Abstract
Chemokines comprise a structurally related family of cytokines that regulate leukocyte trafficking. Because infection with Toxoplasma gondii can induce an important inflammatory reaction that, if left uncontrolled, can lead to death, we investigated the role of the chemokine receptor CCR2 in T. gondii infection. We orally infected CCR2−/− mice with five ME-49 T. gondii cysts and monitored morbidity, survival, and immune response thereafter. The CCR2−/− mice displayed higher susceptibility to infection as all mice died on day 28 after infection. Despite similar Th1 responses, a more evident anti-inflammatory response was induced in the peripheral organs of CCR2−/− mice compared with wild-type C57BL/6 mice. Additionally, CCR2−/− mice presented greater parasitism and a milder inflammatory reaction in their peripheral organs with lesser CD4+ and MAC-1+ and greater CD8+ cell migration. The parasite load decreased in these organs in CCR2−/− mice but remained uncontrolled in the central nervous system. Additionally, we observed down-regulated inducible nitric oxide synthase expression in peripheral organs from CCR2−/− mice that was associated with a small nitric oxide production by spleen macrophages. In conclusion, in the absence of CCR2, another mechanism is activated to control tissue parasitism in peripheral organs. Nevertheless, CCR2 is essential for the activation of microbicidal mediators that control T. gondii replication in the central nervous system.
Chemokines play an important role in the physiopathology of toxoplasmosis in murine and in vitro models.1,2,3,4,5 In the oral Toxoplasma gondii infection, enterocytes can initiate innate immunological events that lead to a robust inflammatory process in the gut.3 Tissue samples isolated from the small intestine of T. gondii infected mice display a significant increase in chemokine secretion including monocyte chemotactic protein 1 (CCL2) and interferon (IFN)-γ inducible protein (CXCL10), and to a lesser extent, both macrophage inflammatory proteins 1α and β (CCL3 and CCL4), as well as CXCL2 regulated on activation and normally T-cell-expressed (CCL5).6 The CXCL2 secreted by enterocytes is involved in the trafficking of neutrophils and clearance of the invading parasite, since CXCL2-deficient mice fail to recruit neutrophils to the site of infection and exhibit higher parasite burden following T. gondii inoculation.7 The expression of several chemokine receptors such as CCR1, CCR2, CCR5, and CXCR3 was observed in the ileum of T. gondii-infected mice, being that CCR5 expression is markedly increased in antigen-primed CD8+ intraepithelial lymphocytes.8 In addition to promoting cell migration by inducing a different set of chemokines and chemokine receptors, T. gondii produces specific chemokine-like factors, in particular cyclophilin-18 (C-18), that stimulates production of interleukin (IL)-12 through the CCR5 chemokine receptor.9
CCL2 is a chemokine that can be produced by many cells, including macrophages, dendritic cells, endothelial cells, and fibroblasts, and expression is promoted after exposure to inflammatory stimuli such as IL-1, tumor necrosis factor (TNF)-α, or IL-4.10 CCR2 is the only known functional receptor for CCL2 expressed in monocytes and on activated and memory T cells, including both Th1 and Th2 cells,11,12 and also has a vital role in host defense to a number of pathogens.2,13,14,15,16,17 Likewise, the lack of CCR2 impairs the control of infectious diseases such as from Mycobacterium tuberculosis16 and infection of Leishmania major.13,17 Recent studies have shown the requirement of CCR2 and a substantial role for CCL2 in the recruitment of Gr-1+ inflammatory monocytes in mice inoculated by intraperitoneal route with tachyzoites to the local control of T. gondii replication.2
Infection with T. gondii is naturally acquired through the oral route by ingestion of undercooked or raw meat containing cysts of the parasite or through ingestion of water or food contaminated with cysts or oocysts.18 Although in immunocompetent individuals the infection with the parasite causes little or no overt signs of disease, in patients with immunodeficiency, or during congenital infection, T. gondii may emerge as a serious infection, which, if not treated, can lead to host death.19,20 IL-12, TNF-α, and IFN-γ, and the generation of reactive nitrogen intermediates show an important role as mediators of host resistance to early T. gondii infection in mice.20 Resistance in both acute and chronic infection with T. gondii in the murine model is highly dependent on endogenous IFN-γ.21 Depending on genetic susceptibility in mice, the peroral infection with T. gondii can lead to CD4+ T cell-dependent, interferon-γ-mediated necrosis of the small intestine.22 The oral route of T. gondii infection is thought to reflect the typical infection that occurs in naturally acquired toxoplasmosis in humans. To understand the role of CCR2 in acute and chronic T. gondii infection we inoculated CCR2-deficient mice with five cysts of ME-49 strain by oral route. This experimental procedure allowed us to analyze the effect of the absence of CCR2 in the morbidity, mortality, parasite multiplication, inflammatory lesions, and phenotype cell migration in peripheral organs and in the central nervous system (CNS) of T. gondii-infected mice. In the present investigation, we observed that the absence of CCR2 resulted in a higher susceptibility in the knockout mice with a down-regulation of microbicidal mechanisms and uncontrolled parasite burden in the CNS.
Materials and Methods
Animals
Female C57BL/6 (wild type; WT) and CCR2-deficient mice (CCR2−/−), 8 to 10 weeks old, were bred and maintained under standard conditions in the animal facility of the Department of Biochemistry and Immunology, School of Medicine of Ribeirão Preto, University of São Paulo-USP (Ribeirão Preto, Brazil). CCR2−/− mice were generated by W.A. Kuziel (Proc. Natl. Acad. Sci. USA 1997, Oct 94: 12053–12058 and were kindly supplied). CCR2−/− mice were backcrossed with C57BL/6 mice for over 20 generations. All procedures were in accordance with international guidelines for the use of animals and received prior approval by the animal ethics committee of the University of São Paulo.
Parasites and Challenge Infection
The low-virulent ME-49 strain of T. gondii was used to infect animals. Cysts were harvested from the brains of C57BL/6 that had been inoculated 1 month beforehand with approximately 20 cysts by the intraperitoneal route. For the experimental infection CCR2−/− and C57BL/6 mice were orally infected with five cysts in a volume of 0.2 ml and mortality monitored daily for 2 months.
Experimental Procedure and Histological Analysis
CCR2−/− and C57BL/6 mice were orally infected with five T. gondii cysts, and group of three mice were euthanized by cervical dislocation 8 and 23 days after infection. Blood samples were collected for serological assays, and tissue samples, such as CNS (particularly the brain), small intestine, lung, liver, and spleen were collected, fixed in 10% buffered formalin, and processed routinely for paraffin embedding and sectioning. The small intestine was cut into four pieces, and each piece was rolled on itself to make a “Swiss roll.” The peripheral organs and the CNS were examined histologically. For each organ, tissue sections of 4 μm thickness were mounted on slides and the sections stained with hematoxylin and eosin. All of the analysis were done at a magnification of 400× in a blind manner by two investigators.
Quantification of Tissue Parasitism by Immunohistochemistry
The tissue parasitism was quantified by immunohistochemistry as previously described.23,24 Deparaffinized sections were incubated for 30 minutes at 37°C in phosphate-buffered saline (PBS) and 1% bovine serum albumin and then overnight at 4°C with polyclonal rabbit antibody against antigens of T. gondii ME-49 strain or nonimmune rabbit serum as control. After incubation with biotinylated sheep anti-rabbit antibodies, the sensitivity was improved with avidin-biotin complex (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, CA). The reaction was developed with H2O2 plus 3,3′-diaminobenzidine tetrahydrochloride (Zymed Laboratories Inc., CA) for 5 minutes, and the slides were examined under light microscopy. The tissue parasitism was scored by counting the number of parasitophorous vacuoles from the entire small intestine or per section in the CNS or from 40 microscopic fields in the lung, liver, and spleen (1 × 400) from two histological sections of each mouse and from at least three mice per group.
Immunohistochemical Analysis for Detection of CCL2, CCR2, CD4+, CD8+, MAC-1+, and Inducible Nitric Oxide Synthase (iNOS) Expression in Tissue Samples
To detect CCL2 and CCR2 expression by immunohistochemistry, deparaffinized sections were subjected to antigenic unmasking in microwave oven and then incubated for 30 minutes at 37°C in PBS/1% bovine serum albumin (Sigma Aldrich, St. Louis, MO) to reduce nonspecific binding and then incubated with rat IgG anti-mouse CCL2 and goat IgG anti-CCR2 (Abcam, Inc.) at 4°C overnight. For CCL2 analysis, the slides were first incubated in PBS/saponin 0.01%. Secondary biotinylated antibodies and the streptavidin to improve the sensitivity were both from Dako Cytomation, LSAB (Dako North America). The reaction was visualized by incubating the sections with 3,3′-diaminobenzidine tetrahydrochloride (Zymed Laboratories Inc.). Control slides were incubated with nonimmune rat serum. The expression of CCL2 and CCR2 was measured by counting the positive cells in 40 microscopic fields.
To detect CD4+, CD8+, MAC-1+ (monocytes/macrophages, granulocytes, and NK cells), and iNOS positive cells, tissue samples were removed, embedded in tissue-freezing medium (Tissue-Tek, Miles Laboratories), and stored in liquid N2. Five-μm frozen tissue sections were obtained and fixed with ice-cold acetone for 10 minutes. The slides were placed in a humidified chamber, and the endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 30 minutes, followed by incubation with PBS plus 3% (P/V) nonfat milk (Nestle, São Paulo, Brazil). The slides were washed with PBS and incubated overnight with rat IgG anti-mouse CD4, MAC-1, and iNOS antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), rat IgG anti-mouse CD8 biotin-labeled antibodies (Caltag Laboratories), or normal rat IgG (controls) diluted 100 times in PBS. After successive rinsing with PBS, the sections were incubated for 30 minutes with biotin-labeled goat anti-rat antibody (Santa Cruz Biotechnology) and washed three times in PBS. Next, the sections were incubated with avidin-biotin-peroxidase complex (ABC kit, PK-4000; Vector Laboratories, Inc.), for 30 minutes at 37°C, the color developed with 3,3′-diaminobenzidine (Vector Laboratories, ON, Canada), and the slides counterstained with Mayer’s hematoxylin. The phenotypic analysis was evaluated in 40 microscopic fields.
Flow Cytometry Assay (Fluorescence-Activated Cell Sorting)
The small intestine inflammatory cell infiltrates were also evaluated by flow cytometry assay as described previously.25 Viability was assessed by Trypan blue exclusion and cells were counted in a Neubauer chamber. Leukocytes obtained were incubated with the following antibodies: anti-CD8 phycoerythrin-labeled (BD Biosciences PharMingen) and anti-CD11b phycoerythrin-labeled (e-Bioescience), anti-CD4, anti-CD11c, anti-FITC-conjugated (BD Biosciences PharMingen), and anti-CD3 peridinin chlorophyll protein-conjugated (Biosciences PharMingen). Leukocytes were acquired (FACSCanto II, BD Bioscience, San Jose, CA) and analyzed by FlowJo software (TreeStar) according to size forward scatter and granulosity side scatter scatter dot plot. Single- or two-color staining was used to identify T lymphocytes (CD3+, CD3+CD4+, CD3+CD8+), phagocytes (CD11b+), and dendritic cells (CD11c+). The isotype antibody controls used were rat IgG2a-PerCP, rat IgG2b-fluorescein isothiocyanate and rat IgG1-PE (BD Biosciences PharMingen). Results are expressed as mean ± SEM of the percentage of each antibody specific stained subpopulation within the gated cells obtained from small intestine of five different animals per group.
Cytokine Measurement in the Serum Samples and Intestinal Homogenates
The concentrations of cytokines were measured by sandwich enzyme-linked immunosorbent assay (ELISA). The small intestine tissue samples of 100 mg obtained from WT and CCR2−/− mice on day 8 of infection were homogenized with an Omni TH homogenizer in 0.5 ml of PBS containing protease inhibitors (0.1 mmol/L phenylmethylsulfonyl fluoride, 0.1 mmol/L benzamidine chloride, 10 μg/ml aprotinin A, and 100 μg/ml leupeptin). Each sample was then centrifuged for 10 minutes at 3000 × g, and the supernatant was used for ELISA. The IL-12p70, IFN-γ, IL-10, and transforming growth factor (TGF)-β (OpTEIA, BD Bioscience, San Diego, CA), TNF-α, IL-4, and CCL2 (Duoset R&D Systems, Minneapolis, MN), were assayed according to instructions from the manufacturer. The concentrations of cytokines in the samples were calculated from a standard curve of murine recombinant cytokine. The sensitivities of detection in the ELISAs were 31.25 pg/ml (IL-12p70), 15.63 pg/ml (IFN-γ, IL-10, TNF-α, and CCL2), 62.5 pg/ml (TGF-β), and 3.9 pg/ml (IL-4).
Spleen Macrophage Cultures
To isolate spleen macrophages, the WT and CCR2−/− mice were euthanized and their spleens were removed. The suspensions of spleen cells were washed in RPMI 1640 medium and treated for 4 minutes with lysing buffer (9 volumes of 0.16 mol/L NH4Cl and 1 volume of 0.17 mol/L Tris-HCl, pH 7.5). The erythrocyte-free cells were then washed three times and adjusted to 1 × 106 cells/ml in RPMI supplemented with 5% fetal bovine serum. The cell suspension was distributed in triplicate in 96-well culture plate and incubated for 3 hours at 37°C in a humidified 5% CO2 incubator. The nonadherent cells were removed by exhaustive washing with Hanks’ solution, and the adherent cells were stimulated with IFN-γ (1, 5, or 25 U/ml) and MCP-1 (2, 10, or 50 ng/ml) and the culture supernatants were collected after 48 hours for determination of NO production.
Nitrite Assay
NO production by spleen macrophage was measured by accumulation of nitrite, nitric oxide’s metabolite, in supernatants collected after 48 hours of culture, as described previously.26 Briefly, 0.05 ml of Griess reagent (0.1% naphthyl ethylenediamine and 1% sulfanilamide in 2.5% phosphoric acid, prepared with reagents from Sigma) was added to 0.05 ml of supernatant, and absorbance was read at 540 nm using an automated plate reader. Nitrite concentration was calculated from a NaNO2 standard curve.
Statistical Analysis
The Kaplan-Meier method was used to compare the survival rates of the studied groups, and the survival curves were compared using log-rank and χ2 tests that generate a two-tail P value to test the null hypothesis that they were identical in the overall groups of animals. Histological analysis, tissue parasitism, and cytokine levels produced by groups of animals were compared by using Mann-Whitney U test. Statistical analysis and graphs were performed using GraphPad prism version 4.0 (GraphPad Software, San Diego, CA). Values of P < 0.05 were considered statistically significant.
Results
The Expression of CCR2 and CCL2 Are Increased in Small Intestine after Oral T. gondii Infection
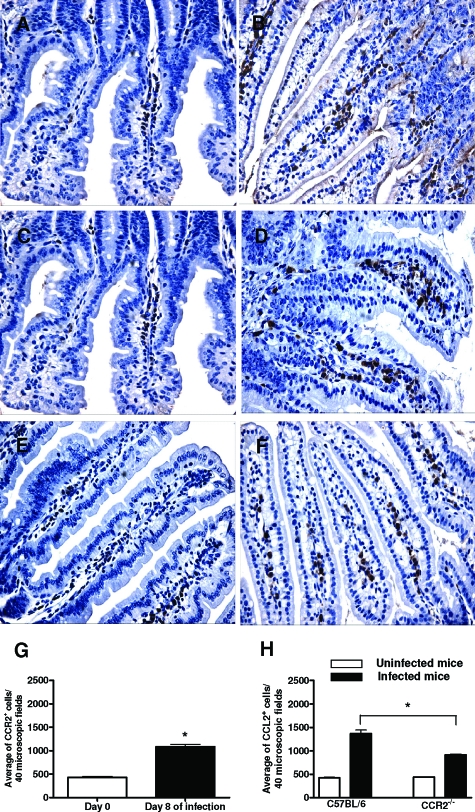
In our experiments, we evaluated the role of CCR2 in the resistance to the infection with five T. gondii cysts in the predominant stage of parasite transmission by the natural (oral) route of infection. Firstly, we evaluated the CCR2 and CCL2 expression in the intestinal tissue samples from WT mice uninfected or infected with T. gondii. We found that the number of CCR2+ cells in WT mice was significantly increased on day 8 after inoculation of the parasite (Figure 1, B and G) compared with uninfected mice, which presented only a small number of CCR2+ cells in the lamina propria (LP) (Figure 1A). In relation to the expression of CCL2, WT (Figure 1C) and CCR2−/− (Figure 1E) mice presented a small amount of CCL2+ cells in the LP of the small intestine. However, an increase of these cells was observed on day 8 after T. gondii infection (Figure 1, D and F). In addition, infected WT mice presented a higher number of CCL2+ cells (Figure 1D) compared with CCR2−/−-infected mice (Figure 1F), as clearly shown after quantification of these cells (Figure 1H). Next, we assayed the CCL2 production in the small intestine and in serum samples from WT and CCR2−/−-infected mice by ELISA. In accordance with previous results, the infection with T. gondii led to increased levels of CCL2 in the serum samples and in the small intestine at day 8 after infection (data not shown). Interestingly, systemic CCL2 production was increased in CCR2−/− compared with WT mice. Therefore, we suggest that after T. gondii infection the increase of CCL2 and CCR2 expression could be involved in the migration of inflammatory cells in the site of infection.
Figure 1.
Immunohistochemical staining for CCR2 and CCL2 and quantification of positive cells in the small intestine of CCR2−/− and WT mice infected with five ME-49 T. gondii cysts by oral route. The CCR2 positive cells were found in the small intestine from WT noninfected mice (A), and the expression was higher on day 8 after infection (B). WT (C) and CCR2−/− (E) uninfected mice presented immunohistochemistry staining for CCL2 in the small intestine, and the expression was higher in the organ in WT (D) and CCR2−/− (F) on day 8 after infection. Number of infiltrating CCR2+ cells (G) in the small intestine from WT mice and CCL2+ cells (H) in the small intestine from WT and CCR2−/− mice. The positive cells were counted by immunohistochemistry in 40 microscopic fields. Data are representative of at least two independent experiments with three mice per group and two noncontiguous sections from each mouse. Original magnification, ×40. *, significantly different (P < 0.05).
Absence of CCR2 Leads to an Increased Susceptibility to T. gondii Infection
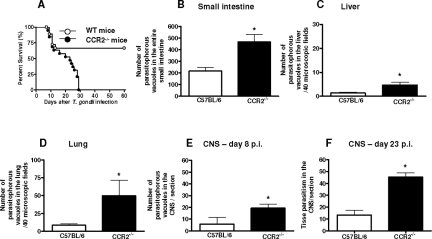
To analyze the role of CCR2 in the mortality of mice in oral T. gondii infection, the animals were inoculated with five T. gondii cysts by gavage. For these experiments, eight animals from each group were used, and the mortality was monitored daily until 60 days after infection. We found that 70% of WT mice survived until 60 days after infection. In contrast, the CCR2−/− mice presented profound susceptibility to infection (χ2 = 12.47; P = 0.0004; df = 1), presenting 100% mortality 28 days after T. gondii inoculation (Figure 2A). These findings indicate that CCR2−/− mice are highly susceptible to oral infection with T. gondii.
Figure 2.
Mortality rates and tissue parasitism of CCR2−/− and WT mice orally infected with five ME-49 T. gondii cysts. The mortality rate for eight mice from each group was determined (A). CCR2−/− mice were significantly more susceptible to toxoplasmosis than were WT mice (χ2 = 12.47; P = 0.0004; df = 1). The tissue parasitism in the small intestine (B), liver (C), lung (D), and CNS (E, F) were detected by immunohistochemistry staining and scored by counting the number of parasitophorous vacuoles per 40 microscopic fields in the peripheral organs and number of parasitophorous vacuoles and cyst-like structures per section in the CNS. Data are representative of at least two independent experiments of three mice per group that provided similar results (*P < 0.05).
It is well known that oral infection with the ME-49 strain of T. gondii causes a high proliferation of parasites in the small intestine that disseminate through the blood vessels to other organs.18 To determine whether the decreased resistance of CCR2−/− mice to T. gondii infection was due to increased parasite replication, the small intestine, liver, lung and CNS of WT and CCR2−/− mice were analyzed for tissue parasitism. We found that CCR2−/− mice exhibited higher parasite load in the small intestine (Figure 2B), liver (Figure 2C), lung (Figure 2D), and CNS (Figure 2E) compared with WT mice at day 8 after infection. As the majority of CCR2−/− mice succumbed to T. gondii infection around 23 days after parasite inoculation, we evaluated the tissue parasitism in the organs of CCR2−/− and WT mice at this time of infection. We found that the tissue parasitism decreased in the peripheral organs in both lineages of mice. However, the CCR2−/− mice presented a remarkably increase in the parasite load in the CNS, showing four times more parasites compared to WT mice (Figure 2F). Therefore, CCR2−/− mice present impaired ability to control parasite replication in the CNS at the initial chronic phase of infection. Similar data were observed when we measured the amount of parasites using real-time polymerase chain reaction.
The Absence of CCR2 Attenuates Lesions in the Peripheral Organs but Not in the CNS of T. gondii-Infected Mice
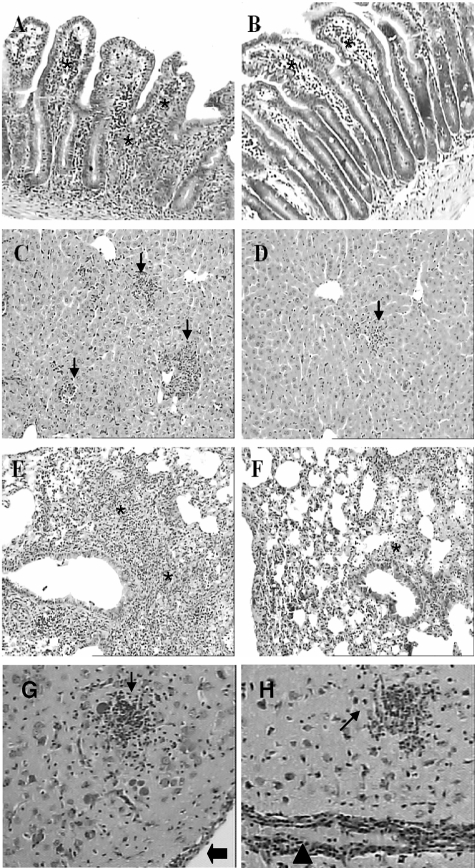
Since CCR2−/− mice are more susceptible to oral infection with five T. gondii cysts, we investigated whether the susceptibility was associated with histopathological lesions. On day 8 after infection the small intestine, liver, and lungs were the most inflamed organs in both CCR2−/− and WT mice. The small intestine presented a moderate inflammatory infiltration in the LP, epithelium, and submucosa (Figure 3, A and B). In some areas, a reduced length and increased thickness of the villi was also observed. The liver presented lesions that were characterized mainly by mononuclear inflammatory foci scattered by parenchyma and portal areas (Figure 3, C and D). The lungs of T. gondii-infected mice presented an interstitial pneumonia with mononuclear inflammatory infiltration in the alveolar septa (Figure 3, E and F). In all of the peripheral organs analyzed on day 8 after infection, the lesions were more severe in WT (Figure 3, A, C, and E) compared to CCR2−/− mice (Figure 3, B, D, and F). The CNS was also analyzed for histopathological changes on day 8 after T. gondii inoculation, but no significant inflammatory lesions were observed in this period of infection. On day 23 after T. gondii infection, the inflammatory lesions were partially controlled in the peripheral organs in both lineages of mice. However, in this phase of infection the CNS presented important inflammatory alterations, which were characterized by diffuse infiltrates of mononuclear cells, glial nodules, vascular cuffing by lymphocytes, and focal infiltrates in the meninges. The lesions were similar in the CNS in WT (Figure 3G) and CCR2−/− mice (Figure 3H).
Figure 3.
Histological changes in the small intestine, liver, lung, and CNS of CCR2−/− and WT mice infected perorally with T. gondii. WT (A, C, E) and CCR2−/− (B, D, F) mice were inoculated with T. gondii and the peripheral organs were collected on day 8 after infection. Observed were inflammatory infiltrates in the lamina propria, epithelium, and submucosa (asterisks) in the small intestine (A, B); inflammatory foci (arrows) in the parenchyma of the liver (C, D); and inflammatory cell infiltration within the alveolar walls (asterisks) in the lung (E, F). The inflammatory reaction was milder in the peripheral organs of CCR2−/− (B, D, F) compared to WT mice (A, C, E). The CNS from WT (G) and CCR2−/− mice (H) was analyzed on day 23 after infection. The lesions characterized by glial nodules (small arrows), vascular cuffing (arrowheads) and inflammatory cells in the meninges (large arrows) were similar in both lineages of mice in this period of infection. Slides were stained with hematoxylin and eosin. Original magnification, ×10.
CCR2 Modulates CD4+ and MAC-1+ Cell Migration in Response to T. gondii Infection
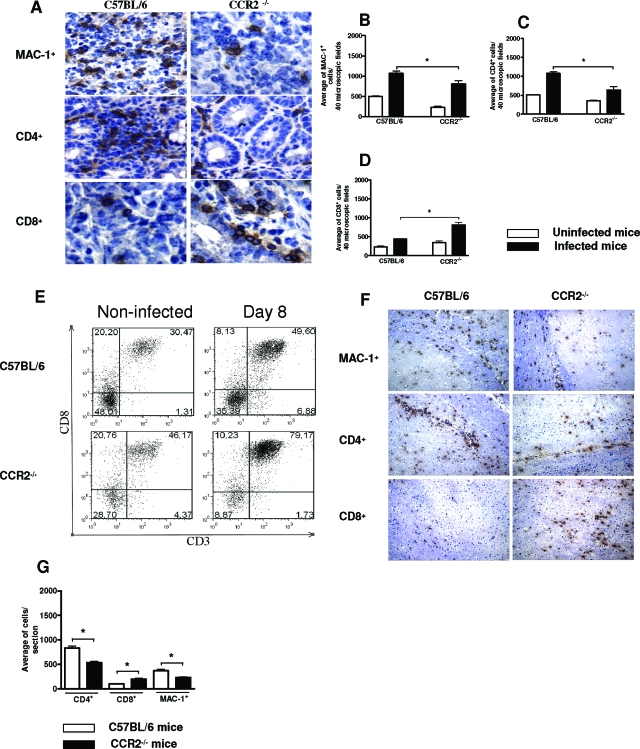
To determine the subsets of infiltrating cells in the small intestine on day 8 and in the CNS on day 23 after T. gondii inoculation, we counted the number of CD4+, CD8+, and MAC-1+ cells in WT and CCR2−/−-infected mice by immunohistochemistry (Figure 4). In accordance with histological analysis, there was a reduction in MAC-1+ (Figure 4, A and B) and CD4+ (Figure 4, A and C) cell migration in the small intestine of CCR2−/− compared to WT mice. In contrast, CCR2−/− showed an increased number of CD8+ cells in this organ when compared to WT mice (Figure 4, A and D). The presence of a leukocyte subpopulation within the small intestine was also demonstrated by fluorescence-activated cell sorting analysis. Confirming our immunohistochemical data, CD3+CD4+ T and CD11b+CD11c− cells were higher in the small intestine in WT compared to CCR2−/− mice. In contrast, CD3+CD8+ T cells were higher in the intraepithelial cell subpopulation in the organs from CCR2−/− compared to WT mice (Figure 4E). In the CNS, the same pattern of cell migration was observed on day 23 after infection (Figure 4, F and G). These results indicate that CCR2 is involved in CD4+ and MAC-1+ but not CD8+ cell migrations in the sites of T. gondii replication.
Figure 4.
Immunohistochemical staining, number of CD4+, CD8+, and MAC-1+ cells into the small intestine and CNS, and flow cytometry analysis of small intestine of CCR2−/− and WT mice after oral T. gondii infection with five cysts. The small intestine (A) and CNS (F) were collected on days 8 and 23 after infection, respectively, and CD4+, CD8+, and MAC-1+ cells were detected by immunohistochemistry. Original magnifications: small intestine, ×40; CNS, ×10. The positive MAC-1+ (B), CD4+ (C,) and CD8+ (D) cells were counted in 40 microscopic fields in the small intestine and per sagittal section in the CNS (G). Original magnification ×40. E: Flow cytometry analysis of the intraepithelial lymphocytes in the small intestine on day 8 after infection. The data represent the mean ± SD and are representative of at least two independent experiments with three mice per group that provided similar results. *, significantly different from values obtained from WT mice (P < 0.05).
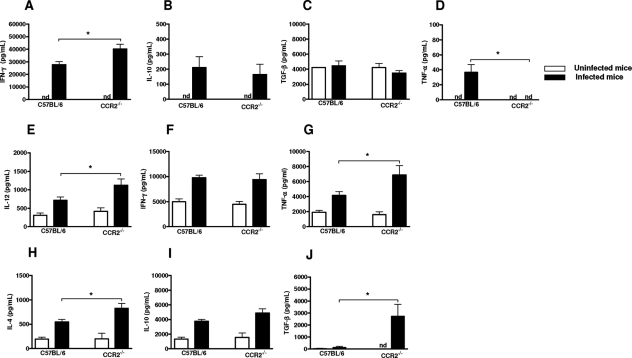
T. gondii Infection Induces an Enhanced and Concomitant Proinflammatory and Anti-Inflammatory Immune Response in CCR2−/− Mice
Since CCR2−/− mice proved to be more susceptible to T. gondii infection, we wanted to know whether systematic and local cytokine production could be involved in the susceptibility. The proinflammatory IL-12, IFN-γ, and TNF-α and anti-inflammatory IL-4, IL-10, and TGF-β were measured in serum samples and intestinal homogenates on day 8 after infection. The levels of IFN-γ were significantly higher in infected WT and CCR2−/− mice compared to noninfected control mice (Figure 5A). Elevated levels of IFN-γ were observed in serum samples from CCR2−/− and WT mice, and it was found to be higher in CCR2−/− compared to WT mice (Figure 5A). In contrast, the levels of TNF-α were higher in WT compared to CCR2−/− mice (Figure 5D), and infected WT mice showed higher levels of the cytokine compared to noninfected mice. Regarding anti-inflammatory cytokines in serum samples, the infection with the parasite induced an increased IL-10 production in both lineages of mice, and the infection did not systemically alter TGF-β (Figure 5, B and C).
Figure 5.
Levels of IFN-γ, TNF-α, IL-4, IL-10, IL-12p70, and TGF-β in the serum samples (A–D) and intestinal homogenate (E–J) from CCR2−/− and WT mice on day 8 after T. gondii infection with five cysts by oral route. The cytokine levels were measured by ELISA. The values shown were the mean and SD of five mice per data point. The experiments were repeated twice and provided similar results. *, significantly different from values obtained from WT mice (P < 0.05); nd, not detected.
T. gondii infection induced increased IL-12, IFN-γ, and TNF-α production in small intestine homogenates in both CCR2−/− and WT mice (Figure 5, E–G). Interestingly, the production of IL-12 and TNF-α locally was higher in CCR2−/− compared to WT mice (Figure 5, E and G). IL-4 and IL-10 levels were also increased in the small intestine when animals were infected with T. gondii, and CCR2−/− showed more elevated levels of IL-4 than those observed in WT mice (Figure 5H). In accordance with the smaller inflammatory cell infiltrates in the small intestine, the production of TGF-β, a cytokine that is known to control the exacerbated inflammatory immune response in the gut,27 was lower in WT compared to CCR2−/− mice (Figure 5J), and infected knockout mice showed higher levels of the cytokine compared to noninfected animals. These data suggest that despite the higher systemic production of proinflammatory cytokines (IFN-γ) in the small intestine (IL-12 and TNF-α) of CCR2−/− mice, the higher production of anti-inflammatory cytokines locally could counteract the detrimental effect of an exacerbated proinflammatory immune response at this site of infection.
CCR2 Modulates iNOS Expression and NO Production in T. gondii Infection
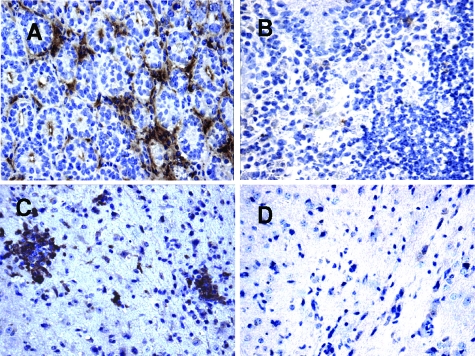
Reactive nitrogen intermediates, including nitric oxide (NO), have been identified in the control of protozoan replication.28,29 Thus, we investigated the expression of iNOS, the enzyme that induces NO production, by immunohistochemistry in the small intestine on day 8 and in the CNS on day 23 after T. gondii infection. A high number of cells from WT mice expressing iNOS in the small intestine on day 8 (Figure 6A) and in the CNS on day 23 after infection (Figure 6C) probably are macrophages and microglial cells, respectively, in accordance with previous data that showed that these cells express iNOS in T. gondii infection.30 Surprisingly, in CCR2−/− mice, cells expressing iNOS was seldom found in the organs from this lineage of mice (Figure 6, B and D), indicating that this pathway of parasite control is not functional in this lineage. In accordance with the iNOS tissue expression, the spleen macrophages from CCR2−/− mice showed a very low NO production when stimulated with IFN-γ or CCL2 (data not shown).
Figure 6.
Immunohistochemical staining for iNOS in the small intestine and in the CNS of WT (A, C) and CCR2−/− (B, D) mice infected with five ME-49 T. gondii cysts by oral route. The animals were infected and the small intestine were collected on day 8 (A, B) and the CNS on day 23 (C, D) after infection for iNOS immunostaining. Original magnification, ×40.
Discussion
The dominant immune response during toxoplasmosis is a Th1 response that results in a prominent leukocyte migration to the site of infection, but if uncontrolled, the response may lead to immunopathology. In the present investigation, we wanted to determine the role of CCR2 in cell migration and control of the parasite in peripheral organs and CNS of T. gondii-infected mice. For this purpose, CCR2−/− mice were orally infected with five cysts of the parasite and the morbidity, survival, and immune response were evaluated. In accordance with previous studies, we observed a high inflammatory cell infiltration expressing CCR28 and CCL2 in the ilea of T. gondii-infected C57BL/6 mice in the acute phase, indicating that this chemokine and chemokine receptor are involved in inflammatory cell migration to the small intestine. In parallel, the CCL2 concentration was elevated in the serum samples and in the small intestine homogenate in the same period of infection. The levels of the chemokine in the sera were higher in CCR2−/− compared to WT mice, which is consistent with the fact that CCR2 is the primary receptor for CCL2 in the mouse31 and that in the absence of the receptor, the concentration of the unbinding chemokine in the serum samples increases.
CCR2−/− mice presented accelerated death associated with high parasite rates in the peripheral organs and CNS in acute phase and also in the CNS at the initial phase of chronic infection. As we have previously shown, there is parasite dissemination in the peripheral organs of the C57BL/6, TNFp55 receptor−/−, and iNOS−/− mice infected with T. gondii; the decreased parasite load in the majority of these sites is at the beginning of the chronic phase of infection but not in the CNS of knockout mice.32 Similarly, in our present work, it was also observed that the tissue parasitism was decreased in the peripheral organs from WT and CCR2−/− mice but increased with the progression of infection in the CNS of CCR2−/− mice. Thus, in the peripheral organs, microbicidal mechanisms that are independent of CCR2 are important to control the parasite, but in the CNS, the chemokine receptor seems to be essential to control T. gondii replication.
It is well known that mice lacking the CCR2 chemokine receptor present defects in macrophage recruitment.15 Oral infection with T. gondii induces an important inflammatory reaction in the small intestine, liver, lung, and CNS in C57BL/6 mice that is controlled in peripheral organs from day 14 of infection and onward.33 In our experiments, it was verified that on day 8 after T. gondii infection the inflammatory reaction in peripheral organs was milder in the knockout compared to WT mice, indicating that the CCR2 is important to promote inflammatory cell migration to these sites. Interestingly, in the small intestine from CCR2−/− mice the CCL2+ cells were lower compared to WT mice, reflecting a smaller cell migration in this lineage of mice and the dependence of the receptor on recruitment of the inflammatory cells. In this organ on day 8 after infection, the inflammatory infiltrates were composed of CD4+, CD8+, and MAC-1+ cells; the CD4+and MAC-1+ cells infiltrate less and CD8+ cells infiltrate more in CCR2−/− compared to WT mice. The infection of C57BL/6 mice with 100 T. gondii cysts by oral route induces a CD8+ and TCR-γδ T cell migration in the epithelia of the small intestine, whereas in the LP the majority of infiltrated cells are CD4+ and TCR-αβ T cells.34 Additionally, it was determined that the CD4+ T cell phenotype in the LP is the cell population responsible for the severe histopathological changes in the intestine, leading to tissue necrosis and subsequent mortality on day 7 of infection.22,31 In other experiments, it was verified that CD8+ T lymphocytes in the intestinal epithelia are able to modulate the inflammatory activity of CD4+ T cells from LP.27 In our studies, we observed smaller CD4+ T lymphocyte and MAC-1+ cell infiltrations in the small intestine of CCR2−/− mice that were associated with a smaller inflammatory reaction, in contrast to greater CD8+ T lymphocyte migrations to this site. Small intestinal intraepithelial and lamina propria lymphocytes migrate to thymus-expressed chemokine (TECK/CCL25), and this attraction is mediated by CCR9, a chemoattractant receptor expressed at high levels by essentially all CD4+ and CD8+ T lymphocytes in the small intestine.35 In vivo-primed effector CD8αβ+ T cells displayed regionalized differences in their entry to the small intestine epithelium with enhanced CCR9-independent entry to the ileum.36 CCR1, CCR2, CCR5, and CXCR3 are increased in Toxoplasma-infected ileum, and in particular, CCR5 expression was markedly increased in Toxoplasma-primed CD8+ intraepithelial T lymphocytes.8 Our results demonstrate that despite the absence of CCR2, other chemokine receptors are involved in CD8+ T lymphocyte migrations to the small intestine. Thus, smaller CD4+ cell migration to the LP, in addition to a greater CD8+ cell migration to the epithelia, could contribute to the decrease in inflammatory lesions in the small intestine in knockout mice.
Because our experiments demonstrate the greater susceptibility of CCR2−/− mice, we hypothesized an impaired development of Th1 protective immune response in infected knockout mice. The results of the present experimental work show that the IL-12 and IFN-γ normally developed in infected CCR2−/− mice; the IFN-γ in serum samples and IL-12 and TNF-α levels in the small intestine are higher than those observed in WT mice. Previous studies have demonstrated that CCR2−/− mice have markedly reduced T cell IFN-γ responses as well as defects in clearance of intracellular pathogens.15,31 The immune response pattern of infected animals depends on the biological features of the involved infectious organisms, and in the case of Toxoplasma, the parasite is known to induce a strong Th1 immune response. As we demonstrated, despite the absence of CCR2, the parasite induced a Th1 type response. Th1 CD4+ T cells express CCR2, CCR5, and CXCR3;37 thus, our data suggest that in the absence of CCR2, other chemokine receptors or other immune mechanisms are involved in the Th1 immune response activation. We also observed that the IL-4 and TGF-β were present in higher levels in the small intestine of CCR2−/− compared to WT infected mice. The elevated levels of IL-4 and TGF-β are in accordance with the smaller cell migration and inflammatory changes verified in the small intestine of the knockout mice, and TGF-β is particularly associated with the modulation of CD4+ T cell activity in the LP.27 Thus, in addition to the smaller cell migration observed in the small intestine in the absence of CCR2, the secretion of IL-4 and TGF-β might contribute to the small inflammatory changes at this site in CCR2−/− mice. Th2 CD4+ T cells express CCR2, CCR3, CCR4, and CCR8,37 and in the absence of CCR2 other chemokine receptors or immune mechanisms could be involved in Th2 activation. Therefore, we can conclude that both Th1 and Th2 immune mediators are activated by T. gondii infection in the absence of CCR2 receptors.
As the inflammatory reaction and parasite multiplication were decreased in the peripheral organs in the absence of the CCR2, we investigated the morbidity of the major important site of T. gondii infection in the initial chronic phase, the CNS. The inflammatory reaction in the CNS was similar in both lineages of mice on day 23 after infection, when the majority of knockout mice were dying, which suggests that other mechanisms including adhesion molecules and other chemokines and chemokine receptors in addition to CCR2-CCL2 are involved in cell migration to this organ. Accordingly, in toxoplasmic encephalitis in C57BL/6 mice, there is a significant intracerebral transcription of CXCL10, CXCL9, CCL5, CCL2, CCL3, and CCL4, which are dependent on IFN-γ, and these levels were parallel with increasing numbers of CD4+ and CD8+ T cells, as well as macrophages, granulocytes, and B cells.5 Despite similar inflammatory lesions in the CNS on day 23 after infection, the CCR2−/− mice presented an uncontrolled tissue parasitism. As we observed in the small intestine, the CNS from CCR2−/− mice also presented a smaller CD4+ and MAC-1+ cell migration. This is in accordance with previous studies that have demonstrated the important role of these cell phenotypes to activate microbicidal mechanisms in the CNS in toxoplasmic encephalitis.38,39 Since iNOS expression associated with NO production is an important microbicidal mechanism against T. gondii replication, we verified the iNOS expression in the CNS and the NO production by spleen macrophages. Interestingly, the iNOS expression was almost absent in CCR2−/− mice. This is in accordance with previous studies that show that dendritic cells (Tip-DC) CD11bINT CD11c+ producing TNF-α and iNOS are absent in CCR2-deficient mice with urinary tract infections.40 In addition, in our experiments the spleen macrophages from CCR2−/− mice, despite being stimulated with IFN-γ, did not produce NO sufficiently, showing the important role of CCR2 in inducing iNOS expression and consequently the NO production. In conclusion, despite the tissue parasitism being partially controlled in the peripheral organs, the microbicidal mechanisms are not effective in the CNS of CCR2−/− mice and parasite replication remains unchecked in this site in the absence of CCR2, contributing to a greater susceptibility in the animals.
Footnotes
Address reprint requests to Dr. Neide Maria Silva, Institute of Biomedical Sciences, Universidade Federal de Uberlândia, Av. Pará, 1720, Bloco 4C, Uberlândia, Minas Gerais, Brazil 38 400-902. E-mail: nmsilva@icbim.ufu.br.
Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. N.M.S. and J.S.S. are research fellows from the Conselho Nacional de Pesquisa Científica e Tecnológica (CNPq). L.B. received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
References
- Brenier-Pinchart M-P, Villena I, Mercier C, Durand F, Simon J, Cesbron-Delauw M-F, Pelloux H. The Toxoplasma surface protein SAG1 triggers efficient in vitro secretion of chemokine ligand 2 (CCL2) from human fibroblasts. Microbes Infect. 2006;8:254–261. doi: 10.1016/j.micinf.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Robben PM, Laregina M, Kuziel WA, Sibley DL. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L, Courret N, Darche S, Luangsay S, Mennechet LM, Rachinel N, Ronet C, Buzoni-Gatel D. Toxoplasma gondii and mucosal immunity. Int J Parasitol. 2004;34:401–409. doi: 10.1016/j.ijpara.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Rachinel N, Buzoni-Gatel D, Dutta C, Mennechet FJD, Luangsay S, Minns LA, Grigg ME, Tomavo S, Boothroyd JC, Kasper LH. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J Immunol. 2004;173:2725–2735. doi: 10.4049/jimmunol.173.4.2725. [DOI] [PubMed] [Google Scholar]
- Strack A, Asensio VC, Campbell IL, Schluter D, Deckert M. Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-gamma. Acta Neuropathol. 2002;103:458–468. doi: 10.1007/s00401-001-0491-7. [DOI] [PubMed] [Google Scholar]
- Mennechet FJD, Kasper LH, Rachinel N, Wen L, Vandewalle A, Buzoni-Gatel D. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol. 2002;168:2988–2996. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol. 2001;167:6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- Luangsay S, Kasper LH, Rachinel N, Minns LA, Mennechet FJD, Vandewalle A, Buzoni-Gatel D. CCR5 mediates specific migration of Toxoplasma gondii – primed CD8+ lymphocytes to inflammatory intestinal epithelial cells. Gastroenterology. 2003;125:491–500. doi: 10.1016/s0016-5085(03)00903-x. [DOI] [PubMed] [Google Scholar]
- Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Souza C, Fairlamb A, Ribeiro JM, Sher A. Molecular mimicry of a CCR5 binding-domain the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- Coillie EV, Damme JV, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokines Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokines receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and (Th2s). J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad SM, Strauss-Ayali D, Field AE, Mack M, Mosser DM. Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages. Infect Immun. 2007;75:653–665. doi: 10.1128/IAI.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison JL, Kuziel WA, Manning JE, Lane TE. Chemokine CC Receptor 2 is important for acute control of cardiac parasitism but does not contribute to cardiac inflammation after infection with Trypanosoma cruzi. J Infect Dis. 2006;193:1584–1588. doi: 10.1086/503812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones MP, Estrada CA, Jimenez F, Martinez H, Willimon O, Kuziel WA, Ahuja SK, Ahuja SS. CCL2-independent role of CCR2 in immune responses against Leishmania major. Parasite Immunol. 2007;29:211–217. doi: 10.1111/j.1365-3024.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57Bl/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185:96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Denkers EY. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1999;1:699–708. doi: 10.1016/s1286-4579(99)80071-9. [DOI] [PubMed] [Google Scholar]
- Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Kang H, Parmley S, Lin S, Park D. Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect. 2000;5:455–462. doi: 10.1016/s1286-4579(00)00318-x. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NM, Tafuri WL, Alvarez-Leite JI, Mineo JR, Gazzinelli RT. Toxoplasma gondii: the in vivo expression of BAG-5 and cyst formation is independent of TNF receptor and inducible nitric oxide synthase functions. Microbes Infect. 2002;4:261–270. doi: 10.1016/s1286-4579(02)01537-x. [DOI] [PubMed] [Google Scholar]
- Welter A, Mineo JR, Silva DAO, Lourenço EV, Ferro EAV, Roque-Barreira MC, Silva NM. An opposite role is exerted by the acarian Mycoptes musculinus in the outcome of Toxoplasma gondii infection according to the route of the protozoa inoculation. Microbes Infect. 2006;8:2618–2628. doi: 10.1016/j.micinf.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Arstila T, Arstila TP, Calbo S, Selz F, Malassis-Seris M, Vassalli P, Kourilsky P, Guy-Grand D. Identical T cell clones are located within the mouse gut epithelium and lamina propria and circulate I the thoracic duct lymph. J Exp Med. 2000;191:823–834. doi: 10.1084/jem.191.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Tannenbaum SR, Goldman P. Nitrite biosynthesis in germfree and conventional rat. Science. 1981;212:56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Mennechet FJD, Kasper LH, Rachinel N, Minns LA, Luangsay S, Vandewalle A, Buzoni-Gatel D. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur J Immunol. 2004;34:1059–1067. doi: 10.1002/eji.200324416. [DOI] [PubMed] [Google Scholar]
- Adams LB, Hibbs JB, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii: role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- Langermans JAM, Van Der Hulst MBE, Nibbering PH, Hiemstra PS, Fransen L, Van Furth R. IFN-γ induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-α. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- Deckert M, Sedgwick JD. Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol. 2006;111:548–558. doi: 10.1007/s00401-006-0062-z. [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese-Jr RV, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LFL, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;62:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes ES, Silva NM, Ruas LP, Mineo JR, Loyola AM, Hsu DK, Liu F-T, Chammas R, Roque-Barreira MC. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am J Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. Immune responses to Toxoplasma gondii in the gut. Immunobiology. 1999;201:229–239. doi: 10.1016/S0171-2985(99)80063-1. [DOI] [PubMed] [Google Scholar]
- Kunkel E, Campbell JL, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression on tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–767. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstad H, Svensson M, Cucak H, Kotarsky K, Agace K. Differential homing mechanisms regulate regionalized effector CD8alphabeta+ T cell accumulation within the small intestine. Proc Natl Acad Sci USA. 2007;104:10122–10127. doi: 10.1073/pnas.0700269104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer. Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- Gazzinelli RT, Hu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactive chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gutgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]