plus E2 does not, indicating that IGF-1 is necessary for the normal embryonic and postnatal development of the mammary gland.7
To clarify the role of tissue overexpression of IGF-1 in mammary development and tumorigenesis, we used a transgenic animal in which IGF-1 is under the control of the bovine keratin 5 (BK5) promoter.26,27 The BK5 promoter has been well characterized in multiple transgenic models and has been shown to direct constitutive transgene expression to the basal layer of stratified epithelia, where endogenous K5 is normally expressed.27,28,29 In the mammary gland, BK5-driven transgenes are expressed in the K5-positive myoepithelial cells,30 which are specialized cells adjacent to the ductal epithelium. Expression of IGF-1 by the myoepithelial cells in this model exposes the mammary epithelium in a paracrine/juxtacrine manner that recapitulates, to a greater extent than previous models, the contribution of stromal IGF-1 to breast development and tumorigenesis in a hormonally relevant in vivo milieu.
Materials and Methods
Animals and Treatments
The BK5.IGF-1 transgenic mice26,27 were maintained on an outbred ICR background, and all transgenics were hemizygous for the transgene. Age-matched, nontransgenic littermates were used as wild-type controls for all experiments. Animals were fed ad libitum with AIN-76A, a defined high sugar, fat, and protein food formula.31 At 6 weeks of age, virgin BK5.IGF-1 and wild-type female control mice were segregated into three dosing groups (initially starting with ∼50 mice per group): 1) 20 μg/mouse/day 7,12-dimethylbenz[a]anthracene (DMBA) (the lowest known efficacious dose),32 2) 10 μg/mouse/day DMBA (subefficacious dose) and 3) vehicle alone (corn oil). Starting at 7 to 9 weeks of age, mice were treated with DMBA or vehicle via oral gavage, 5 days per week, for 6 weeks.32 Animals were checked daily for general health condition and palpated for tumors three times per week. Following detection by palpation, mammary tumors were measured and harvested for later analysis. Mice were sacrificed by CO2 asphyxiation when they reached 14 months of age or when tumor size reached 1.5 cm as specified in institutional animal care and use committee-approved protocols.
Whole Mounts
Mammary glands were harvested and pressed between glass plates and fixed in 10% neutral buffered formalin for at least 1 week. Then, glands were dehydrated using a series of ethanol solutions (70%, 95%, and 100%) for 1 hour each and cleared in xylene for 2 hours. Following rehydration, tissue was immersed in fresh toluidine blue (1% aqueous) and stored in fresh phosphate-buffered saline (PBS) until photographs were taken.
Histological Analysis
For histological analysis, whole mammary glands and tumors were fixed in 10% neutral buffered formalin and embedded in paraffin before sectioning. Sections of 5 μm were cut, deparaffinized, rehydrated, and stained with hematoxylin and eosin. The Cardiff/Annapolis classification system was used to guide evaluation of histological phenotypes.33 To evaluate proliferation, selected mice received an intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU) in PBS (100 mg/kg body weight) 2 hours before sacrifice. BrdU was immunolocalized in paraffin embedded sections, as described previously.34 For determination of apoptosis, neutral buffered formalin-fixed, paraffin-embedded sections were stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay (ApoTag, Intergen, Purchase, NY) according to the manufacturer’s protocol. The number of BrdU-positive or terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cells, as well as the total number of cells, were counted in 5 high-power fields of each slide of two different mammary glands (inguinal and thoracic)/mouse in groups of three or four mice of each genotype and age. To identify mast cells within the mammary tissue, sections were stained with 0.1% toluidine blue for 10 minutes, rinsed in water, and dehydrated.
Immunohistochemistry
Keratin 5 (K5) and keratin 8 (K8) expression were examined in mammary buds from perinatal mice and tumors. Frozen sections (5 μm) of ventral skin were fixed in 4% paraformaldehyde for 20 minutes and then rinsed in PBS. All incubations were performed at room temperature. Sections were blocked for 30 minutes in 15% donkey serum and then incubated for 30 minutes with antibodies to either K5 (1:500; 2 μg/ml; Berkeley Antibody Company, Richmond, CA) or K8 (1:10; TROMA-1, Developmental Studies Hybridoma Bank, National Institute of Child Health and Human Development, Bethesda, MD). Following washing, the slides were then incubated with either fluorescein isothiocyanate-labeled donkey anti-rabbit IgG (K5) or Cy5-labeled donkey anti-rat IgG (K8) (1:200; 2.5 μg/ml; Jackson Immunoresearch Laboratories, West Grove, PA), for 30 minutes, while being shielded from light. The slides were mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA) medium, coverslipped, and viewed by confocal microscopy (Olympus Flowview confocal microscope).
To assess expression of K5 and K8 in formalin-fixed mammary glands and tumors, slides were deparaffinized and rehydrated, and endogenous peroxidase was blocked with 3% H2O2 for 10 minutes. For antigen retrieval, slides were boiled in 10 mmol/L citrate buffer, pH 6, for 10 minutes in a microwave oven. All further manipulations were performed at room temperature. For K5 antibody staining, slides were blocked in 10% goat serum for 30 minutes and then incubated with anti-K5 (1:500; 2 μg/ml) for 1 hour. Then slides were washed and incubated with horseradish peroxidase (HRP)-labeled anti-rabbit IgG (1:500; 3 μg/ml; Vector Laboratories) for 30 minutes, washed again and then incubated with 3,3′-diaminobenzidene (BioGenex, San Ramon, CA), followed by counterstaining and coverslipping. To immunolocalize K8, slides were blocked with 10% rabbit serum for 30 minutes and then incubated with anti-K8 (1:10) for 2 hours. Following washing, slides were incubated with biotinylated rabbit-anti-rat IgG (1:200; 2.5 μg/ml; Vector Laboratories) for 30 minutes and then incubated with streptavidin-HRP (BioGenex) for 30 minutes, followed by 3,3′-diaminobenzidene.
To detect the human IGF-1 transgene in the mammary glands of BK5.IGF-1 transgenic mice, formalin-fixed sections were incubated in 10 mmol/L citrate buffer, pH 6, for 10 minutes as above, blocked with Biocare blocking reagent (Biocare Medical, Concord, CA), and incubated with 1:500 dilution of goat polyclonal anti-human IGF-1 antibody (0.5 μg/ml; Abcam, Cambridge, MA) at 4°C overnight. Sections were then incubated with Goat Probe followed by Goat Polymer-HRP (Biocare Medical) and color developed with 3,3′-diaminobenzidene (Dako, Carpinteria, CA).
Phospho-Akt expression was determined in formalin-fixed sections of prepubertal mammary glands from wild-type and BK5.IGF-1 transgenic mice pretreated with 10 mmol/L citrate buffer. Slides were then incubated with rabbit polyclonal anti-phospho-Akt (Ser473) (1:50; 4 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) followed by HRP-linked anti-rabbit IgG (Dako) and 3,3′-diaminobenzidene.
Estrogen receptor α (ER) protein expression was evaluated using the standard avidin-biotin-complex immunoperoxidase method with the VECTASTAIN Elite ABC kit (Vector Laboratories) in frozen sections fixed with Zamboni’s (picrate paraformaldehyde) for 5 minutes. Following washing in PBS, the sections were quenched with 0.3% H2O2 in PBS/0.3% bovine serum albumin, blocked with 15% normal goat serum and then incubated with the primary antibody and HRP-labeled goat anti-rabbit secondary antibody (1:500; 3 μg/ml; Vector Laboratories). Two polyclonal ER antibodies were used; one obtained commercially from Zymed (South San Francisco, CA), the other a generous gift from Dr. Geoffrey Greene (ER-21).35 Dr. Greene’s peptide antibody (ER-21) was used at 5 μg/ml final concentration, the Zymed antibody at a 1:300 dilution. Both yielded the same results. Sections were then incubated with the final ABC reagent, washed again, incubated with aqueous 3,3′-diaminobenzidene (20 μg/ml) for 1.0 to 1.5 minutes and counterstained with hematoxylin. Frozen sections of mouse uterus were used as positive ER tissue controls. Negative tissue controls were 1) female mouse kidney sections, 2) uterine slides incubated with ER-21 antibody that had been preabsorbed with the ER-21 peptide, or 3) uterine slides incubated with preimmune rabbit serum rather than the Zymed antibody.
RNA Extraction and Reverse Transcription
Tumor tissues were homogenized with a Brinkmann Polytron bench-top homogenizer and RNA extracted with an Absolutely RNA Miniprep kit (Stratagene, La Jolla, CA) according to manufacturer’s instructions. After homogenization, whole mammary preparations were centrifuged at 18,000 × g at 4°C for 30 minutes to let the fat separate before proceeding with the protocol. After spectrophotometric quantitation (A260/280), 5 μg of total RNA was reverse transcribed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and random primers (Promega, Madison, WI). The resulting cDNA was diluted to 1 ng/μl working solution. A normal tissue standard was produced from whole mammary tissue from a young, untreated wild-type mouse and used as a calibrator for the relative standard curves. This normal tissue was extracted, reverse transcribed, diluted to 5, 1, and 0.2 ng/μl working solutions, and stored at −70°C in single-use aliquots.
Real-Time Quantitative Polymerase Chain Reaction (QPCR)
Real-time QPCR was performed using the ABI Prizm 7700 sequence detection system (Applied Biosystems, Foster City, CA). The primers and probes were designed with Primer Express software (Applied Biosystems) according to the manufacturer’s instructions and cross-evaluated using the Oligo 4.0 program. The ER primers and probe used were: forward primer (5′-CCATGACCCTTCACACCAAAG-3′), reverse primer (5′-CCAGCTCGTTCCCTTGGAT-3′) and TaqMan probe (5′-FAM-CTCGGGAATGGCCTTGCTG1CAC-TAMRA-3′). The progesterone receptor primers and probe used were: forward primer (5′-TGCCAGTCCGCTTCTAAAGAG-3′), reverse primer (5′-AAAACCGTGAATCTTCCTTTGG-3′), and TaqMan probe (5′-FAM-ACCTCGAGCACTGGAAGGCACCG -TAMRA-3′). The cyclin D1 primers and probe used were: forward primer (5′-CCAGAGGCGGATGAGAACAA-3′), reverse primer (5′-GGCACAGAGGGCCACAAA-3′) and TaqMan probe (5′-FAM- CAGACCATCCGCAAGCATGCACAG-TAMRA-3′). TATA box binding protein was used as a reference gene. The TATA box binding protein primers and probe used were: forward primer (5′-GGTGGCAGCATGAAGTGACA-3′), reverse primer (5′-GCACAGAGCAAGCAACTCACA-3′), and TaqMan probe (5′-FAM-CCTCTGCACTGAAATCACCTGCAGCA-TAMRA-3′). Primers and probe for human IGF-1 were obtained from Applied Biosystems TaqMan gene expression assays. All assays, including target and reference genes, were run in duplicates on the same plate. The relative standard curve for ER was generated using the whole mammary gland from a young, untreated wild-type mouse and the curve for human IGF-1 from a whole mammary gland from a BK5.IGF-1 transgenic mouse. Three points, consisting of 25 ng, 5 ng, and 1 ng sample size per well, were added to the plate to generate a linear standard curve. The average Cτ (threshold cycle) of each sample was plotted against the log of nanograms of cDNA added to the wells of the standard curve, and the reverse log of that number was normalized to its corresponding TATA box binding protein value. The result is a unitless number that reflects the amount of target gene in different samples relative to the randomly chosen calibrator.
Western Analysis of Cyclin D1 Expression
Frozen mammary tumors were homogenized in whole cell extract buffer (10 mmol/L Hepes, pH 7.9, 0.4 mol/L NaCl, 0.1 mmol/L EDTA, 5% glycerol, 0.02 mmol/L sodium orthovanadate, and protease inhibitors) and protein concentrations in the lysates quantitated using the BCA assay (Pierce, Rockford, IL). Equal amounts (50 μg) of protein from each tumor lysate were loaded onto a 10% polyacrylamide/sodium dodecyl sulfate gel and electrophoresed. Proteins were electroblotted onto polyvinylidene difluoride membrane (Pierce) at 100 V for 1 hour. The membrane was blocked with 5% nonfat dry milk in PBS with 0.1% Tween 20 (PBS-T) at room temperature for 1 hour and then incubated with 1:1000 dilution of mouse anti-cyclin D1 (Cell Signaling, Danvers, MA) in 4% bovine serum albumin, 0.1% Tween 20, Tris-buffered saline for 1 hour. The blot was incubated with 1:2500 to 1:5000 dilution of HRP-labeled anti-mouse IgG secondary antibody (Cell Signaling) and proteins detected using ECL Plus (Amersham, GE Health care, Piscataway, NJ). The blot was stripped and reprobed with 1:15,000 dilution of anti-glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling) in 5% milk in PBS-T for loading control.
Statistical Analysis
BrdU and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analyses were evaluated using the analysis of variance and Student’s t-test. Incidence was analyzed using the Fisher’s exact test. Kaplan-Meier survival analysis was used for time-to-tumor analysis with log-rank tests of significance. All tests were performed using SPSS version 10 for Macintosh (SPSS, Inc, Chicago, IL).
Results
Keratins 5 and 8 and IGF-1 Transgene Expression in Mammary Glands
To characterize the expression of the BK5.IGF-1 transgene both temporally and spatially, we initially used confocal microscopy to evaluate K5 and K8 expression, markers of myoepithelial and epithelial cells, respectively, in glands from newborn and 2.5-day-old wild-type mice. Rudimentary mammary ductal structures that were present from birth (Figure 1A) were further developed by 2.5 days old (Figure 1B) and had the expected organization of myoepithelial (K5+) and epithelial (K8+) cells. Double immunofluorescent staining allows identification of any cells expressing both antigens, as they appear yellow. As seen in Figure 1, A and B, mammary glands from very young mice have distinct and separate populations of K5- and K8-expressing cells without evidence of yellow fluorescent cells expressing both keratin proteins.
Figure 1.
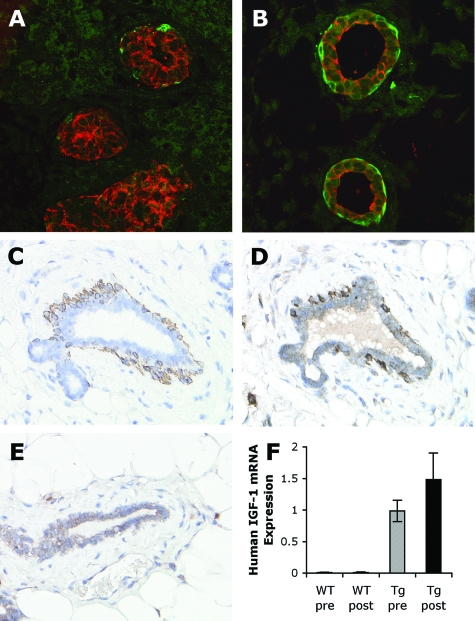
K5, K8, and IGF-1 transgene expression in mammary glands. Dual immunofluorescent detection of K5 (green) and K8 (red) antigens in mammary glands from female wild-type perinatal mice, sacrificed on the day of birth, day 0 (A) and at 2.5 days old (B). Serial sections of a postpubertal BK5.IGF-1 transgenic mammary gland immunostained for K5 (C) and IGF-1 (D). Dark staining of IGF-1 was seen in the K5-positive myoepithelial cells. In comparison, there was only light IGF-1 immunostaining in the ductal cells of postpubertal wild-type mammary gland (E). Human IGF-1 mRNA expression was determined in wild-type (WT) and BK5.IGF-1 transgenic (Tg) pre- and postpubertal mammary glands by QPCR (n = 6 for each group) (F). As expected, there was no detectable human IGF-1 mRNA in wild-type glands, but human IGF-1 mRNA was expressed in both pre- and postpubertal transgenic mammary glands. All mRNA levels were normalized to the expression of TATA box binding protein mRNA as described in Materials and Methods. Original magnifications: A and B, ×60; C–E, ×40.
The human IGF-1 transgene in BK5.IGF-1 transgenic mice has been shown to be expressed in the basal layer of the epidermis27 and prostate.26 To verify IGF-1 transgene expression in the mammary glands of BK5.IGF-1 transgenic mice, serial sections from postpubertal mice were immunostained for K5 or with an antibody that recognizes human IGF-1 (but also cross-reacts with mouse IGF-1). As shown in Figure 1 (C and D), IGF-1 was strongly expressed in the K5-positive myoepithelial cells. There was also light staining in the ductal epithelial cells as well as in some stromal cells, which indicates cross-reactivity with endogenous mouse IGF-1, since similar light staining was seen in the wild-type mammary gland (Figure 1E).
In addition, transgene expression at the mRNA level was evaluated in mammary glands from pre- and postpubertal transgenic mice by QPCR using human IGF-1-specific primers and probe. As shown in Figure 1F, the results clearly demonstrate that the BK5 promoter directed transgene expression in the mammary gland and that expression was maintained postpubertally. Together these results agree with a previous study that showed in BK5.COX-2 transgenic mice, the COX-2 transgene is overexpressed in the myoepithelial cells of the mammary gland.30
Phenotype of BK5.IGF-1 Transgenic Mammary Glands
To determine whether IGF-1 transgene expression affected mammary gland development or phenotype, whole mounts and histological sections of mammary glands were examined. Whole mounts from wild-type and transgenic mice (Figure 2, A and B) revealed that mammary glands from 5-week-old BK5.IGF-1 mice displayed increased alveolar end bud formation and ductal dilation compared to age-matched, wild-type littermates. These stimulatory and proliferative effects of IGF-1 were also demonstrated in histological sections (Figure 2, C and D). When compared to age-matched virgin wild-type mice, glands from transgenics showed profound ductal hyperplasia with reactive stromal thickening, as well as an influx of mast cells (Figure 2, E and F) and increased vascularization (Figure 2, G and H).
Figure 2.
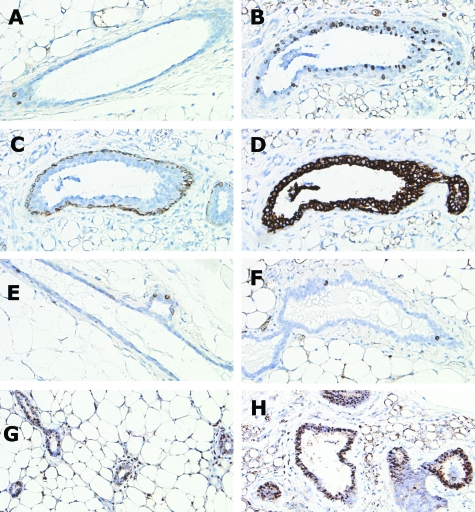
Whole mounts and histological sections from wild-type and BK5.IGF-1 transgenic mice. Whole mounts of mammary gland from 5-week-old wild-type mouse (A) and from 5-week-old BK5.IGF-1 transgenic mouse (B). H&E-stained sections of gland from virgin, adult (postpubertal) wild-type mouse (C) and from age-matched, virgin BK5.IGF-1 transgenic mouse (D). Toluidine blue staining for mast cells in wild-type (E) and BK5.IGF-1 transgenic (F) postpubertal mammary glands. Arrows in F indicate dark-staining mast cells in the transgenic gland. Blood vessels present in H&E-stained sections demonstrate increased vascularity in postpubertal mammary gland of transgenic mice (H) compared to wild-type gland (G). Original magnifications: A and B, ×1; C and D, ×10; E–H, ×40.
Proliferation, measured as BrdU incorporation, was assessed in glandular mammary epithelium in pre- and postpubertal animals. BrdU incorporation was significantly (P < 0.05) higher in prepubertal (4 to 5 weeks old) transgenic glands (17.73% ± 7.94% BrdU-positive cells) compared to age-matched wild-type glands (1.64% ± 1.66% BrdU-positive cells) (Figure 3, A and B). The majority of proliferating cells in the glands of prepubertal transgenic mice were K8-positive (Figure 3, B–D), which strongly suggests that IGF-1 transgene expression in the myoepithelial cells induced proliferation in the adjacent mammary epithelial cells. Conversely, there was no significant difference between BrdU incorporation in glands from transgenic and wild-type postpubertal (7 to 8 weeks old) animals, both of which had very low levels (P = 0.071) (Figure 3, E and F). Apoptosis, determined by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analysis, was also low in glands from both age groups and differences between transgenic and wild-type animals were not significant (data not shown). As discussed above, this lack of increased proliferation in postpubertal transgenics was not due to loss of transgene expression.
Figure 3.
Immunohistochemical expression of K5, K8, BrdU, and phospho-Akt in sections of mammary glands from wild-type and BK5.IGF-1 transgenic mice. BrdU immunostaining in wild-type prepubertal gland (A). Serial sections of BK5.IGF-1 transgenic prepubertal gland demonstrating immunolocalization of BrdU (B), K5 (C), and K8 (D). There were more BrdU positive cells in the prepubertal transgenic gland and most of the BrdU positive cells were also K8 positive. BrdU immunostaining of postpubertal wild-type (E) and transgenic (F) glands was not significantly different. Immunostaining for phospho-Akt in prepubertal wild-type (G) and transgenic (H) glands. Phospho-Akt staining was more intense in the ductal epithelial cells of transgenic compared to wild-type glands. Original magnifications, ×40.
To further investigate the response of mammary epithelial cells to transgenic IGF-1, prepubertal glands from wild-type and BK5.IGF-1 transgenic mice were immunostained for phospho-Akt, a known downstream mediator of IGF-1 signaling.36 As shown in Figure 3, G and H, staining for phospho-Akt was more intense in the ductal epithelium of transgenic compared to wild-type glands. Taken together, these results show that transgenic IGF-1 expression in myoepithelial cells resulted in activation of Akt signaling and induction of proliferation in the adjacent K8-positive mammary ductal epithelial cells.
Mammary Tumorigenesis
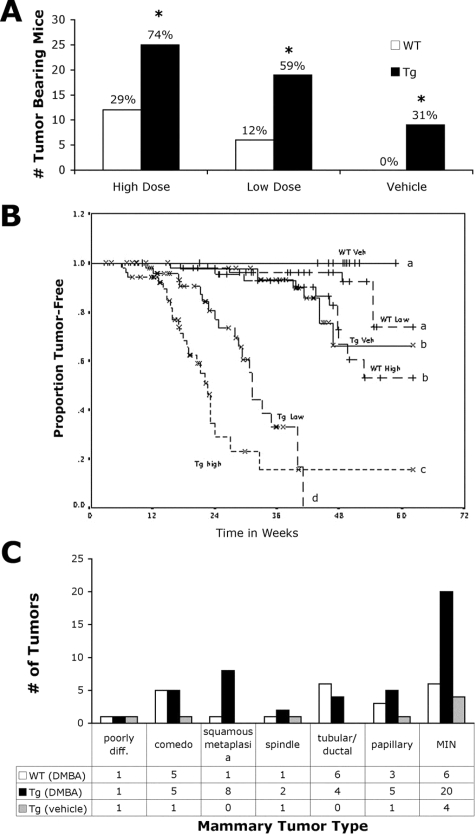
To examine the impact of paracrine IGF-1 overexpression on mammary tumorigenesis, we compared the susceptibility of wild-type and BK5.IGF-1 transgenic animals to chemical carcinogenesis with DMBA. A low-dose, extended duration carcinogenesis regimen that preferentially induces mammary tumorigenesis was used.32 There was a significantly higher incidence of mammary tumors in BK5.IGF-1 transgenic mice compared to wild-types at both doses of carcinogen (Figure 4A). Seventy-four percent of BK5.IGF-1 transgenic animals treated with daily doses of 20 μg of DMBA developed mammary tumors compared to 29% of the wild-type mice. In the 10 μg dose group, 59% of the transgenics developed mammary tumors beginning by week 11, but only 12% of the wild-type mice developed mammary carcinomas at this dose. Interestingly, 31% of the vehicle-treated transgenic animals, but none of the vehicle-treated wild-type group, developed spontaneous mammary carcinomas by 55 weeks. In each case, the differences were highly significant (P < 0.001, Fisher’s exact test).
Figure 4.
Incidence, latency and histological classification of mouse mammary tumors in the BK5.IGF-1 model. Tumor incidence in transgenic (Tg) and wild-type (WT) mice treated with corn oil vehicle or DMBA at 20 μg/mouse/day (high dose) or 10 μg/mouse/day (low dose) (A). Actual numbers of tumor-bearing mice are graphed with the percentage of tumor-bearing mice shown above each bar. The final numbers of mice in each arm were: WT, 41 in high dose, 50 in low dose, and 52 in vehicle only groups; Tg, 34 in high dose, 32 in low dose, and 30 in vehicle only groups. *P < 0.001 versus similarly treated wild-type mice (Fisher’s exact test). Kaplan-Meier analysis of tumor development in all treatment groups (B). Veh, vehicle only. Curves denoted by different letters are significantly different, P < 0.02 (log rank test). Because some mice died or were sacrificed due to other health problems (especially thymoma development in transgenic mice) before termination of the experiment at 55 weeks, final proportions of tumor-free mice do not exactly match the percent incidence values in A. Graphical representation showing the number of each histological tumor type that developed in wild-type mice treated with DMBA [WT (DMBA)], in transgenic mice treated with DMBA [Tg (DMBA)] and in transgenic mice treated with vehicle only [Tg (vehicle)] (C). Poorly diff., poorly differentiated; MIN, mammary intraepithelial neoplasia.
Kaplan-Meier analysis demonstrated that, in addition to the increased incidence, latency of the mammary tumors was also significantly (P < 0.02) decreased in transgenic mice compared to wild-type mice, in all dosing groups (Figure 4B). Additionally, DMBA-induced tumors developed earlier than spontaneous tumors in BK5.IGF-1 transgenic mice.
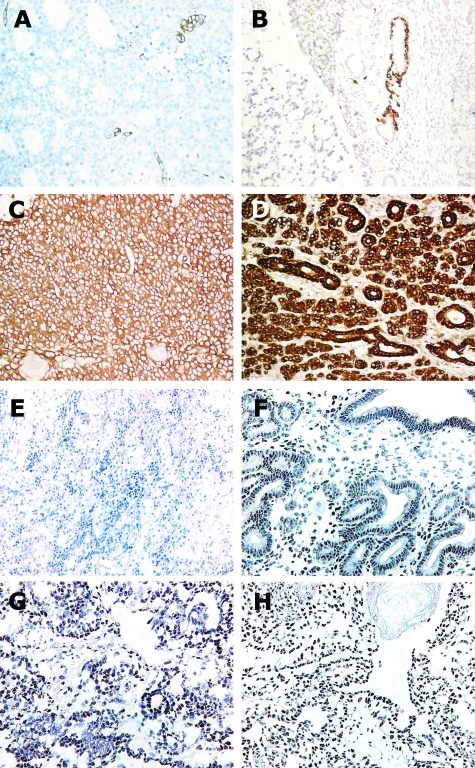
Histological analysis of the mammary tumors indicated they were moderately differentiated adenocarcinomas, some with a “ductal/tubular” or “papillary” phenotype and others with a predominantly adenosquamous phenotype with areas of squamous metaplasia and keratinization (Figure 5, A and B, respectively). Tumors from BK5.IGF-1 transgenic mice tended to have more squamous metaplasia (32%, excluding mammary intraepithelial neoplasia), while the wild-type tumors more frequently had the “ductal” or “papillary” phenotype (53%, excluding mammary intraepithelial neoplasia) (Figure 4C). Several tumors displayed areas of central necrosis with marked infiltration of inflammatory cells consistent with a comedo type of morphology (Figure 5C). The comedo type tumors were somewhat more common in wild-type than transgenic mice (29% in wild-type versus 20% in transgenic mice). Glands were also evaluated for the presence of microscopic tumors. Small invasive lesions, as well as those reminiscent of the mammary intraepithelial neoplasia, were identified (data not shown). Tumors from both wild-type and transgenic mice were composed predominantly of K8-expressing ductal epithelial cells (Figure 6, C and D). K5-expressing cells were confined to small nests within the tumors (Figure 6, A and B), indicating that the cells expressing IGF-1 were not the predominant cells targeted for transformation by the carcinogen.
Figure 5.
Histological subtypes of mammary tumors. Photomicrographs of H&E-stained sections from DMBA-treated BK5.IGF-1 transgenic and wild-type mice. The ductal phenotype (A) was seen most commonly in tumors from wild-type animals, while transgenic tumors frequently had areas of squamous metaplasia and keratinization (B). Relatively more comedo-type tumors (C) were seen in wild-type rather than transgenic mice. Original magnifications: A and C, ×10; B, ×20.
Figure 6.
Immunohistochemical detection of K5 (A, B) and K8 (C, D) antigens in mammary tumors from DMBA-treated wild-type (A, C) and vehicle-treated BK5.IGF-1 transgenic (B, D) mice. Immunostaining of ER in mammary tumors from BK5.IGF-1 transgenic and wild-type mice. Negative (kidney) (E) and positive (uterus) (F) tissue controls. Representative sections of ER immunolocalization in mammary adenocarcinomas from a wild-type (G) and a BK5.IGF-1 transgenic (H) mouse. Original magnifications: A–E, ×10; F–H, ×20.
ER and Cyclin D1 Expression in Mammary Tumors
ER expression in mammary tumors was evaluated by immunolocalization in frozen sections and by QPCR (Figure 6, G and H, and 7A, respectively). Although heterogeneous, immunohistological analysis indicated that tumors from BK5.IGF-1 transgenic and wild-type mice had areas of strong ER positivity with anticipated nuclear localization (Figure 6, G and H). QPCR data showed that, on average, tumors had lower ER mRNA levels than those detected in whole mammary glands of postpubertal mice, in which both ductal and stromal elements contribute (Figure 7A). Despite this, injection of 17β-estradiol into wild-type and transgenic tumor-bearing animals stimulated an increase in progesterone receptor mRNA expression in mammary tumors of more than sixfold, indicating that ER in the tumors was functional (Figure 7B).
Figure 7.
ER, progesterone receptor, and cyclin D1 expression in tissues and tumors, measured by QPCR (A–C) and Western analysis (D). ER mRNA expression in whole postpubertal mammary glands and mammary tumors from wild-type (WT) and BK5.IGF-1 transgenic (Tg) mice (A). Progesterone receptor mRNA expression in tumors (average of 10 different samples) from untreated wild-type and transgenic mice and in tumors from one wild-type and one transgenic mouse 6 hours after intraperitoneal injection of 17β-estradiol (20 μg/kg) (B). Cyclin D1 mRNA expression in tumors from wild-type and BK5.IGF-1 transgenic mice (C). Numbers of samples analyzed are noted within each bar. All mRNA levels were normalized to the expression of TATA box binding protein mRNA as described in Materials and Methods. Western blot analysis of cyclin D1 protein levels in individual mammary tumors from wild-type and BK5.IGF-1 transgenic mice (D). Blot was reprobed with antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to demonstrate equal loading.
Cyclin D1 is a downstream target of both IGF-1 and estrogen signaling.37,38,39 To investigate the proliferative capacity of tumor cells, cyclin D1 mRNA expression was assessed by QPCR. As shown in Figure 7C, tumors from transgenic mice had on average greater than twofold more cyclin D1 message than tumors from wild-type mice, although this was not statistically significant due to large intertumor variability. Similarly, cyclin D1 protein levels were higher in transgenic tumors (relative mean intensity normalized to glyceraldehyde-3-phosphate dehydrogenase band ± SD, 1.51 ± 0.15) compared to tumors from wild-type mice (1.00 ± 0.15, Figure 7D), which was significant (P < 0.001).
Discussion
In contrast to transgenic models using MMTV and whey acidic protein promoters that drive expression to the luminal cells, the bovine keratin 5 (BK5) promoter directs IGF-1 transgene expression in the mammary gland to the myoepithelial cells. The constitutive expression is detectable at birth and persists throughout early gland development and adulthood. In this model, mammary epithelial cells are exposed to high levels of transgenic human IGF-1 via a paracrine/juxtacrine mechanism. Published reports indicate that paracrine exposure of breast epithelium is an important route of IGF-1-mediated stimulation in the breast.8,20,21 Thus, this animal model recapitulates, at least in part, the paracrine IGF-1 exposure seen in breast cancer patients and women at increased risk. The unique aspect of this model is that it allows us to assess the impact of increased tissue expression of IGF-1 on mammary development and tumorigenesis in vivo in an appropriate endogenous hormonal milieu. Glands from BK5.IGF-1 mice had ductal hyperplasia, dilated ducts, and increased alveolar bud formation, demonstrating the strong proliferative effects of IGF-1 on the adjacent ductal epithelium.
BrdU incorporation was significantly higher in prepubertal transgenics compared to wild-types but there was no significant difference in postpubertal adult animals. This suggests that the proliferative effects of estrogen and IGF-1 were not additive and that much of the hyperplasia seen in the transgenics resulted from prepubertal exposure to IGF-1. The BrdU incorporation and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analyses indicate that paracrine overexpression of IGF-1 stimulates proliferation without increasing apoptosis. As transgenic IGF-1 expression did not stimulate increased mammary epithelial proliferation during the period when mice were being treated with DMBA (beginning at 7 to 9 weeks old), IGF-1 was not acting as a classical tumor promoter in this model. It may be that BK5.IGF-1-driven ductal proliferation and accelerated prepubertal mammary development increased the number of carcinogen target cells, leading to accelerated and increased DMBA-induced tumorigenesis. Others have shown that cells in the terminal end buds are the targets for carcinogen-induced transformation40 and that breast cancer risk depends on the number of target cells.41 In addition, the reactive stroma and chronic activated state induced in the mammary gland by IGF-1 transgene expression were also likely contributors to the development of both DMBA-induced and spontaneous mammary tumors in BK5.IGF-1 transgenic mice. Further experiments are needed to address these issues.
The increased vascularization that was seen in BK5.IGF-1 transgenic mammary glands can also be attributed to IGF-1 overexpression. Oh et al showed that IGF-1 induces the expression of angiogenic factors in breast epithelial cells.42 It has also been shown that insulin and IGF-1 increase vascular endothelial growth factor gene expression in fibroblasts.43
Tumor incidence and latency data clearly demonstrated that the paracrine/juxtacrine exposure in BK5.IGF-1 transgenic mice rendered the ductal epithelial cells more susceptible to mammary carcinogenesis with DMBA. In addition, data presented here showed that vehicle-treated transgenic mice also developed mammary cancer, demonstrating that mammary tumorigenesis did not require the administration of an exogenous carcinogen.
Spontaneous tumors also develop in other K5-expressing tissues of BK5.IGF-1 transgenic mice, including the thymus and cervix (J. DiGiovanni, unpublished studies), prostate,26 and skin.27 It should be noted that spontaneous tumors arise only in K5-positive tissues in these transgenic mice, suggesting that local overexpression of IGF-1, rather than circulating levels, is driving tumorigenesis. Indeed, endogenous mouse IGF-1 levels in the serum of transgenic BK5.IGF-1 animals are markedly reduced, probably due to negative feedback, leading to total serum IGF-1 (human + mouse) levels being similar in transgenic and wild-type mice (Shirley SH et al, manuscript in preparation).
Reports demonstrate that the effects of paracrine IGF-1 transgene expression on mammary gland hyperplasia and spontaneous tumorigenesis are mediated through activation of its receptor, IGF-1R. Studies also show that transgenic mice that overexpress a constitutively active IGF-1R in the mammary epithelia, via the MMTV promoter, also develop mammary gland hyperplasia and spontaneous mammary tumors.44 One arm of IGF-1 signaling through IGF-1R leads to Akt activation and we showed that mammary ductal epithelium of prepubertal BK5.IGF-1 transgenic mice have increased levels of Akt phosphorylation compared to wild-type mice (Figure 3, G and H). Transgenic mice that overexpress a constitutively activated Akt (myrAkt) in the mammary glands (MMTV promoter) have also been shown to have increased susceptibility to DMBA-induced mammary tumors.45 This suggests that in our BK5.IGF-1 transgenic mice the IGF-1/phosphatidylinositol 3-kinase/Akt signaling pathway may be important for enhanced carcinogen-induced tumorigenesis.
While about 60% of human breast cancers express ER and are hormonally responsive, the majority of genetically engineered mouse models of mammary tumorigenesis develop mammary cancers that are ER negative and hormone-independent.22 Clinically, human tumors in which at least 10% of the cells express estrogen receptor are classified as ER positive; by this standard, the BK5.IGF-1 mouse mammary cancers were strongly receptor positive. Heterogeneity of ER expression, as seen in the mouse mammary tumors (Figure 6, G and H), has also been reported in human breast cancers.46,47 Although whole glands had higher average mRNA levels of ER than the mammary tumors, injection of 17β-estradiol induced a more than sixfold increase in progesterone receptor expression in the tumors, indicating that the tumoral ER was functional.
The rapid development of mammary tumors in BK5.IGF-1 transgenic mice correlated with increased cyclin D1 expression in the tumors compared to wild-type tumors. Others have shown that IGF-1 induces cyclin D1 mRNA and protein expression.38,39,48 Induction of cyclin D1 by IGF-1 occurs both at the transcriptional level and also by increasing the stability of cyclin D1 mRNA.48,49 Induction of cyclin D1 nuclear accumulation, as well as mRNA stabilization, by IGF-1 requires the phosphatidylinositol 3-kinase/Akt pathway.39,49 In the skin of BK5.IGF-1 transgenic mice, cyclin D1 expression is up-regulated compared to skin of wild-type mice and inhibition of phosphatidylinositol 3-kinase not only reduces cyclin D1 expression, but also epidermal proliferation.36 Although many other compounds, including estradiol, can also induce cyclin D1 expression,49 it seems most likely that the IGF-1 transgene expression in the mammary gland of transgenic mice contributed to up-regulated cyclin D1 expression in the mammary tumors.
Cross-talk between the ER and IGF-1 signaling pathways has been demonstrated extensively in vitro.50,51,52,53 IGF-1-stimulates phosphorylation of serines 118 and 167 of ER, which can enhance receptor activation both in the presence and absence of ligand.54,55 In turn, estrogen up-regulates the expression of IGF-1R and its substrate, insulin receptor substrate-1, at both the protein and message level, enhancing the activation of IGF-1 signal transduction pathways.56,57 Cell culture studies indicate that increased exposure to IGF-1 may render breast cancer cells refractory to proliferative inhibition by antiestrogens, suggesting that IGF-1 may promote hormonal independence.49,50,58,59 Both IGF-1 and ER stimulate proliferation in normal and tumor cells, and evidence indicates that the combined activities of IGF-1 and estrogen are required to achieve maximum expression of cyclin D1.37,38,39,60 Taken together, data from this study suggest that markers of IGF-1 and ER stimulation, such as cyclin D1, may identify tumors likely to be refractory to antiestrogens, even if they are strongly ER positive. The detection of functional ER in DMBA-induced mammary tumors in transgenic BK5.IGF-1 and wild-type mice indicates that this model may provide a unique opportunity to evaluate hormonal regulation and cross-talk between ER and IGF-1 in vivo.
In conclusion, the current study demonstrated that paracrine exposure of the mammary epithelium to IGF-1 overexpression led to mammary gland hyperplasia, spontaneous mammary tumorigenesis, and enhanced susceptibility to chemical carcinogenesis. The BK5.IGF-1 model does not completely recapitulate all of the aspects of human mammary cancer, as IGF-1 is overexpressed in the myoepithelial cells rather than stromal cells, but this mouse model more closely resembles the paracrine IGF-1 exposure demonstrated in human breast cancers as compared to MMTV- or whey acidic protein-driven IGF-1 transgenic models. This model should be useful for future therapeutic prevention and intervention studies that target the IGF-1 signaling pathway.
Acknowledgments
We gratefully acknowledge the contributions of Dr. Lezlee Coghlan, Dr. Kaoru Kiguchi, Ms. Linda Beltrán, and Ms. Amanda Robinson.
Footnotes
Address reprint requests to Dr. Robin Fuchs-Young, University of Texas M.D. Anderson Cancer Center, Science Park–Research Division, 1808 Park Road 1C, P.O. Box 389, Smithville, TX 78957. E-mail: rfyoung@mdanderson.org.
Supported by U.S. Army Medical Research and Materiel Command grant DAMD17-01-0303 (to K.K.O.), grant CA 104114 (to RFY), grant CA37111 (to J.D.G.), NIEHS Center grant ES07784, and NCI Cancer Center Support grant CA16672.
Current address for Dr. Krisztina Kovács de Ostrovich: University of Pittsburgh, 13th Floor Biomedical Science Tower, 200 Lothrop Street, Pittsburgh, PA 15213.
Current address for Dr. Dennis Johnston: Department of Statistical Science, Baylor University, P.O. Box 97328, Waco, TX 76798.
References
- Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Johnson MD, May FE, Westley BR. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990;265:21172–21178. [PubMed] [Google Scholar]
- Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB. Postnatal mammary gland morphogenesis. Microsc Res Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wood TL, Richert MM, Stull MA, Allar MA. The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands. J Mammary Gland Biol Neoplasia. 2000;5:31–42. doi: 10.1023/a:1009511131541. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W. IGF-I: An essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N. Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol. 1989;3:509–517. doi: 10.1210/mend-3-3-509. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–1228. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Vadgama JV, Wu Y, Datta G, Khan H, Chillar R. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology. 1999;57:330–340. doi: 10.1159/000012052. [DOI] [PubMed] [Google Scholar]
- Yu H, Jin F, Shu XO, Li BD, Dai Q, Cheng JR, Berkel HJ, Zheng W. Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:705–712. [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Pollak M. Circulating IGF-I: New Perspectives for a New Century. Trends Endocrinol Metab. 1999;10:136–141. doi: 10.1016/s1043-2760(98)00126-x. [DOI] [PubMed] [Google Scholar]
- Brabant G, Wallaschofski H. Normal levels of serum IGF-I: Determinants and validity of current reference ranges. Pituitary. 2007;10:129–133. doi: 10.1007/s11102-007-0035-9. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I. IGF binding protein-3, and breast cancer risk: eight years on. Endocrine-Related Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:763–768. doi: 10.1158/1055-9965.EPI-06-0960. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Holly JM, Hunter DJ, Pollak MN, Hankinson SE. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocrine-Related Cancer. 2006;13:583–592. doi: 10.1677/erc.1.01149. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Torres JV, Nihei N, Barrett JC. The insulin-like growth factor-1 elevates urokinase-type plasminogen activator-1 in human breast cancer cells: a new avenue for breast cancer therapy. Mol Carcinog. 2000;27:10–17. doi: 10.1002/(sici)1098-2744(200001)27:1<10::aid-mc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Dorudi S, Phillips SM, Feakins RM, Jenkins PJ. Local expression of insulin-like growth factor-I affects angiogenesis in colorectal cancer. Tumour Biol. 2002;23:130–138. doi: 10.1159/000064029. [DOI] [PubMed] [Google Scholar]
- Marshman E, Streuli CH. Insulin-like growth factors and insulin-like growth factor binding proteins in mammary gland function. Breast Cancer Res. 2002;4:231–239. doi: 10.1186/bcr535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev D, Yee D. The IGF system and breast cancer. Endocrine-Related Cancer. 2001;8:197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nature Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Hadsell DL, Greenberg NM, Fligger JM, Baumrucker CR, Rosen JM. Targeted expression of des(1-3) human insulin-like growth factor I in transgenic mice influences mammary gland development and IGF-binding protein expression. Endocrinology. 1996;137:321–330. doi: 10.1210/endo.137.1.8536631. [DOI] [PubMed] [Google Scholar]
- Hadsell DL, Murphy KL, Bonnette SG, Reece N, Laucirica R, Rosen JM. Cooperative interaction between mutant p53 and des(1-3)IGF-I accelerates mammary tumorigenesis. Oncogene. 2000;19:889–898. doi: 10.1038/sj.onc.1203386. [DOI] [PubMed] [Google Scholar]
- Weber MS, Boyle PL, Corl BA, Wong EA, Gwazdauskas FC, Akers RM. Expression of ovine insulin-like growth factor-1 (IGF-1) stimulates alveolar bud development in mammary glands of transgenic mice. Endocrine. 1998;8:251–259. doi: 10.1385/ENDO:8:3:251. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, Moats S, Ramirez A, Jorcano JL, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanni J, Bol DK, Wilker E, Beltrán L, Carbajal S, Moats S, Ramirez A, Jorcano J, Kiguchi K. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- Ramírez A, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Lu J, Hammann B, Santos M, Moral M, Cascallana JL, Lara MF, Rho O, Carbajal S, Traag J, Beltrán L, Martínez-Cruz AB, García-Escudero R, Lorz C, Ruiz S, Bravo A, Paramio JM, DiGiovanni J. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. 2007;67:10879–10888. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K, Berger I, Ackermann K, Ehemann V, Zoubova S, Aulmann S, Pyerin W, Fürstenberger G. Cystic duct dilatations and proliferative epithelial lesions in mouse mammary glands upon keratin 5 promoter-driven overexpression of cyclooxygenase-2. Am J Pathol. 2005;166:575–584. doi: 10.1016/S0002-9440(10)62279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri JG. AIN-76 diet. J Nutr. 1979;109:925–926. doi: 10.1093/jn/109.5.925. [DOI] [PubMed] [Google Scholar]
- Qing WG, Conti CJ, LaBate M, Johnston D, Slaga TJ, MacLeod MC. Induction of mammary cancer and lymphoma by multiple, low oral doses of 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:553–559. doi: 10.1093/carcin/18.3.553. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Eldridge SR, Tilbury LF, Goldsworthy TL, Butterworth BE. Measurement of chemically induced cell proliferation in rodent liver and kidney: a comparison of 5-bromo-2′-deoxyuridine and [3H]thymidine administered by injection or osmotic pump. Carcinogenesis. 1990;11:2245–2251. doi: 10.1093/carcin/11.12.2245. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (α and β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18:602–611. [PubMed] [Google Scholar]
- Wilker E, Lu J, Rho O, Carbajal S, Beltrán L, DiGiovanni J. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinogenesis. 2005;44:137–145. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- Hamelers IH, van Schaik RF, van Teeffelen HA, Sussenbach JS, Steenbergh PH. Synergistic proliferative action of insulin-like growth factor I and 17 beta-estradiol in MCF-7S breast tumor cells. Exp Cell Res. 2002;273:107–117. doi: 10.1006/excr.2001.5430. [DOI] [PubMed] [Google Scholar]
- Dupont J, Karas M, LeRoith D. The potentiation of estrogen on insulin-like growth factor I action in MCF-7 human breast cancer cells includes cell cycle components. J Biol Chem. 2000;275:35893–35901. doi: 10.1074/jbc.M006741200. [DOI] [PubMed] [Google Scholar]
- Hamelers IHL, van Schaik RFMA, Sipkema J, Sussenbach JS, Steenbergh PH. Insulin-like growth factor I triggers nuclear accumulation of cyclin D1 in MCF-7S breast cancer cells. J Biol Chem. 2002;277:47645–47652. doi: 10.1074/jbc.M208727200. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environm Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami H-O, Persson I, Ekbom A, Wolk A, Pontén J, Trichopoulos D. The aetiology and pathogenesis of human breast cancer. Mutat Res. 1995;333:29–35. doi: 10.1016/0027-5107(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kucab JE, Bushel PR, Martin K, Bennett L, Collins J, DiAugustine RP, Barrett JC, Afshari CA, Dunn SE. Insulin-like growth factor-1 inscribes a gene expression profile for angiogenic factors and cancer progression in breast epithelial cells. Neoplasia. 2002;4:204–217. doi: 10.1038/sj.neo.7900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele C, Rochford JJ, Filippa N, Giorgetti-Peraldi S, Van Obberghen E. Insulin and insulin-like growth factor-I induce vascular endothelial growth factor mRNA expression via different signaling pathways. J Biol Chem. 2000;275:21695–21702. doi: 10.1074/jbc.M000805200. [DOI] [PubMed] [Google Scholar]
- Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, Camuso AE, Gottardis M, Greer AF, Ho CP, Hurlburt W, Li A, Saulnier M, Velaparthi U, Wang C, Wen M-L, Westhouse RA, Wittman M, Zimmermann K, Rupnow BA, Wong TW. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- Blanco-Aparicio C, Pérez-Gallego L, Pequeño B, Leal JFM, Renner O, Carnero A. Mice expressing myrAKT1 in the mammary gland develop carcinogen-induced ER-positive mammary tumors that mimic human breast cancer. Carcinogenesis. 2007;28:584–594. doi: 10.1093/carcin/bgl190. [DOI] [PubMed] [Google Scholar]
- Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat. 2002;74:199–211. doi: 10.1023/a:1016361932220. [DOI] [PubMed] [Google Scholar]
- Hayashi S-I, Imai K, Suga K, Kurihara T, Higashi Y, Nakachi K. Two promoters in expression of estrogen receptor messenger RNA in human breast cancer. Carcinogenesis. 1997;18:459–464. doi: 10.1093/carcin/18.3.459. [DOI] [PubMed] [Google Scholar]
- Furlanetto RW, Harwell SE, Frick KK. Insulin-like growth factor-I induces cyclin-D1 expression in MG63 human osteosarcoma cells in vitro. Mol Endocrinol. 1994;8:510–517. doi: 10.1210/mend.8.4.8052269. [DOI] [PubMed] [Google Scholar]
- Dufourny B, van Teeffelen HAAM, Hamelers IHL, Sussenbach JS, Steenbergh PH. Stabilization of cyclin D1 mRNA via the phosphatidylinositol 3-kinase pathway in MCF-7 human breast cancer cells. J Endocrinol. 2000;166:329–338. doi: 10.1677/joe.0.1660329. [DOI] [PubMed] [Google Scholar]
- Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:11491–11494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13:787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- Sato T, Wang G, Hardy MP, Kurita T, Cunha GR, Cooke PS. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology. 2002;143:2673–2679. doi: 10.1210/endo.143.7.8878. [DOI] [PubMed] [Google Scholar]
- Lee AV, Weng CN, Jackson JG, Yee D. Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J Endocrinol. 1997;152:39–47. doi: 10.1677/joe.0.1520039. [DOI] [PubMed] [Google Scholar]
- Aronica SM, Katzenellenbogen BS. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- Group EBCTC Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy, 133 randomized trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:1–15, 71–85. [PubMed] [Google Scholar]
- Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- Hadsell DL, Bonnette SG. IGF and insulin action in the mammary gland: lessons from transgenic and knockout models. J Mammary Gland Biol Neoplasia. 2000;5:19–30. doi: 10.1023/a:1009559014703. [DOI] [PubMed] [Google Scholar]
- Yang X, Edgerton SM, Kosanke SD, Mason TL, Alvarez KM, Liu N, Chatterton RT, Liu B, Wang Q, Kim A, Murthy S, Thor AD. Hormonal and dietary modulation of mammary carcinogenesis in mouse mammary tumor virus-c-erbB-2 transgenic mice. Cancer Res. 2003;63:2425–2433. [PubMed] [Google Scholar]