Abstract
CD31(−) CD45(−) side population (SP) cells are a minor SP subfraction that have mesenchymal stem cell-like properties in uninjured skeletal muscle but that can expand on muscle injury. To clarify the role of these SP cells in muscle regeneration, we injected green fluorescent protein (GFP)-positive myoblasts with or without CD31(−) CD45(−) SP cells into the tibialis anterior muscles of immunodeficient NOD/scid mice or dystrophin-deficient mdx mice. More GFP-positive fibers were formed after co-transplantation than after transplantation of GFP-positive myoblasts alone in both mdx and NOD/scid muscles. Moreover, grafted myoblasts were more widely distributed after co-transplantation than after transplantation of myoblasts alone. Immunohistochemistry with anti-phosphorylated histone H3 antibody revealed that CD31(−) CD45(−) SP cells stimulated cell division of co-grafted myoblasts. Genome-wide gene expression analyses showed that these SP cells specifically express a variety of extracellular matrix proteins, membrane proteins, and cytokines. We also found that they express high levels of matrix metalloproteinase-2 mRNA and gelatinase activity. Furthermore, matrix metalloproteinase-2 derived from CD31(−) CD45(−) SP cells promoted migration of myoblasts in vivo. Our results suggest that CD31(−) CD45(−) SP cells support muscle regeneration by promoting proliferation and migration of myoblasts. Future studies to further define the molecular and cellular mechanisms of muscle regeneration will aid in the development of cell therapies for muscular dystrophy.
Regeneration of skeletal muscle is a complex but well-organized process involving activation, proliferation, and differentiation of myogenic precursor cells, infiltration of macrophages to remove necrotic tissues, and remodeling of the extracellular matrix.1,2,3 Muscle satellite cells are myogenic precursor cells that are located between the basal lamina and the sarcolemma of myofibers in a quiescent state, and are primarily responsible for muscle fiber regeneration in adult muscle.4 Recent studies also demonstrated that a fraction of satellite cells self-renew and behave as muscle stem cells in vivo.5,6 On the other hand, several research groups reported multipotent stem cells derived from skeletal muscle. These include muscle-derived stem cells,7 multipotent adult precursor cells,8 myogenic-endothelial progenitors,9 CD34(+) Sca-1(+) cells,10 CD45(+) Sca-1 (+) cells,11 mesoangioblasts,12 and pericytes,13 and all were demonstrated to contribute to muscle regeneration as myogenic progenitor cells.
Side population (SP) cells are defined as the cell fraction that efficiently effluxes Hoechst 33342 dye and therefore shows a unique pattern on fluorescence-activated cell sorting (FACS) analysis.14 Muscle SP cells are proposed to be multipotent15,16 and are clearly distinguished from satellite cells.17 Previous reports showed that muscle SP cells participated in regeneration of dystrophic myofibers after systemic delivery15 and gave rise to muscle satellite cells after intramuscular injection into cardiotoxin (CTX)-treated muscle.17 Muscle SP cells adapted to myogenic characteristics after co-culture with proliferating satellite cells/myoblasts in vitro,17 and expressed a satellite cell-specific transcription factor, Pax7, after intra-arterial transplantation.18 However, the extent to which muscle SP cells participate in muscle fiber regeneration as myogenic progenitor cells is still primarily unknown. Importantly, Frank and colleagues19 recently showed that muscle SP cells secret BMP4 and regulate proliferation of BMP receptor1α (+) Myf5high myogenic cells in human fetal skeletal muscle, raising the possibility that SP cells in adult muscle play regulatory roles during muscle regeneration.
Previously we showed that skeletal muscle-derived SP cell fraction are heterogeneous and contain at least three subpopulations: CD31(+) CD45(−) SP cells, CD31(−) CD45(+) SP cells, and CD31(−) CD45(−) SP cells.20 These three SP subpopulations have distinct origins, gene expression profiles, and differentiation potentials.20 CD31(+) CD45(−) SP cells account for more than 90% of all SP cells in normal skeletal muscle, take up Ac-LDL, and are associated with the vascular endothelium. CD31(+) CD45(−) SP cells did not proliferate after CTX-induced muscle injury. Bone marrow transplantation experiments demonstrated that CD31(−) CD45(+) SP cells are recruited from bone marrow into injured muscle. A few of them are thought to participate in fiber formation.21 Cells of the third SP subfraction, CD31(−) CD45(−), constitute only 5 to 6% of all SP cells in adult normal skeletal muscle, but they actively expand in the early stages of muscle regeneration and return to normal levels when muscle regeneration is completed. Although CD31(−) CD45(−) SP cells are the only SP subset that exhibited the capacity to differentiate into myogenic, adipogenic, and osteogenic cells in vitro,20 their myogenic potential in vivo is limited compared with satellite cells. Therefore, we hypothesized that CD31(−) CD45(−) SP cells might play critical roles during muscle regeneration other than as myogenic stem cells.
In the present study, we demonstrate that the efficacy of myoblast transfer is markedly improved by co-transplantation of CD31(−) CD45(−) SP cells in both regenerating immunodeficient NOD/scid and dystrophin-deficient mdx mice. We also show that CD31(−) CD45(−) SP cells increased the proliferation and migration of grafted myoblasts in vivo and in vitro. We further show that CD31(−) CD45(−) SP cell-derived matrix metalloproteinase (MMP)-2 greatly promotes the migration of myoblasts in vivo. Our findings would provide us insights into the molecular and cellular mechanisms of muscle regeneration, and also help us develop cell therapy for muscular dystrophy.
Materials and Methods
Animals
All experimental procedures were approved by the Experimental Animal Care and Use Committee at the National Institute of Neuroscience. Eight- to twelve-week-old C57BL/6 mice and NOD/scid mice were purchased from Nihon CLEA (Tokyo, Japan). MMP-2-null mice were obtained from Riken BioResource Center (Tsukuba, Japan).22 GFP-transgenic mice (GFP-Tg) were kindly provided by Dr. M. Okabe (Osaka University, Osaka, Japan). C57BL/6-background mdx mice were generously given by Dr. T. Sasaoka (National Institute for Basic Biology, Aichi, Japan) and maintained in our animal facility.
Isolation of Muscle SP Cells
To evoke muscle regeneration, CTX (10 μmol/L in saline; Sigma, St. Louis, MO) was injected into the tibialis anterior (TA) (50 μl), gastrocnemius (150 μl), and quadriceps femoris muscles (100 μl) of 8- to 12-week-old GFP-Tg mice, C57BL/6 mice, MMP-2-null mice, and their wild-type littermates; 3 days later, SP cells were isolated from the muscles as described by Uezumi and colleagues.20 In brief, limb muscles were digested with 0.2% type II collagenase (Worthington Biochemical, Lakewood, NJ) for 90 minutes at 37°C. After elimination of erythrocytes by treatment with 0.8% NH4Cl in Tris-buffer (pH 7.15), mononucleated cells were suspended at 106 cells per ml in Dulbecco’s modified Eagle’s medium (Wako, Richmond, VA) containing 2% fetal bovine serum (JRH Biosciences, Inc., Kansas City, KS), 10 mmol/L Hepes, and 5 μg/ml Hoechst 33342 (Sigma), incubated for 90 minutes at 37°C in the presence or the absence of 50 μmol/L Verapamil (Sigma), and then incubated with phycoerythrin (PE)-conjugated anti-CD31 antibody (1:200, clone 390; Southern Biotechnology, Birmingham, AL) and PE-conjugated anti-CD45 (1:200, clone 30-F11; BD Pharmingen, Franklin Lakes, NJ) for 30 minutes on ice. Dead cells were eliminated by propidium iodide staining. Analysis and cell sorting were performed on an FACS VantageSE flow cytometer (BD Bioscience, Franklin Lakes, NJ). APC-conjugated anti-CD90, Sca-1, CD34, CD49b, CD14, CD124, c-kit, CD14 (BD Pharmingen), CD44 (Southern Biotechnology Associates), and CD133 (eBioscience, San Diego, CA) were used at 1:200 dilution.
Preparation of Satellite Cell-Derived Myoblasts and Macrophages
Satellite cells were isolated from GFP-Tg mice or C57BL/6 mice by using SM/C-2.6 monoclonal antibody23 and expanded in vitro in Dulbecco’s modified Eagle’s medium containing 20% fetal bovine serum and 2.5 ng/ml of basic fibroblast growth factor (Invitrogen, Carlsbad, CA) for 4 days before transplantation. Macrophages were isolated from C57BL/6 mice 3 days after CTX injection. Mononucleated cells were stained with anti-Mac-1-PE (1:200, clone M1/70; BD PharMingen) and anti-F4/80-APC (1:200, clone CI, A3-1; Serotec, Oxford, UK). Mac-1(+) F4/80(+) cells were isolated by cell sorting as macrophages.
Cell Transplantation
To induce muscle regeneration, 100 μl of 10 μmol/L CTX was injected into the TA muscle of NOD/scid muscles, and 24 hours later, 30 μl of cell suspensions containing 3 × 104 myoblasts, 3 × 104 CD31(−) CD45(−) SP cells, or 3 × 104 GFP(+) myoblasts plus 2 × 104 CD31(−) CD45(−) SP cells were directly injected into the TA muscles of 8-week-old NOD/scid or mdx mice. At several time points after transplantation, the muscles were dissected, fixed in 4% paraformaldehyde for 30 minutes, immersed in 10% sucrose/phosphate-buffered saline (PBS) and then in 20% sucrose/PBS, and frozen in isopentane cooled with liquid nitrogen.
Retrovirus Transduction in Vitro
Red fluorescent protein (DsRed) cDNA (BD Biosciences, San Diego, CA) was cloned into a retrovirus plasmid, pMXs, kindly provided by Dr. T. Kitamura of the University of Tokyo, Tokyo, Japan.24 Viral particles were prepared by introducing the resultant pMXs-DsRed into PLAT-E retrovirus packaging cells,25 and the filtered supernatant was added to the myoblast culture. The next day, DsRed(+) myoblasts were collected by flow cytometry.
Immunohistochemistry
We cut the entire TA muscle tissues on a cryostat into 6-μm cross sections, and observed all serial sections under fluorescence microscopy. We then selected two or three sections in which GFP(+) cells were found most frequently. The sections were then blocked with 5% goat serum (Cedarlane, Hornby, Canada) in PBS for 15 minutes, and then reacted with anti-GFP antibody (Chemicon International, Temecula, CA), anti-laminin α2 antibody (4H8-2; Alexis, San Diego, CA), anti-phospho-histone H3 antibody (Upstate Biotechnology, Lake Placid, NY), or anti-DsRed antibody (Clontech, Palo Alto, CA) at 4°C overnight. Dystrophin was detected using a monoclonal antibody, Dys-2 (Novocastra, Newcastle on Tyne, UK), and a M.O.M. Kit (Vector Laboratories, Burlingame, CA). The sections were then incubated with appropriate combinations of Alexa 488-, 568-, or 594-labeled secondary antibodies (Molecular Probes, Eugene, OR) and TOTO-3 (Molecular Probes), and photographed using a confocal laser-scanning microscope system TCSSP (Leica, Heidelberg, Germany). The area occupied by GFP(+) cells or myofibers was measured by using Image J software (National Institutes of Health, Bethesda, MD) on cross sections from three independent experiments, and defined as the distribution area.
RNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from muscles using TRIzol (Invitrogen). First strand cDNA was synthesized using a QuantiTect reverse transcription kit (Qiagen, Hilden, Germany). The levels of GFP mRNA and 18S rRNA were quantified using SYBR Premix Ex Taq (Takara, Otsu, Shiga, Japan) on a MyiQ single-color system (Bio-Rad Laboratories, Richmond, CA) following the manufacturer’s instructions. Primer sequences for real-time PCR were: 18s rRNA, forward: 5′-TACCCTGGCGGTGGGATTAAC-3′, reverse: 5′-CGAGAGAAGACCACGCCAAC-3′ and EGFP, forward: 5′-GACGTAAACGGCCACAAGTT-3′, reverse: 5′-AAGTCGTGCTGCTTCATGTG-3′. The expression levels of MMP-2 and MMP-9 were evaluated by conventional reverse transcriptase (RT)-PCR using the following primers: MMP-2, forward: 5′-TGCAAGGCAGTGGTCATAGCT-3′, reverse: 5′-AGCCAGTCGGATTTGATGCT-3′.
Cell Proliferation Assay
CD31(−) CD45(−) SP cells or 10T1/2 cells were cultured in Dulbecco’s modified Eagle’s medium containing 20% fetal bovine serum for 5 days, and the supernatants were collected as conditioned medium. Myoblasts were plated on 96-well culture plates at a density of 5000 cells/well and cultured in conditioned medium for 3 days. BrdU was then added to the culture medium (final concentration, 10 μmol/L). Twenty-four hours later, BrdU uptake was quantified by a cell proliferation enzyme-linked immunosorbent assay, a BrdU kit (Roche Diagnostics, Meylan, France), and Lumi-Image F1 (Roche).
Gene Expression Profiling
Total RNAs were extracted from CD31(−) CD45(−) SP cells, macrophages, or myoblasts using an RNeasy RNA isolation kit (Qiagen). cDNA synthesis, biotin-labeled target synthesis, MOE430A GeneChip (Affymetrix, Santa Clara, CA) array hybridization, staining, and scanning were performed according to standard protocols supplied by Affymetrix. The quality of the data presented in this study was controlled by using the Microarray Suite MAS 5.0 (Affymetrix). The MAS-generated raw data were uploaded to GeneSpring software version 7.0 (Silicon Genetics, Redwood City, CA). The software calculates signal intensities, and each signal was normalized to a median of its values in all samples or the 50th percentile of all signals in a specific hybridization experiment. Fold ratios were obtained by comparing normalized data of CD31(−) CD45(−) SP cells and macrophages or myoblasts.
In Situ Zymography
CD31(−) CD45(−) SP cells, myoblasts, and macrophages were isolated from regenerating muscles 3 days after CTX injection by cell sorting and collected by a Cytospin3 centrifuge (ThermoShandon, Cheshire, UK) on DQ-gelatin-coated slides (Molecular Probes). The slides were then incubated for 24 hours at 37°C in the presence or absence of GM6001 (a broad-spectrum inhibitor of MMPs, 50 μmol/L; Calbiochem, San Diego, CA) or E-64 (a cysteine protease inhibitor, 50 mmol/L; Calbiochem). Fluorescence of fluorescein isothiocyanate was detected with excitation at 460 to 500 nm and emission at 512 to 542 nm.
Statistics
Statistical differences were determined by Student’s unpaired t-test. For comparison of more than two groups, one-way analysis of variance was used. All values are expressed as means ± SE. A probability of less than 5% (P < 0.05) or 1% (P < 0.01) was considered statistically significant.
Results
Marker Expression on Muscle-Derived CD31(−) CD45(−) SP Cells
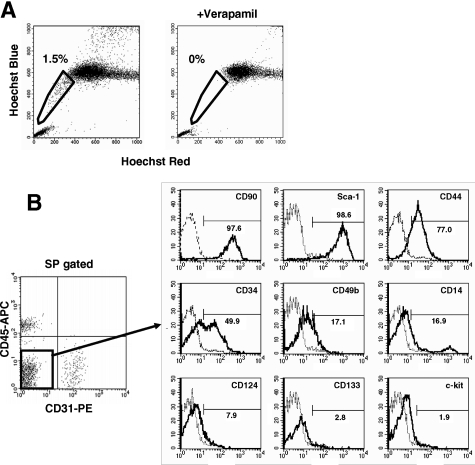
When incubated with 5 μg/ml of Hoechst 33342 dye at 37°C for 90 minutes, 1 to 3% of muscle mononuclear cells show the SP phenotype (Figure 1A). Previously, we reported that muscle SP cells can be further divided into three subpopulation, CD31(−) CD45(−) cells, CD31(−) CD45(+) cells, and CD31(+) CD45(−) SP cells (Figure 1B).20 The CD31(−) CD45(−) SP cells did not express Pax3, Pax7, or Myf5, indicating that they are not yet committed to the muscle lineage.20 RT-PCR suggested that CD31(−) CD45(−) SP cells have mesenchymal cell characteristics.20 To further clarify the properties of CD31(−) CD45(−) SP cells, we analyzed their cell surface markers. CD31(−) CD45(−) SP cells were negative for CD124, CD133, CD14, c-kit (Figure 1B), and CD184 (data not shown), weakly positive for CD34 and CD49b, and strongly positive for Sca-1, CD44, and CD90 (Figure 1). The FACS patterns shown in Figure 1B suggested that CD31(−) CD45(−) SP cells are a homogeneous cell population. CD14 is an exception. A small fraction of CD31(−) CD45(−) SP cells were strongly positive for CD14, but the majority weakly expressed this marker. The function of CD14high CD31(−) CD45(−) SP cells remains to be determined.
Figure 1.
Cell surface markers on CD31(−) CD45(−) SP cells from regenerating muscle. A: Mononuclear cells were prepared from limb muscles of C57BL/6 mice at 3 days after CTX injection, incubated with 5 μmol/L Hoechst 33342 with (right) or without (left) Verapamil, and analyzed by a cell sorter. SP cells are shown by polygons. The numbers indicate the percentage of SP cells in all mononuclear cells. B: Left: Expression of CD45 and CD31 on muscle SP cells. Right: The expression of surface markers (CD90, Sca-1, CD44, CD34, CD49b, CD14, CD124, CD133, and c-kit) on CD31(−) CD45(−) SP cells was further analyzed by FACS. The x axis shows the fluorescence intensity, and the y axis indicates cell numbers. Solid lines are with antibodies; dotted lines are negative controls.
Efficiency of Myoblast Transplantation Is Increased by Co-Transplantation of Muscle CD31(−) CD45(−) SP Cells in NOD/scid Mice
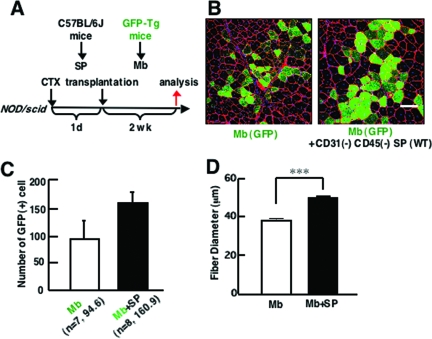
To clarify the functions of CD31(−) CD45(−) SP cells during muscle regeneration, we isolated myoblasts from GFP-transgenic mice (GFP-Tg) and injected them (3 × 104 cells/muscle) with or without CD31(−) CD45(−) SP cells (2 × 104 cells/muscle) into TA muscles of immunodeficient NOD/scid mice (Figure 2A). CTX was injected into recipient muscles 24 hours before cell transplantation to induce muscle regeneration. Two weeks after transplantation, the contribution of grafted myoblasts to muscle regeneration was investigated by immunodetection of GFP(+) myofibers. Co-transplantation of GFP(+) myoblasts with nonlabeled CD31(−) CD45(−) SP cells produced a higher number of GFP(+) myofibers than transplantation of GFP(+) myoblasts alone (Figure 2, B and C). Furthermore, the average diameter of GFP(+) myofibers was significantly larger in co-transplanted muscles than in muscles transplanted with myoblasts alone (Figure 2D). These results suggest that more myoblasts participated in myofiber formation after co-transplantation than after single transplantation, injected SP cells promoted growth of regenerating myofibers, or both.
Figure 2.
Co-transplantation of myoblasts and CD31(−) CD45(−) SP cells into skeletal muscle of immunodeficient NOD/scid mice promotes myofiber formation by transplanted myoblasts. A: Schematic protocol of co-transplantation experiments. CTX was injected into TA muscle 1 day before transplantation. Then, GFP(+) myoblasts (Mb) alone or with a mixture of GFP(+) myoblasts and CD31(−) CD45(−) SP cells derived from wild-type (WT) mice were transplanted to CTX-injected TA muscles of 8- to 12-week-old NOD/scid mice, and sampled 2 weeks after transplantation. B: Cross-sections of transplanted TA muscles stained with anti-GFP (green) and anti-laminin-α2 chain (red) antibodies. Nuclei were stained with TOTO3 (blue). C: The number of GFP(+) fibers per cross section of transplanted TA muscle. Values are means with SE (seven to eight mice in each group). **P < 0.01. D: Average diameters of GFP(+) fibers in the TA muscles transplanted with myoblasts (Mb) or myoblasts plus CD31(−) CD45(−) SP cells (Mb + SP). Values are means with SE. ***P < 0.001. Scale bar = 80 μm.
Co-transplantation of Myoblasts with Muscle CD31(−) CD45(−) SP Cells Significantly Increased Efficiency of Myoblast Transplantation in mdx Mice
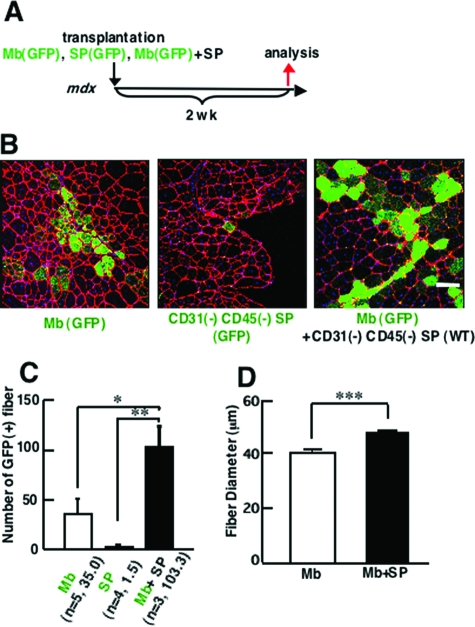
Next, co-transplantation experiments were performed using 8-week-old dystrophin-deficient mdx mice as a host. Three kinds of transplantations were performed: 3 × 104 myoblasts derived from GFP-Tg mice, 3 × 104 CD31(−) CD45(−) SP cells derived from GFP-Tg mice, or a mixture of GFP(+) 3 × 104 myoblasts and 2 × 104 CD31(−) CD45(−) SP cells derived from C57BL/6 mice (Figure 3A).
Figure 3.
Co-transplantation of CD31(−) CD45(−) SP cells and myoblasts improves efficiency of myoblast transfer in dystrophin-deficient mdx mice. A: Schematic protocol of experiments. GFP(+) myoblasts alone (3 × 104), GFP(+) CD31(−) CD45(−) SP cells alone (3 × 104 cells), or a mixture of GFP(+) myoblasts (3 × 104) and CD31(−) CD45(−) SP cells (2 × 104) were directly injected into TA muscles of 8-week-old mdx mice, and the muscles were sampled 2 weeks after transplantation. B: Cross-sections of transplanted TA muscles stained with anti-GFP (green) and anti-laminin-α2 chain (red) antibodies. Nuclei were stained with TOTO3 (blue). C: The number of GFP(+) fibers per cross section. Myoblasts gave rise to more myofibers when co-transplanted with CD31(−) CD45(−) SP cells (Mb + SP) than when transplanted alone (Mb). Transplantation of only GFP(+) SP cells resulted in formation of few myofibers (SP). Values are means with SE (n = 3 to 5 mice). *P < 0.05, **P < 0.01. D: Average diameters of GFP(+) fibers in the TA muscles transplanted with myoblasts (Mb) or with myoblasts plus CD31(−) CD45(−) SP cells (Mb + SP). Values are means with SE. ***P < 0.001. Scale bar = 80 μm.
When analyzed at 2 weeks after transplantation, a much higher number of GFP(+) myofibers were detected on cross-sections after co-transplantation of myoblasts and CD31(−) CD45(−) SP cells than after transplantation of GFP(+) myoblasts alone (Figure 3, B and C). On the other hand, transplantation of GFP(+) SP cells alone resulted in formation of few GFP(+) myofibers. This observation is consistent with our previous report.20 Co-transplantation of myoblasts and CD31(−) CD45(−) SP cells also gave rise to more myofibers expressing dystrophin at the sarcolemma in dystrophin-deficient mdx muscles than transplantation of myoblasts alone (data not shown). Again, the diameter of GFP(+) myofibers was significantly larger in co-transplanted muscles than in muscles transplanted with myoblasts or CD31(−) CD45(−) SP cells alone (Figure 3D).
The transplantation efficiency of myoblasts in mdx mice was 40 to 60% lower than that in NOD/scid mice. In the present study, mdx mice were not treated with any immunosuppressant. Although cellular infiltration was not evident when examined 2 weeks after transplantation (data not shown), some immune reaction might be evoked and eliminate myoblasts transplanted into mdx muscle.
Localization of Transplanted Myoblasts and CD31(−) CD45(−) SP Cells after Intramuscular Injection
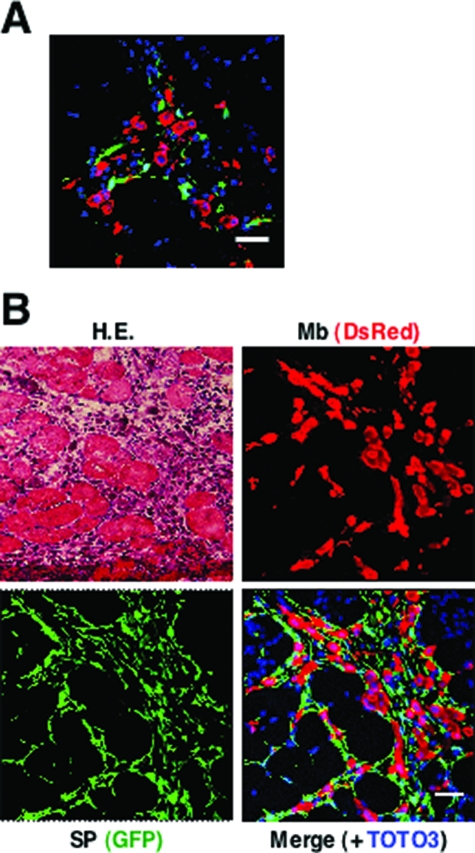
To examine the interaction between grafted myoblasts and CD31(−) CD45(−) SP cells during muscle regeneration, we labeled C57BL/6 myoblasts with a retrovirus vector expressing a red fluorescent protein, DsRed. CD31(−) CD45(−) SP cells were isolated from GFP-Tg mice. We then injected a mixture of DsRed(+) myoblasts and GFP(+) CD31(−) CD45(−) SP cells into CTX-injected NOD/scid TA muscles. At 24 hours after transplantation, DsRed(+) myoblasts and GFP(+) CD31(−) CD45(−) SP cells were observed clearly (Figure 4A). At 48 hours after transplantation, immunohistochemistry revealed that grafted CD31(−) CD45(−) SP cells expanded, and surrounded both grafted myoblasts and damaged myofibers, but rarely fused with myoblasts (Figure 4B).
Figure 4.
Behavior of GFP+ CD31(−) CD45(−) SP cells and DsRed-labeled myoblasts after transplantation. A: NOD/scid TA muscles were injected with CTX 24 hours before transplantation. Then, myoblasts transduced with a retrovirus vector expressing DsRed were injected together with GFP(+) CD31(−) CD45(−) SP cells into the muscles. The muscles were dissected 24 hours after the transplantation, sectioned, and stained with anti-DsRed (red) and anti-GFP antibodies (green). Nuclei were stained with TOTO3 (blue). B: Representative image of DsRed(+) myoblasts and GFP(+) SP cells 48 hours after co-transplantation. One serial section was stained with H&E. Scale bars = 40 μm.
CD31(−) CD45(−) SP Cells Promote Proliferation of Myoblasts in Vivo and in Vitro
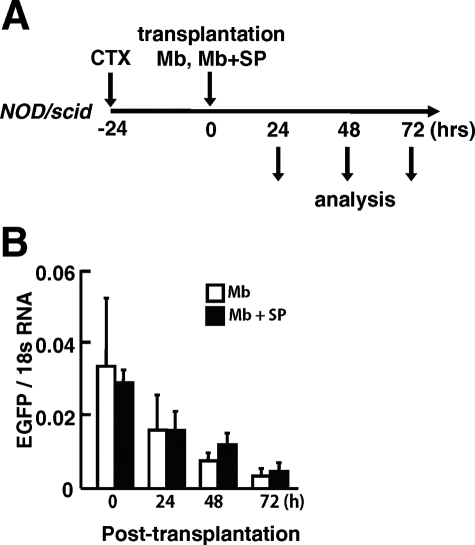
Next, to clarify the mechanism by which co-transplanted CD31(−) CD45(−) SP cells increased the contribution of grafted myoblasts to myofiber regeneration, we investigated the survival of grafted myoblasts after transplantation (Figure 5). GFP(+) myoblasts were injected into TA muscles of NOD/scid mice with or without unlabeled CD31(−) CD45(−) SP cells. At 24, 48, and 72 hours after transplantation, injected TA muscles were dissected, and the GFP mRNA level in injected muscles was evaluated by using real-time PCR (Figure 5A). There was a decline of the GFP mRNA level of injected muscles from 24 to 72 hours after injection (Figure 5B) with no differences in survival rates between single transplantation and co-transplantation.
Figure 5.
Survival of injected myoblasts in NOD/scid mice. A: Experimental design. GFP(+) myoblasts alone (3 × 104 cells) or a mixture of GFP(+) myoblasts (3 × 104 cells) and nonlabeled CD31(−) CD45(−) SP cells (2 × 104 cells) were injected into previously CTX-injected TA muscles of NOD/scid mice. The muscles were then sampled at 0, 24, 48, and 72 hours after transplantation. B: The mRNA level of GFP at each time point was quantified by real-time PCR. The y axis shows GFP mRNA levels normalized to 18s RNA with SE (n = 4 to 5).
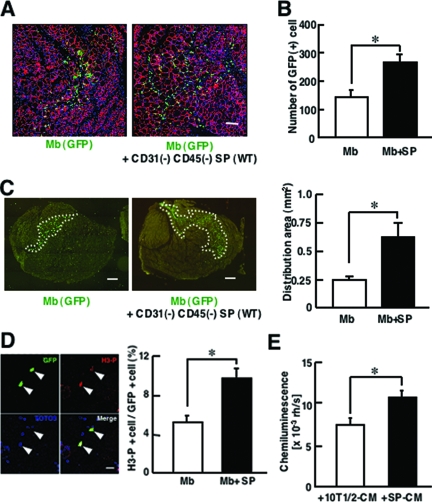
At 48 and 72 hours after transplantation, however, GFP mRNA levels were slightly higher in co-injected muscle than in muscle injected with myoblasts alone (Figure 5B). Therefore, we directly counted the number of GFP(+) myoblasts at 72 hours after transplantation. As shown in Figure 6, A and B, many more GFP(+) myoblasts were detected in co-transplanted muscles than in myoblast-transplanted muscles (Figure 6, A and B). In addition, GFP(+) cells were more widely spread in the co-injected muscles than in muscles transplanted with myoblasts alone (Figure 6C).
Figure 6.
CD31(−) CD45(−) SP cells promote proliferation of myoblasts in vitro and in vivo. A: Representative images of cross sections of 72-hour samples stained with anti-GFP (green) and anti-laminin-α2 chain (red) antibodies. GFP(+) myoblasts are more widely scattered in injected muscle when co-transplanted with CD31(−) CD45(−) SP cells, compared with single transplantation. B: The number of GFP(+) cells per cross section of TA muscles injected with myoblasts or myoblasts and CD31(−) CD45(−) SP cells. Values were means with SE (n = 4 to 5). *P < 0.05. C: Left: Representative distributions of GFP(+) myoblasts/myotubes 72 hours after transplantation. Right: Distribution area (marked by white dotted lines in left panels) was measured by Image J software. Values were means with SE (n = 4 to 5). *P < 0.05. D: GFP(+) myoblasts were transplanted into CTX-injected TA muscles of NOD/scid mice with (Mb + SP) or without CD31(−) CD45(−) SP cells (Mb). Forty-eight hours after transplantation, the muscles were dissected, sectioned, and stained with anti-phosphorylated histone-H3 (H3-P) (red) and anti-GFP (green) antibodies. Arrowheads indicate H3-P(+) GFP(+) cells. The right graph shows the percentage of H3-P(+) cells in GFP(+) myoblasts in single-transplanted muscle (Mb) or in co-transplanted muscle (Mb + SP). The values are means with SE (n = 3). *P < 0.05. E: Myoblasts were cultured for 3 days in conditioned medium of either CD31(−) CD45(−) SP cells (SP-CM) or 10T1/2 cells (10T1/2-CM) and then cultured for an additional 24 hours in the presence of BrdU. The vertical axis shows BrdU uptake by myoblasts. Values are means with SE (n = 6). *P < 0.05. Scale bars: 100 μm (A); 200 μm (C); 80 μm (D).
To determine whether CD31(−) CD45(−) SP cells promote proliferation of implanted myoblasts, we dissected the muscles at 48 hours after transplantation, and stained the cross-sections with anti-phosphorylated histone H3 antibody, a marker of the mitotic phase of the cell cycle. Co-transplantation of myoblasts with CD31(−) CD45(−) SP cells significantly increased the percentage of mitotic GFP(+) cells compared with transplantation of myoblasts alone (Figure 6D). These observations suggest that co-injection of CD31(−) CD45(−) SP cells promoted proliferation of grafted myoblasts.
Next, to examine whether CD31(−) CD45(−) SP cells directly promote proliferation of myoblasts or not, we performed an in vitro proliferation assay using primary myoblasts and conditioned medium (CM) of CD31(−) CD45(−) SP cells and CM of 10T1/2 cells. BrdU uptake analysis showed that SP-CM more strongly stimulated the proliferation of myoblasts than 10T1/2-CM did (Figure 6E). The results suggest that CD31(−) CD45(−) SP cells promote proliferation of injected myoblasts at least in part by producing soluble factors.
Gene Expression Profiling of CD31(−) CD45(−) SP Cells
To identify the growth factor produced by CD31(−) CD45(−) SP cells that promotes proliferation of myoblasts, we extracted total RNAs from CD31(−) CD45(−) SP cells, myoblasts, and macrophages isolated from regenerating muscles 3 days after CTX injection, and examined the gene expression in these three cell populations by microarray. Eventually, we identified 192 genes that were expressed at more than 10-fold higher levels in CD31(−) CD45(−) SP cells than in either macrophages or myoblasts. We categorized the 192 genes based on gene ontology, and found that CD31(−) CD45(−) SP cells preferentially express extracellular matrix proteins and cytokines and their receptors (see Supplementary Table S1 at http://ajp.amjpathol.org). We found numerous genes involved in wound healing and tissue repair on the gene list, suggesting that CD31(−) CD45(−) SP cells play a regulatory role in the muscle regeneration process. Interestingly, the gene list contained both muscle proliferation or differentiation-promoting (follistatin),26 and inhibitory factors (eg, insulin-like growth factor binding proteins,27 Nov28). The list also contains regulators of TGF-β (eg, thrombospondins,29 Prss11,30 Ltbp331), which would consequently attenuate or stimulate proliferation and differentiation of myoblasts.
CD31(−) CD45(−) SP Cell-Derived MMP-2 Promotes the Migration of Myoblasts
Genome-wide gene expression analysis revealed that CD31(−) CD45(−) SP cells highly express matrix metalloproteinases (see Supplementary Table S1 and Supplementary Figure S1 at http://ajp.amjpathol.org). MMPs are a group of zinc-dependent endopeptidases that degrade extracellular matrix components, thereby facilitating cell migration and tissue remodeling.32,33 Furthermore, MMPs are known to release growth factors stored within the extracellular matrix and process growth factor receptors, resulting in stimulation of cell proliferation.34,35,36 Among the MMPs up-regulated in CD31(−) CD45(−) SP cells, we paid special attention to MMP-2 (also called gelatinase A or 72-kDa type IV collagenase). In CTX-injected muscle, MMP-2 activity was shown to be increased concomitantly with the transition from the regeneration phases characterized by the appearance of young myotubes to maturation of the myotubes into multinucleated myofibers37,38 MMP-2 was also activated in the endomysium of regenerating fibers in dystrophin-deficient muscular dystrophy dogs.39 Furthermore, MMP-2 transcripts were found in the areas of fiber regeneration, and were localized to mesenchymal fibroblasts in DMD skeletal muscle.40
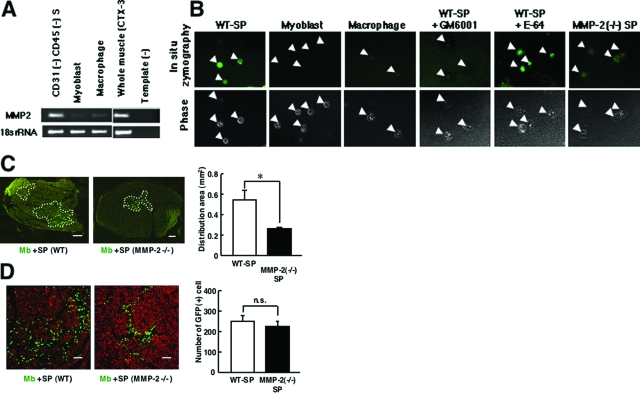
We confirmed that the mRNA level of MMP-2 was much higher in CD31(−) CD45(−) SP cells than in macrophages or myoblasts (Figure 7A). Next, we examined the gelatinolytic activity in CD31(−) CD45(−) SP cells, macrophages, and myoblasts by DQ-gelatin zymography. The cells were directly isolated from regenerating muscle. High gelatinolytic activity was detected in CD31(−) CD45(−) SP cells, compared to myoblasts or macrophages (Figure 7B). Importantly, the signal in MMP-2-null SP cells was considerably weak, compared with wild-type SP cells. The results indicate that DQ-gelatin was degraded mainly (but not exclusively) by MMP-2 in the assay. We hardly detected the green fluorescence in wild-type SP cells in the presence of a broad-spectrum inhibitor of MMPs, GM6001, but not a potent inhibitor of cysteine proteases, E-64, suggesting that other MMPs contribute to gelatin degradation to some extent in the assay. Collectively, these results indicate that CD31(−) CD45(−) SP cells have high MMP-2 activity.
Figure 7.
MMP-2 derived from CD31(−) CD45(−) SP cells promotes the migration of myoblasts in vivo. A: RT-PCR analysis of the expression of MMP-2 in CD31(−) CD45(−) SP cells, myoblasts, macrophages, and regenerating muscles. 18s rRNA is shown as an internal control. Template (−) is a negative control. B: In situ zymography of wild-type CD31(−) CD45(−) SP cells (WT-SP), myoblasts, macrophages, and MMP-2(−/−) CD31(−) CD45(−) SP cells (MMP-2(−/−) SP) in the presence or absence of GM6001 (50 μmol/L) or E-64 (50 μmol/L). Cells were freshly isolated from regenerating muscles 3 days after CTX injury and collected on the glass slides. Top panels are fluorescent signals from digested DQ-gelatin. Phase contrast images of the cells (arrowheads) are shown in bottom panels. C: Left: Representative images of GFP(+) myoblasts 72 hours after co-transplantation of GFP+ myoblasts and CD31(−) CD45(−) SP cells from wild-type (WT) or from MMP-2-null mice (MMP-2 −/−) into CTX-injected TA muscles of NOD/Scid mice. Right: Distribution areas shown by white dotted lines in the left panels were measured by ImageJ (National Institutes of Health). Values are means with SE (n = 5 to 6). *P < 0.05. D: Left: Representative immunohistochemistry of cross-sections of the TA muscle 72 hours after co-transplantation. Right: The number of GFP(+) cells per cross section of the TA muscle injected with GFP(+) myoblasts and CD31(−) CD45(−) SP cells derived from wild-type littermates (Mb-SP) or MMP-2-null mice (MMP-2(−/−) SP). Values are means with SE (n = 5 to 6). Scale bars: 200 μm (C); 100 μm (D).
MMP-2 is reported to mediate cell migration and tissue remodeling.32,33 To directly investigate the effects of MMP-2 on the migration and proliferation of transplanted myoblasts, we injected GFP(+) myoblasts with CD31(−) CD45(−) SP cells prepared from wild-type mice or from MMP-2-null mice into CTX-injected TA muscles of NOD/scid mice. There was no difference in the yield of CD31(−) CD45(−) SP cells from regenerating muscle between wild-type and MMP-2-null mice (data not shown). Consistent with this observation, MMP-2-null CD31(−) CD45(−) SP cells proliferated as vigorously as wild-type in vitro (data not shown). At 72 hours after transplantation, GFP(+) myoblasts were more widely spread in the muscle co-injected with wild-type CD31(−) CD45(−) SP cells than in the muscles co-injected with MMP-2-deficient CD31(−) CD45(−) SP cells (Figure 7C). In contrast, there was no difference in the number of GFP(+) myoblasts between two groups (Figure 7D). These results strongly suggest that MMP-2 derived from CD31(−) CD45(−) SP cells significantly promotes migration of myoblasts, but does not influence the proliferation of myoblasts.
Discussion
We previously reported a novel SP subset: CD31(−) CD45(−) SP cells.20 They are resident in skeletal muscle and are activated and vigorously proliferate during muscle regeneration. RT-PCR analysis suggested that CD31(−) CD45(−) SP cells are of mesenchymal lineage, and indeed they differentiated into adipocytes, osteogenic cells, and muscle cells after specific induction in vitro.20 In the present study, we further characterized CD31(−) CD45(−) SP cells and found that co-transplantation of CD31(−) CD45(−) SP cells markedly improves the efficacy of myoblast transfer to dystrophic mdx mice. Our findings suggest that endogenous CD31(−) CD45(−) SP cells support muscle regeneration by stimulating proliferation and migration of myoblasts.
Are CD31(−) CD45(−) SP Cells Mesenchymal Stem Cells?
Analysis of cell surface antigens on CD31(−) CD45(−) SP cells suggests that they are a homogeneous population. Several reports showed that mesenchymal stem cells (MSCs) express CD44, CD90, but not CD31, CD45, or CD14.41,42 The expression patterns of these markers on CD31(−) CD45(−) SP cells and their differentiation potentials into osteogenic cells, adipocytes, and myogenic cells suggest that CD31(−) CD45(−) SP cells are closely related to MSCs.20 On the other hand, the expression of PDGFRβ,20 CD44, CD49b, CD90, and the lack of CD133 expression on CD31(−) CD45(−) SP cells are similar to those of human pericytes.13 Unlike human pericytes, however, CD31(−) CD45(−) SP cells have limited myogenic potential in vivo.13,20 The relationship between CD31(−) CD45(−) SP cells and MSCs or pericytes remains to be determined in a future study.
CD31(−) CD45(−) SP Cells Promote Proliferation of Myogenic Cells
In the present study, we demonstrated that the efficiency of myoblast transfer is greatly improved by co-transplantation of CD31(−) CD45(−) SP cells. Transplanted CD31(−) CD45(−) SP cells proliferated in the injection site and surrounded both engrafted myoblasts and damaged myofibers, but rarely fused with myoblasts (Figure 4). Transplantation of CD31(−) CD45(−) SP cells alone contributed little to myofiber formation. Therefore, the improvement in efficiency of myoblast transfer by co-transplantation is not attributable to differentiation of CD31(−) CD45(−) SP cells into muscle fibers.
Because the conditioned medium from CD31(−) CD45(−) SP cells modestly stimulated the proliferation of myoblasts in vitro, when compared with CM of 10T1/2 cells, it is possible that CD31(−) CD45(−) SP cells stimulated proliferation of myoblasts by secreting growth factors. CD31(−) CD45(−) SP cells are found in close vicinity to myoblasts 48 hours after transplantation. Therefore, even low levels of growth factors produced by CD31(−) CD45(−) SP cells may effectively stimulate the proliferation of myoblasts. Importantly, several reports showed that MSCs secrete a variety of cytokines and growth factors, which suppress the local immune system, inhibit fibrosis and apoptosis, enhance angiogenesis, and stimulate mitosis and differentiation of tissue-specific stem cells.43 On the gene list, we found a variety of cytokines/chemokines and their regulators (see Supplementary Table S1 at http://ajp.amjpathol.org). These molecules may directly or indirectly stimulate proliferation of myoblasts.
MMP-2 Derived from CD31(−) CD45(−) SP Cells Promotes the Migration of Myoblasts
Transplanted GFP(+) myoblasts were more widely spread in injected muscle when co-injected with CD31(−) CD45(−) SP cells than when transplanted alone (Figure 6C). MMP-2 is a candidate molecule that promotes migration of myoblasts. MMP-2 plays a critical role in myogenesis44 and is up-regulated in muscle regeneration (see Supplementary Figure S2 at http://ajp.amjpathol.org).38 MMP-2 expression is also detected in regenerating areas of dystrophic muscles.39,40 Importantly, El Fahime and colleagues45 reported that forced expression of MMP-2 in normal myoblasts significantly increased migration of myoblasts in vivo. In the present study, we demonstrated that CD31(−) CD45(−) SP cells highly express MMP-2 (see Figure 7A and Supplementary Table S1 at http://ajp.amjpathol.org). Gelatin zymography confirmed that CD31(−) CD45(−) SP cells have high gelatinolytic activities (Figure 7B). Importantly, CD31(−) CD45(−) SP cells prepared from wild-type mice promoted the migration of transplanted myoblasts, but those from MMP-2-null mice did not (Figure 7C). Our results suggest that CD31(−) CD45(−) SP cells promote the migration of myoblasts via MMP-2 secretion. CD31(−) CD45(−) SP cells highly express MMP-2, 3, 9, 14, and 23 during regenerating muscle (see Supplementary Figures S1 and S2 and Supplementary Table S1 at http://ajp. amjpathol.org). Therefore, it remains to be determined whether MMPs other than MMP-2 also promote the migration of myoblasts. MMPs are reported to promote cell proliferation by releasing local growth factors stored within the extracellular matrix and process growth factor receptors.34,35,46 In the present study, however, MMP-2 derived from CD31(−) CD45(−) SP cells did not stimulate the proliferation of myoblasts in vivo (Figure 7D). The factors that stimulate the proliferation of myoblasts remain to be determined in a future study. MMP-3, -9, -14, and -23 are candidates that play a role in stimulating the proliferation of myoblasts.
CD31(−) CD45(−) SP Cells Are the Third Cellular Component of Muscle Regeneration
Our results suggest that transplanted CD31(−) CD45(−) SP cells stimulate myogenesis of co-transplanted myoblasts by supporting their proliferation and migration. Our results also suggest that endogenous CD31(−) CD45(−) SP cells promote muscle regeneration by the same mechanisms. Muscle regeneration is a complex, highly coordinated process in which not only myogenic cells but also inflammatory cells such as macrophages play critical roles.3 Based on our finding that CD31(−) CD45(−) SP cells regulate myoblast proliferation and migration, we propose that CD31(−) CD45(−) SP cells are a third cellular component of muscle regeneration. In addition, gene expression analysis on CD31(−) CD45(−) SP cells revealed that CD31(−) CD45(−) SP cells express a wide range of regulatory molecules implicated in embryonic development, tissue growth and repair, angiogenesis, and tumor progression, suggesting that CD31(−) CD45(−) SP cells are a versatile player in regeneration of skeletal muscle. Future studies of ablation of endogenous CD31(−) CD45(−) SP cells in the mouse will likely further clarify the mechanisms by which CD31(−) CD45(−) SP cells promote muscle regeneration.
Acknowledgments
We thank Satoru Masuda and Chika Harano for technical support.
Footnotes
Address reprint requests to Yuko Miyagoe-Suzuki, M.D., Ph.D., Department of Molecular Therapy, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1 Ogawa-higashi, Kodaira, Tokyo 187-8502, Japan. E-mail: miyagoe@ncnp.go.jp.
Supported by the Ministry of Health, Labor, and Welfare (grant 16b-2 for research on nervous and mental disorders, health science research grant h16-genome-003 for research on the human genome and gene therapy, grants h15-kokoro-021, H18-kokoro-019 for research on brain science); the Ministry of Education, Culture, Sports, Science, and Technology (grants-in-aid for scientific research 16590333 and 18590392); and the Japan Space Forum (ground-based research program for space utilization).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Orimo S, Hiyamuta E, Arahata K, Sugita H. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve. 1991;14:515–520. doi: 10.1002/mus.880140605. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D'Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del Nido P, McGowan FX, Li S, Flint A, Chamberlain J. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve. 2006;34:44–52. doi: 10.1002/mus.20560. [DOI] [PubMed] [Google Scholar]
- Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, Ramoni MF, Frank MH, Kohane IS, Gussoni E. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J Cell Biol. 2006;175:99–110. doi: 10.1083/jcb.200511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, Takeda S. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun. 2006;341:864–873. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Ojima K, Uezumi A, Miyoshi H, Masuda S, Morita Y, Fukase A, Hattori A, Nakauchi H, Miyagoe-Suzuki Y, Takeda S. Mac-1(low) early myeloid cells in the bone marrow-derived SP fraction migrate into injured skeletal muscle and participate in muscle regeneration. Biochem Biophys Res Commun. 2004;321:1050–1061. doi: 10.1016/j.bbrc.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of β-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83:154–160. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- Tocharus J, Tsuchiya A, Kajikawa M, Ueta Y, Oka C, Kawaichi M. Developmentally regulated expression of mouse HtrA3 and its role as an inhibitor of TGF-beta signaling. Dev Growth Differ. 2004;46:257–274. doi: 10.1111/j.1440-169X.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol. 2005;167:419–428. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Balcerzak D, Querengesser L, Dixon WT, Baracos VE. Coordinate expression of matrix-degrading proteinases and their activators and inhibitors in bovine skeletal muscle. J Anim Sci. 2001;79:94–107. doi: 10.2527/2001.79194x. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzrein M, Garred O, Olsnes S, Sandvig K. Diphtheria toxin endocytosis and membrane translocation are dependent on the intact membrane-anchored receptor (HB-EGF precursor): studies on the cell-associated receptor cleaved by a metalloprotease in phorbol-ester-treated cells. Biochem J. 1995;310:285–289. doi: 10.1042/bj3100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch CB, Strittmatter WJ. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983;32:257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci. 2006;119:3822–3832. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdière-Sahuqué M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda S, Ikeda S. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord. 2007;8:54. doi: 10.1186/1471-2474-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moers A, Zwirner A, Reinhold A, Brückmann O, van Landeghem F, Stoltenburg-Didinger G, Schuppan D, Herbst H, Schuelke M. Increased mRNA expression of tissue inhibitors of metalloproteinase-1 and -2 in Duchenne muscular dystrophy. Acta Neuropathol (Berl) 2005;109:285–293. doi: 10.1007/s00401-004-0941-0. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, Itoh T, Itohara S, Takahashi C, Noda M. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–5048. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]