Abstract
Psoriasis is an inflammatory skin disease driven by aberrant interactions between the epithelium and the immune system. Anti-psoriatic drugs can therefore target either the keratinocytes or the immunocytes. Here we sought to develop an in vitro reconstructed skin model that would display the molecular characteristics of psoriatic epidermis in a controlled manner, allowing the screening of anti-psoriatic drugs and providing a model in which to study the biology of this disease. Human skin equivalents generated from normal human adult keratinocytes after air exposure and stimulation by keratinocyte growth factor and epidermal growth factor displayed the correct morphological and molecular characteristics of normal human epidermis whereas the psoriasis-associated proteins, hBD-2, SKALP/elafin, and CK16, were absent. Skin equivalents generated from foreskin keratinocytes were clearly abnormal both morphologically and with respect to gene expression. When normal skin equivalents derived from adult keratinocytes were stimulated with psoriasis-associated cytokines [tumor necrosis factor-α, interleukin (IL)-1α, IL-6, and IL-22] or combinations thereof, strong expression of hBD-2, SKALP/elafin, CK16, IL-8, and tumor necrosis factor-α was induced as shown by quantitative polymerase chain reaction and immunohistochemistry. Retinoic acid but not cyclosporin A was found to inhibit cytokine-induced gene expression at both the mRNA and protein levels. These results illustrate the potential of this disease model to study the molecular pathology and pharmacological intervention in vitro.
Psoriasis is a highly prevalent inflammatory skin disease that has both environmental and genetic components to its etiology.1 Linkage analysis has been used to identify multiple loci and alleles that confer risk of the disease, with the strongest genetic effect found at chromosome 6p21.3, where haplotypes carrying the HLA-Cw6 allele are associated with an increase in risk.2 Recently we have found that increased β-defensin copy numbers are associated with psoriasis, suggesting that both the adaptive immune system and the epidermal innate immune system are causally involved in the disease.3 Psoriasis is characterized by erythro-squamous plaques, and histological examination of psoriatic lesions shows inflammation, increased proliferation, and disturbed epidermal differentiation.4 At the molecular level, a regenerative epidermal differentiation program is induced that includes expression of psoriasis-associated genes such as cytokeratin 16 (CK16), SKALP/elafin, psoriasin, and β-defensin-2 (hBD-2).5 Furthermore, high levels of proinflammatory cytokines and chemokines have been demonstrated, including interferon-γ, interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6, and IL-22, which are produced by multiple cell types.6,7,8,9,10 A wide array of mechanistically distinct anti-psoriatic therapies is available, including agents that presumably target the adaptive immune system (corticosteroids, UVB, PUVA, calcineurin inhibitors), agents that are thought to be directed to the keratinocyte (retinoids, vitamin D3 derivatives, dithranol) and agents that possibly target multiple cell types (methotrexate, anti-TNF).4,11,12,13 Although the anti-psoriatic armamentarium has been expanded throughout the last years there is still room for improvement with regard to efficacy and side effects. The development of relevant, high-content in vitro models would greatly enhance the evaluation of novel therapeutic agents.
Submerged keratinocyte culture systems have been widely used for biological and pharmacological studies and some of these have been developed for high-throughput screening of anti-psoriatic drugs.14,15,16 Recent studies introduced more advanced systems such as three-dimensional tissue-engineered human skin equivalents.17 Tissue-engineered skin equivalents were initially developed for treatment of skin defects such as burn wounds and ulcers, but have also made a great impact on basic and applied research.17 Commercially available skin equivalents, mostly derived from foreskin keratinocytes, mimic normal skin to a large extent and are used in a wide range of biological studies, skin corrosion testing, and irritation studies.18 el-Ghalbzouri and colleagues19 have described the development of skin equivalents using de-epidermized dermis (DED) and adult keratinocytes. The addition of fibroblasts or defined growth factors to the equivalents stimulated the development of a good morphology of the epithelium. However, in contrast to normal skin, the psoriasis-associated markers SKALP/elafin and cytokeratin 6 were still present in this model. More recent studies have introduced skin equivalent models for diseased skin, by using keratinocytes from psoriasis patients,20 induction of a psoriatic phenotype by inhibition of transglutaminases,21 or by the addition of lymphocytes.22 All these models exhibit features of psoriatic epidermis and some of them were validated by anti-psoriatic agents.
Here we aimed to generate a reconstructed skin model from normal adult human keratinocytes, that would allow controlled induction of psoriasis-associated features and gene expression by the addition of relevant pro-inflammatory cytokines. The system should allow quantitative measurement of established psoriasis markers in the epidermal keratinocytes. Preferably, the starting point should be reconstructed skin that would mimic normal skin both morphologically and with respect to gene expression, as defined by the expression of established marker genes (CK10, involucrin, loricrin) and the absence of psoriasis-associated markers (hBD-2, SKALP/elafin, CK16) and low expression levels of psoriasis-associated pro-inflammatory cytokines such as IL-8 and TNF-α. Here we describe a model system that fulfills these criteria and that is potentially useful for studying biology of the disease and screening of anti-psoriatic drugs.
Materials and Methods
Cell Culture
Cells from the mouse fibroblast cell line 3T3 were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) supplemented with penicillin/streptomycin (50 IU/ml; ICN Biomedicals, Zoetermeer, The Netherlands) and 10% calf serum with iron (Hyclone, Logan, UT).
Keratinocytes were obtained from human abdominal skin derived from donors who underwent surgery for abdominal wall correction. After isolation by trypsin treatment for 16 to 20 hours at 4°C, keratinocytes were cultured in the presence of irradiated (3295 cGy for 4.10 minutes) cells from the 3T3 cell line. 3T3 cells were seeded at a concentration of 3 × 104 cells per cm2 in Greens medium, which consisted of two parts Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) and one part of Ham’s F12 medium (Life Technologies, Inc.) supplemented with 10% fetal bovine serum (Hyclone), l-glutamine (4 mmol/L; Life Technologies, Inc.), penicillin/streptomycin (50 IU/ml; Life Technologies, Inc.), adenine (24.3 μg/ml; Calbiochem, San Diego, CA), insulin (5 μg/ml; Sigma, St. Louis, MO), hydrocortisone (0.4 μg/ml; Merck, Darmstadt, Germany), triiodothyronine (1.36 ng/ml, Sigma) and cholera toxin (10−10 mol/L, Sigma). The next day keratinocytes were added at a concentration of 5 × 104 cells per cm2. After 3 days, medium was replaced by Greens medium containing epidermal growth factor (EGF, 10 ng/ml; Sigma). The cells were then refreshed every 2 to 3 days, and when wells were almost confluent, cells were trypsinized and stored in the liquid nitrogen.
De-Epidermized Dermis (DED)
DED was generated using abdominal skin from donors who underwent surgery for abdominal wall correction. After incubation of the skin for 5 to 10 minutes in phosphate-buffered saline (PBS) at 56°C, the epidermis was separated from the dermis. The dermis was then incubated for 1 month in PBS containing gentamicin (0.5 mg/ml; Life Technologies, Inc.) and antibiotic/antimycotic (Life Technologies, Inc.) at 37°C. Punches were prepared from this DED using an 8-mm biopter. DED still contained basal membrane, as demonstrated by expression of heparin sulfate and collagen type IV.
Generation of Human Skin Equivalents
A hollow metal ring with a diameter of 1 cm was placed on the DED, and 2 × 105 keratinocytes were seeded in the ring in medium containing 5% serum, consisting of two parts Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) and one part Ham’s F12 medium (Life Technologies, Inc.) supplemented with 5% calf serum (Hyclone), l-glutamine (4 mmol/L, Life Technologies, Inc.), penicillin/streptomycin (50 IU/ml, Life Technologies, Inc.), adenine (24.3 μg/ml, Calbiochem), ascorbic acid (50 μg/ml, Sigma), insulin (0.2 μmol/L, Sigma), hydrocortisone (1 μmol/L, Merck), triiodothyronine (1.36 ng/ml, Sigma), and cholera toxin (10−10 mol/L, Sigma). After 1 day, the ring was removed, and the construct was cultured submerged for 3 days. Then, the skin equivalents were placed on a grid, and cultured for 10 days at the air-liquid interface in medium without serum, consisting of two parts Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.) and one part Ham’s F12 medium (Life Technologies, Inc.) supplemented with l-glutamine (4 mmol/L, Life Technologies, Inc.), penicillin/streptomycin (50 IU/ml, Life Technologies, Inc.), adenine (24.3 μg/ml, Calbiochem), l-serine (1 mg/ml, Sigma), l-carnitine (2 μg/ml, Sigma), BSA lipid mix (palmitic acid (25 μmol/L, Sigma), arachidonic acid (7 μmol/L, Sigma), linoleic acid (15 μmol/L, Sigma), vitamin E (0.4 μg/ml, Sigma), ascorbic acid (50 μg/ml, Sigma), insulin (0.1 μmol/L, Sigma), hydrocortisone (1 μmol/L, Merck), triiodothyronine (1.36 ng/ml, Sigma), cholera toxin (10−10 mol/L, Sigma), keratinocyte growth factor (KGF) (5 ng/ml, Sigma) and epidermal growth factor (2 ng/ml, Sigma). Psoriatic skin equivalents were obtained by incubating normal skin equivalents the last 4 days of the air-liquid interface culture with various combinations of cytokines, including IL-1α (Preprotech, Rocky Hill, NJ), TNF-α (Preprotech), IL-6 (2 × 108 IU/ml; Gentaur, Brussels, Belgium), and IL-22 (Preprotech).
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Epidermis was separated for the skin equivalents by dispase (Roche Diagnostics, Mannheim, Germany) treatment for 4 hours at 4°C, and total RNA was isolated from the epidermis using Trizol reagent (Life Technologies, Gaithersburg, MD). Generation of first strand cDNA was performed as described previously.23 The reverse transcriptase reaction products were used for quantitative real-time PCR amplification, which was performed with MyiQ single-color real-time detection system for quantification with SYBR Green and melting curve analysis (Bio-Rad, Richmond, CA). Primers for hBD-2, SKALP/elafin, TNF-α, CXCL-8, and the housekeeping gene human acidic ribosomal protein P0 (hARP) were obtained from Biolegio (Malden, The Netherlands). DNA was PCR-amplified using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) under the following conditions: 2 minutes at 50°C and 10 minutes at 95°C followed by 40 cycles of 15 minutes at 95°C and 1 minute at 60°C, with data collection in the last 30 seconds. All primer concentrations were 300 nmol/L in a total reaction volume of 25 μl. The amount of each mRNA was normalized to the amount of hARP in the same sample. Relative mRNA expression levels of all examined genes were measured using the comparative 2−δδCT method.24
Histology and Immunohistochemistry
Human skin equivalents were fixed in buffered 4% formalin for 4 hours, and processed for routine histology. Skin equivalents were embedded in paraffin, and 6-μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) or processed for immunohistochemical staining using an indirect immunoperoxidase technique with avidin-biotin complex enhancement. To study epidermal proliferation, an antibody directed against Ki-67 (MIB-1; Immunotech, SA, Marseilles, France) was used whereas epidermal differentiation was studied using an antibodies directed against cytokeratin 10 (clone RKSE60; Sanbio, Uden, The Netherlands) and cytokeratin 16 (Novacastra, Newcastle-On Tyne, UK). SKALP/elafin was stained using polyclonal antibodies as described previously25 and hBD-2 was stained using goat anti-hBD-2 polyclonal serum derived from Peprotech (London, UK).
Statistics
Statistical analysis was performed using the Statistica software package (Statsoft Inc., Tulsa, OK). Analysis of qPCR data were done on the δCt values, using a paired t-test or analysis of variance (repeated design), followed by posthoc testing (Duncan’s multiple range test).
Results
A Model for Normal Human Epidermis: Adult Versus Foreskin Keratinocytes
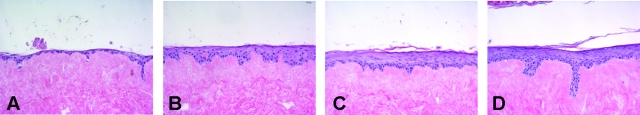
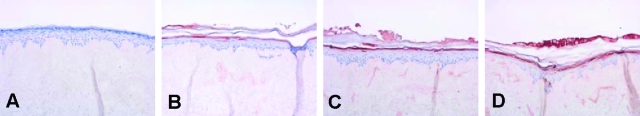
Adult abdominal keratinocytes were seeded on DED, and cultured for 4 days submerged followed by 10 days culturing at the air-liquid interface. To obtain a well-differentiated epithelium that would lack expression of psoriasis-associated genes, the effect of a number of relevant growth factors in a chemically defined medium that lacked serum or bovine pituitary extract, was examined. This was done in various concentrations of these factors, either alone or in combination, and the effect on morphology and expression of SKALP/elafin, hBD-2, and CK16 by the keratinocytes was examined by quantitative real-time PCR (qPCR) and histology. We found that a combination of 5 ng/ml of KGF and 2 ng/ml of EGF in the medium during the air-liquid interface culture was optimal to obtain a good morphology of the epithelium of the skin equivalent, as demonstrated by H&E staining (Figure 1). The cells were shown to attach to the DED in the submerged phase (Figure 1A), followed by formation of a multilayered epithelium during the air-liquid interface culture (Figure 1, B–D). At day 7 of the air-liquid interface culture, differentiation of the epithelium was shown as demonstrated by the development of a granular and a cornified cell layer (Figure 1C). After 10 days culturing at the air-liquid interface, a well-differentiated fully stratified epithelium was formed (Figure 1D). As shown by immunohistochemistry, normal expression of CK10 was noted (Figure 2B) whereas no expression of SKALP/elafin, hBD-2, or CK16 was detected (Figure 2, C–E). This pattern of protein expression, which is also found in normal skin in vivo, was consistently noted in primary cells derived from more than 10 different donors. Because most commercially available skin equivalents use foreskin keratinocytes, we generated skin constructs from neonatal foreskins, for comparison. Under the conditions used here, the constructs containing foreskin keratinocytes displayed parakeratosis (Figure 2F) and consistently expressed the psoriasis-associated marker SKALP/elafin (Figure 2J). Expression of the other marker genes was similar to that found in abdominal keratinocytes (Figure 2, G–I). These results show that adult normal human keratinocytes cultured at an air-liquid interface with defined medium and two defined growth factors (EGF and KGF) exhibit a normal stratification and a protein expression profile found in normal human epidermis.
Figure 1.
Development of normal human skin equivalents. Adult human keratinocytes were seeded on DED, and cultured for 4 days submerged, followed by 0 (A), 3 (B), 7 (C), or 10 (D) days of culturing at the air-liquid interface. Skin equivalents were stained for H&E.
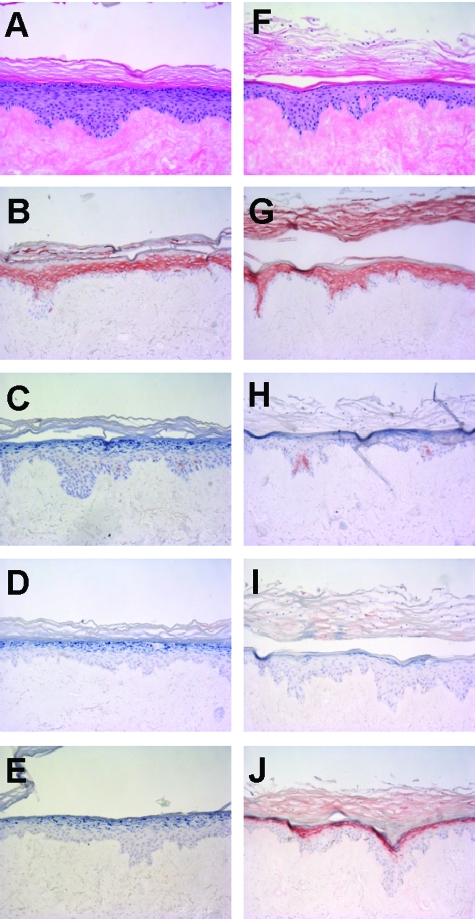
Figure 2.
Comparison of adult and neonatal keratinocytes in the development of normal human skin equivalents. Abdominal (A--E) or foreskin (F--J) human keratinocytes were seeded on DED, and cultured for 4 days submerged, followed by 10 days of culture at the air-liquid interface. Morphology of the skin constructs was studied by H&E staining (A and F), whereas protein expression of CK10 (B and G), CK16 (C and H), hBD-2 (D and I), and SKALP/elafin (E and J) was determined by immunohistochemistry. Note the expression of SKALP/elafin and the presence of parakeratosis in the constructs derived from neonatal foreskin keratinocytes.
Pro-Inflammatory Cytokines Induce a Psoriasis-Associated Gene Expression Signature in Normal Human Skin Equivalents
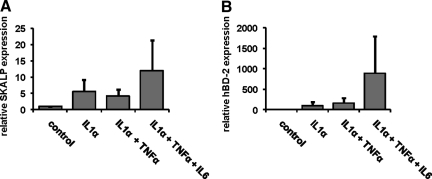
To induce a psoriatic phenotype in the normal human skin equivalents, the constructs were stimulated during the last 4 days of the air-liquid interface culture with various combinations of IL-1α, TNF-α, and IL-6. mRNA was isolated, and gene expression of SKALP/elafin and hBD-2 was determined by qPCR, see Figure 3, A and B, respectively. Treatment of the skin constructs with any of the stimuli induced an increase in expression of SKALP/elafin and/or hBD-2 compared to the control medium. The combination of IL-1α, TNF-α, and IL-6 was found to induce significantly higher expression levels of psoriasis-associated genes than any of the other treatments. TNF-α alone variably induced hBD-2 and SKALP/elafin expression, and high concentrations of TNF-α had a deleterious effect on epidermal morphology (not shown). IL-6 alone moderately affected hBD-2 and SKALP/elafin expression (not shown).
Figure 3.
Effect of cytokine stimulation on SKALP/elafin and hBD-2 gene expression in human skin equivalents. Skin equivalents were stimulated the last 4 days of the air-liquid interface culture with 10 ng/ml of IL-1α, 10 ng/ml of IL-1α and 5 ng/ml of TNF–α, or 10 ng/ml of IL-1α, 5 ng/ml of TNF–α, and 5 ng/ml of IL-6. SKALP/elafin (A) and hBD-2 (B) gene expressions were determined by qPCR. All stimuli induced a significant induction of SKALP/elafin (P < 0.02) and hBD-2 (P < 0.001) gene expression (analysis of variance, repeated design). The cytokine mix of IL-1α, TNF-α, and IL-6 induced a significantly higher expression of hBD-2 than the other stimuli (P < 0.05, Duncan’s multiple range test).
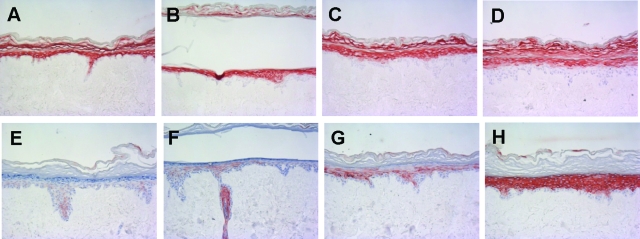
The effect of cytokine stimulation of the normal skin constructs was further investigated at the protein level by immunohistochemical analysis of SKALP/elafin and hBD-2 (Figure 4). In the control skin equivalents, no expression of SKALP/elafin (Figure 4A) and hBD-2 (Figure 4E) could be demonstrated. Stimulation with IL-α alone (Figure 4, B and F), or a combination of IL-1α and TNF-α (Figure 4, C and G) induced weak to moderate expression of SKALP/elafin and hBD-2 protein in the upper layers of the epidermis. Stimulation of the skin construct with the combination of IL-1α, TNF-α, and IL-6 (Figure 4, D and H) induced high expression levels of both SKALP/elafin and hBD-2 protein in the stratum granulosum and stratum spinosum, a pattern also found in lesional psoriatic epidermis. In skin equivalents with high epidermal hBD-2 expression, hBD-2 staining was also found in the acellular dermal compartment (Figure 4H), presumably by the interaction of secreted hBD-2, which is a highly cationic protein, with negatively charged dermal structures such as basal membranes. Cytokine stimulation minimally affected morphology of the skin constructs.
Figure 4.
Effect of cytokine stimulation on SKALP/elafin and hBD-2 protein expression in skin equivalents. Human skin equivalents were stimulated (B–D and F–H) or not (A and E) for 4 days with 10 ng/ml of IL-1α (B and F); 10 ng/ml of IL-1α and 5 ng/ml of TNF-α (C and G); or 10 ng/ml of IL-1α, 5 ng/ml of TNF-α, and 5 ng/ml of IL-6 (D and H). SKALP/elafin (A–D) and hBD-2 (E–H) protein expression was determined by immunohistochemistry.
To study the effects of pro-inflammatory cytokines on epidermal differentiation expression of the normal differentiation marker CK10 and the regeneration-associated marker CK16 was determined by immunohistochemistry (Figure 5). As shown before, control skin constructs showed high CK10 expression in the suprabasal layers (Figure 5A), whereas CK16 was almost absent in the skin constructs (Figure 5E). Stimulation of the constructs with IL-1α, or a combination of IL-1α and TNF-α did not affect CK10 expression (Figure 5, B and C), whereas CK16 expression was slightly increased (Figure 5, F and G). The combination of IL-1α, TNF-α, and IL-6 slightly decreased CK10 expression (Figure 5D), whereas CK16 expression was markedly increased (Figure 5H), showing that cytokine stimulation affects differentiation of the skin constructs in a way that is very similar to that found in lesional psoriatic epidermis.
Figure 5.
Effect of cytokine stimulation on CK10 and CK16 protein expression in skin equivalents. Human skin equivalents were stimulated (B–D and F–H) or not (A and E) for 4 days with 10 ng/ml of IL-1α (B and F); 10 ng/ml of IL-1α and 5 ng/ml of TNF-α (C and G); or 10 ng/ml of IL-1α, 5 ng/ml of TNF-α, and 5 ng/ml of IL6 (D and H). CK10 (A–D) and CK16 (E–H) protein expression was determined by immunohistochemistry.
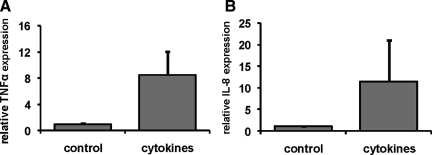
Another hallmark of lesional psoriatic skin are high levels of inflammatory mediators produced both by infiltrate cells and the keratinocytes. We therefore measured the expression of IL-8 and TNF-α in cytokine-stimulated normal human skin equivalents, using qPCR. The mixture of cytokines that induced strong expression of SKALP/elafin, hBD-2, and CK16 was also shown to increase gene expression of both TNF-α (Figure 6A) and IL-8 (Figure 6B), as compared to the control skin construct.
Figure 6.
Effect of pro-inflammatory cytokines on gene expression of inflammatory mediators by keratinocytes in skin equivalents. Skin equivalents were stimulated the last 4 days of the culture with the combination of 10 ng/ml of IL-1α, 5 ng/ml of TNF-α, and 5 ng/ml of IL-6. RNA was isolated and gene expression of TNF-α (A) and IL-8 (B) was determined by qPCR. Stimulation with the cytokine mix significantly induced TNF-α (P < 0.002) and IL-8 (P < 0.02, paired t-test).
Because recent studies have suggested a role for Th17 cell-derived cytokines in psoriasis we studied the effect of IL-22 on the skin equivalents. Figure 7 demonstrates that IL-22 induces a dose-dependent induction of hBD-2 protein expression as determined by immunohistochemistry. No effect was noted on epidermal morphology or cellular proliferation (not shown).
Figure 7.
Effect of IL-22 on hBD-2 protein expression in skin equivalents. Human skin equivalents were stimulated the last 4 days of the culture with 0 (A), 30 (B), 100 (C), or 300 (D) ng/ml of IL-22. hBD-2 protein expression was determined by immunohistochemistry.
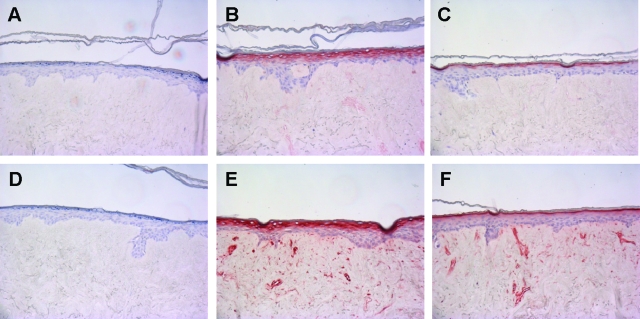
Response of Epidermal Keratinocytes in the Psoriatic Skin Equivalent to Anti-Psoriatic Drugs
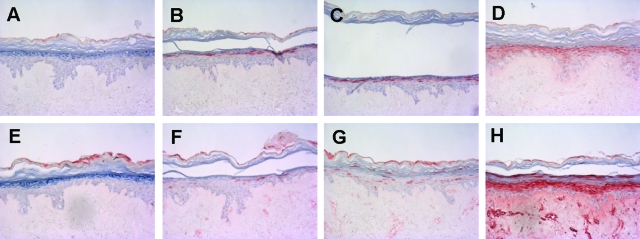
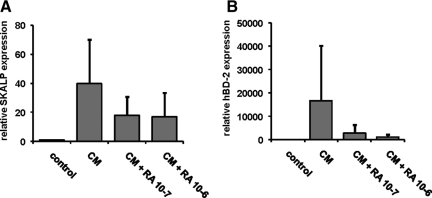
We performed a limited validation of the psoriasis skin equivalent model by testing the effect of selected anti-psoriatic drugs. The prediction would be that drugs known to target the epidermal compartment would show a therapeutic effect, whereas drugs that are mainly active against T cells (absent in our model) would be without effect. Normal skin constructs were stimulated the last 4 days of the air-liquid interface culture with a mixture of IL-1α, TNF-α, and IL-6 in the presence or absence of all-trans retinoic acid (ATRA) or cyclosporine A. Gene expression of SKALP/elafin and hBD-2 in the epithelium was determined by qPCR, and protein expression was determined by immunohistochemistry. ATRA was shown to inhibit cytokine-induced SKALP/elafin (Figure 8A) and hBD-2 (Figure 8B) gene expression as determined by qPCR. Also cytokine-induced SKALP/elafin (Figure 9, A–C) and hBD-2 (Figure 9, D–F) protein expression were inhibited by ATRA, suggesting a therapeutic effect of ATRA, and illustrating the potential use of the model for drug screening. ATRA also inhibited the expression of the normal differentiation marker CK10, which is a known side effect of retinoid therapy in vivo (data not shown). In contrast, no inhibition of cytokine-induced TNF-α and IL-8 expression by ATRA was shown (data not shown). No significant effect of cyclosporine A on hBD-2 and SKALP/elafin expression was noted in concentrations up to 10−6 mol/L (data not shown).
Figure 8.
Effect of all-trans retinoic acid (ATRA) on cytokine-induced SKALP/elafin and hBD-2 gene expression in skin equivalents. Human skin equivalents were stimulated the last 4 days of culture with a mixture of 10 ng/ml of IL-1α, 5 ng/ml of TNF-α, and 5 ng/ml of IL-6 (CM) in the presence or absence of ATRA (10−6 or 10−7 mol/L). RNA was isolated, and gene expression of SKALP/elafin (A) and hBD-2 (B) was determined by qPCR. Treatment with ATRA caused a significant decrease of cytokine-induced expression of SKALP/elafin (P < 0.05) and hBD-2 (P < 0.01, analysis of variance, repeated design). hBD-2 expression was significantly affected by both ATRA concentrations (P < 0.02) whereas SKALP/elafin expression was only affected by the highest ATRA concentration (P < 0.05, Duncan’s multiple range test).
Figure 9.
Effect of all-trans retinoic acid (ATRA) on cytokine-induced SKALP/elafin and hBD-2 protein expression in skin equivalents. Human skin equivalents were stimulated for 4 days of culture with a mixture of 10 ng/ml of IL-1α, 5 ng/ml of TNF-α, and 5 ng/ml of IL-6 in the presence or absence of ATRA (10−6 or 10−7 mol/L). Protein expression of SKALP/elafin (A: no cytokines; B: cytokines; C: cytokines and ATRA) and hBD-2 (D: no cytokines; E: cytokines; F: cytokines and ATRA) was determined by immunohistochemistry. Note the reduction of SKALP/elafin and hBD-2-positive cell layers in the ATRA-treated constructs (C and F) compared to the untreated cytokine-stimulated constructs (B and E).
Discussion
In the present study we sought to develop skin model systems, using a minimal set of relevant growth factors and cytokines, that would reproducibly display morphological and molecular features of normal and psoriatic epidermis. A chemically defined medium with EGF and KGF was found to stimulate development of normal epidermis lacking psoriasis markers, whereas a combination of IL-1α, TNF-α, and IL-6 induced expression of the psoriasis-associated proteins SKALP/elafin, hBD-2, and CK16, and the pro-inflammatory cytokines TNF-α and IL-8. Pharmacological validation of the psoriasis model illustrates the potential use of this model for drug screening, both for therapeutic actions and side effects.
In contrast to the model presented in this study, most commercially available skin equivalents use foreskin keratinocytes.26 We show that foreskin keratinocytes, at least under the conditions used here, form an abnormal epithelial phenotype, as shown by an abnormal morphology and abnormal protein expression. Therefore, adult human keratinocytes instead of foreskin keratinocytes are the preferred cell type to develop normal human skin equivalents. However, because the characteristics of foreskin keratinocytes, including parakeratosis and expression of the psoriasis-associated protein SKALP/elafin, are similar to that of psoriatic skin, one might argue that foreskin keratinocytes as such might be useful for the development of a skin equivalent model mimicking psoriatic skin.
Various submerged keratinocyte culture models have been developed to study inflammatory skin diseases.14,15,16,27,28 Recently, more advanced three-dimensional skin equivalent models displaying characteristics of inflammatory diseases, including keratinocyte hyperproliferation and enhanced expression of inflammatory mediators, have been developed.20,21,22 Fibroblasts, which are included in these models,20,21,22 promote keratinocyte proliferation and differentiation via the secretion of factors such as KGF and GM-CSF.29,30 In our model, we showed that addition of fibroblast-derived KGF to the skin equivalents in combination with EGF results in the correct morphological and molecular characteristics of normal human skin, including the absence of the psoriasis-associated proteins SKALP, hBD-2, and cytokeratin 16. It should be noted that the cytokines used in our model might have different effects, both qualitatively and quantitatively, in skin equivalent models containing fibroblasts. In contrast to our model, the described models make use of either primary keratinocytes from psoriatic donors,20 whose availability is limited, or cell lines,22 which may behave different from primary keratinocytes. In addition, in some models lymphocytes are included, which makes the models complex and less defined.22 We show that a well-defined psoriatic skin equivalent model can be obtained, using relevant pro-inflammatory cytokines and primary keratinocytes obtained from donors without a history of psoriasis.
The development of a psoriatic phenotype in the skin equivalents was studied by expression of the psoriasis-associated proteins SKALP/elafin and hBD-2. The relevance and the specificity of these peptides as surrogate markers for psoriasis is indicated by the high expression levels of both SKALP/elafin and hBD-2 in psoriatic lesions, but not in atopic dermatitis.5,31 Furthermore, β-defensins may also be causally involved in the disease, because an increased copy number of β-defensins was associated with psoriasis.3 In vitro studies have demonstrated that pro-inflammatory cytokines such as IL-1, TNF-α, and IL-6, which are present at high levels in psoriatic lesions,6,7,8,9 induce expression of antimicrobial peptides.32,33 Expression of hBD-2 in submerged cultures of keratinocytes was shown to be induced by TNF-α and IL-1β.33 Furthermore, IL-1α and IL-6 were shown to increase expression of various antimicrobial peptides and to enhance antibacterial properties of keratinocytes in skin equivalents against the skin pathogens Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus.32 Therefore, we used a mixture of the pro-inflammatory cytokines IL-1α, TNF-α, and IL-6 to induce expression of the psoriasis-associated proteins SKALP/elafin and hBD-2 in the skin equivalent model. The specific combinations of pro-inflammatory cytokines were chosen based on pilot experiments. The combination of cytokines was shown to maximally increase expression of psoriasis-associated proteins. Interestingly, although TNF-α increased IL-1α-induced CK-16 expression, IL-1α-induced SKALP/elafin and hBD-2 expression were not affected by TNF-α. Addition of IL-6 to IL-1α and TNF-α was shown to increase both CK-16, and SKALP/elafin and hBD-2 expression. These results suggest that the individual cytokines have specific functions in the pathology of psoriasis.
Recent studies have shown increased levels of IL-22 and oncostatin M in psoriatic lesions.9,10 Both skin equivalent models and in vivo models have been used to study the effects of IL-22 and oncostatin M on the epithelium. IL-22 and oncostatin M were shown to increase expression of psoriasis-associated proteins, including hBD-2, and to induce acanthosis, suggesting that IL-22 and oncostatin M play an important role in psoriasis.34,35,36,37 We show that in addition to the pro-inflammatory cytokines IL-1α, TNF-α, and IL-6, also IL-22 increased hBD-2 expression in the skin equivalents. This shows that in addition to cytokines that have been associated with psoriasis in the context of Th1 responses, also Th17 cell-derived cytokines can induce psoriasis features in the skin equivalent model.
High expression levels of both TNF-α and IL-8 have been demonstrated in psoriatic lesions, and these factors may play an important role in psoriasis by mediating chemotaxis of different inflammatory cell types and regulation of the inflammatory response in keratinocytes.6,7,9 Studies using anti-TNF-α based therapies in psoriasis patients show very promising results, supporting the important role for TNF-α in the pathology of psoriasis.11,12 We show that in addition to increasing expression of the psoriasis-associated proteins SKALP/elafin and hBD-2, the mixture of cytokines significantly increased gene expression of the pro-inflammatory cytokines IL-8 and TNF-α in keratinocytes. These results are in line with studies showing that TNF-α and IL-8 production by keratinocytes is stimulated by cytokines such as IL-1, IL-6, and TNF-α itself.9
In addition to mediating the inflammatory process, pro-inflammatory cytokines have also been demonstrated to affect proliferation of keratinocyte.9 Both IL-8 and IL-6 have been shown to increase proliferation of keratinocytes, suggesting that stimulation of the skin constructs with IL-6, which has direct proliferative activity, and TNF-α and IL-1, which indirectly affect proliferation via production of IL-8, would result in increased proliferation of the skin constructs. However, in the skin constructs produced here, we did not observe increased proliferation after stimulation with the mixture of cytokines. This may be explained by the anti-proliferative effect of TNF-α, or by absence of the relevant psoriasis-specific mitogen in our model system. Alternatively, this may be a generic limitation of working with primary adult keratinocytes, which appear to have little proliferative capacity in vitro anyway. We found that the proliferative potential of our cultures is limited, and that the normal skin equivalents cannot be maintained longer than 4 to 5 weeks after the start of air exposure. This is in contrast with long-term three-dimensional skin cultures described in literature, which last for at least 12 weeks.38 Future studies are directed to address this point in more detail, as it is desirable to obtain a psoriatic skin construct that would include hyperproliferation as a feature. The current isolation methods for primary cells could select for transient amplifying cells rather than stem cells. Obviously, this is a possibility that should be further explored. Alternatively, as already indicated, the use of foreskin keratinocytes could be considered in the psoriasis model because these cells have a larger proliferative capacity, and tend to adopt a psoriatic phenotype anyway, without stimulation. An interesting conclusion that can be drawn from this observation is that, although CK16 is regarded as a hyperproliferation-associated cytokeratin, in our model system CK16 expression is not dependent on a hyperproliferative context.
Validation of the psoriatic skin equivalent model was performed by the addition of all-trans retinoic acid (ATRA) and cyclosporine A. Retinoids have been demonstrated to interfere with terminal differentiation and proliferation of keratinocytes via signaling through nuclear retinoid receptors.39,40 ATRA is well recognized for its reduction of early and late markers of normal differentiation,41 and for its negative regulation of SKALP/elafin in skin raft cultures.42 Furthermore, induction of hBD-2 expression by TNF-α and IL-1β in submerged cultures of keratinocytes was inhibited by ATRA.33 In line with these observations, we show that ATRA inhibits expression of the psoriasis-associated proteins SKALP/elafin and hBD-2 in cytokine-stimulated skin equivalents, and reduces expression of the normal differentiation marker CK10. These results demonstrate the potential use of the psoriatic skin equivalent model for in vitro screening of drugs that act on the epidermis, both for therapeutic effects and side effects. In contrast to ATRA, the immunomodulating drug cyclosporine A which acts on lymphocyte activation did not inhibit cytokine-induced SKALP/elafin and hBD-2 expression.
We conclude that the presented model system is potentially useful for studying biology of psoriasis, and could provide a starting point to investigate the efficacy of potential anti-psoriatic drugs acting on keratinocytes.
Footnotes
Address reprint requests to Dr. G.S. Tjabringa, Department of Dermatology, Radboud University Medical Center, PO Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail: g.tjabringa@derma.umcn.nl.
Supported by the Dutch Program Tissue Engineering (grant number bgt.6739).
Disclosures: Parts of this publication have been submitted as a patent application (application nr PO2005NL50090 20051221).
Present addresses of D.v.R.: Nobilon International BV, Boxmeer, The Netherlands; and E.L.: Global Product Development Neurology, Merck Serono International S.A., Geneva, Switzerland.
References
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NV, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, Voorhees JJ, Elder JT. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, van de Kerkhof PC, Traupe H, de Jongh G, Heijer MD, Reis A, Armour JA, Schalkwijk J. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de Kerkhof PC, Schalkwijk J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163–1173. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–705. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB, Gottlieb AB. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati C, Ameglio F. Cytokines in psoriasis. Int J Dermatol. 1999;38:241–251. doi: 10.1046/j.1365-4362.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB. Psoriasis: emerging therapeutic strategies. Nat Rev Drug Discov. 2005;4:19–34. doi: 10.1038/nrd1607. [DOI] [PubMed] [Google Scholar]
- Krueger G, Ellis CN. Psoriasis—recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol. 2005;53:S94–S100. doi: 10.1016/j.jaad.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Pol A, Bergers M, Van Ruissen F, Pfundt R, Schalkwijk J. A simple technique for high-throughput screening of drugs that modulate normal and psoriasis-like differentiation in cultured human keratinocytes. Skin Pharmacol Appl Skin Physiol. 2002;15:252–261. doi: 10.1159/000066010. [DOI] [PubMed] [Google Scholar]
- Pol A, Bergers M, Schalkwijk J. Comparison of antiproliferative effects of experimental and established antipsoriatic drugs on human keratinocytes, using a simple 96-well-plate assay. In Vitro Cell Dev Biol Anim. 2003;39:36–42. doi: 10.1290/1543-706x(2003)039<0036:coaeoe>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Amigó M, Schalkwijk J, Olthuis D, De Rosa S, Paya M, Terencio MC, Lamme E. Identification of avarol derivatives as potential antipsoriatic drugs using an in vitro model for keratinocyte growth and differentiation. Life Sci. 2006;79:2395–2404. doi: 10.1016/j.lfs.2006.08.003. [DOI] [PubMed] [Google Scholar]
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- Netzlaff F, Lehr CM, Wertz PW, Schaefer UF. The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm. 2005;60:167–178. doi: 10.1016/j.ejpb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- el-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002;147:230–243. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]
- Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, Hutchinson PE, Smith GM, Pringle JH. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Layton CM, Hau Z, Bullock AJ, Johnson TS, Macneil S. Transglutaminase inhibitors induce hyperproliferation and parakeratosis in tissue-engineered skin. Br J Dermatol. 2007;156:247–257. doi: 10.1111/j.1365-2133.2006.07641.x. [DOI] [PubMed] [Google Scholar]
- Engelhart K, El Hindi T, Biesalski HK, Pfitzner I. In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch Dermatol Res. 2005;297:1–9. doi: 10.1007/s00403-005-0575-7. [DOI] [PubMed] [Google Scholar]
- Zeeuwen PL, van Vlijmen-Willems IM, Jansen BJ, Sotiropoulou G, Curfs JH, Meis JF, Janssen JJ, Van Ruissen F, Schalkwijk J. Cystatin M/E expression is restricted to differentiated epidermal keratinocytes and sweat glands: a new skin-specific proteinase inhibitor that is a target for cross-linking by transglutaminase. J Invest Dermatol. 2001;116:693–701. doi: 10.1046/j.1523-1747.2001.01309.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Wingens M, van Bergen BH, Hiemstra PS, Meis JF, van Vlijmen-Willems IM, Zeeuwen PL, Mulder J, Kramps HA, Van Ruissen F, Schalkwijk J. Induction of SLPI (ALP/HUSI-I) in epidermal keratinocytes. J Invest Dermatol. 1998;111:996–1002. doi: 10.1046/j.1523-1747.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Clin Ther. 1997;19:894–905. doi: 10.1016/s0149-2918(97)80043-4. [DOI] [PubMed] [Google Scholar]
- Van Ruissen F, de Jongh GJ, Zeeuwen PL, Van Erp PE, Madsen P, Schalkwijk J. Induction of normal and psoriatic phenotypes in submerged keratinocyte cultures. J Cell Physiol. 1996;168:442–452. doi: 10.1002/(SICI)1097-4652(199608)168:2<442::AID-JCP23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- van Ruissen F, Van Erp PE, de Jongh GJ, Boezeman JB, van de Kerkhof PC, Schalkwijk J. Cell kinetic characterization of growth arrest in cultured human keratinocytes. J Cell Sci. 1994;107:2219–2228. doi: 10.1242/jcs.107.8.2219. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Szabowski A, Stark HJ, Andrecht S, Kolbus A, Schorpp-Kistner M, Angel P, Fusenig NE. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J Invest Dermatol. 2001;116:816–820. doi: 10.1046/j.1523-1747.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Erdag G, Morgan JR. Interleukin-1alpha and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann Surg. 2002;235:113–124. doi: 10.1097/00000658-200201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–529. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E, Guillet G, Dagregorio G, Pene J, Moles JP, Yssel H, Chevalier S, Bernard FX, Gascan H, Lecron JC. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol. 2007;178:4615–4622. doi: 10.4049/jimmunol.178.7.4615. [DOI] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, Boukamp P. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J Investig Dermatol Symp Proc. 2006;11:93–105. doi: 10.1038/sj.jidsymp.5650015. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981;25:617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-Cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Stark HJ, Plein P, Baur M, Fusenig NE. Differential modulation of epidermal keratinization in immortalized (HaCaT) and tumorigenic human skin keratinocytes (HaCaT-ras) by retinoic acid and extracellular Ca2+. Differentiation. 1993;54:201–217. doi: 10.1111/j.1432-0436.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Thacher SM, Patel S, Friant S, Malhotra M, Shafer J, Krasinski G, Asano AT, Teng M, Duvic M, Chandraratna RA. Negative regulation of two hyperproliferative keratinocyte differentiation markers by a retinoic acid receptor-specific retinoid: insight into the mechanism of retinoid action in psoriasis. Cell Growth Differ. 1996;7:1783–1791. [PubMed] [Google Scholar]