Abstract
α-Synuclein (ASYN) is crucial in Parkinson disease (PD) pathogenesis. Increased levels of wild type (WT) ASYN expression are sufficient to cause PD in humans. The manner of post-transcriptional regulation of ASYN levels is controversial. Previously, we had shown that WT ASYN can be degraded by chaperone-mediated autophagy (CMA) in isolated liver lysosomes. Whether this occurs in a cellular and, in particular, in a neuronal cell context is unclear. Using a mutant ASYN form that lacks the CMA recognition motif and RNA interference against the rate-limiting step in the CMA pathway, Lamp2a, we show here that CMA is indeed involved in WT ASYN degradation in PC12 and SH-SY5Y cells, and in primary cortical and midbrain neurons. However, the extent of involvement varies between cell types, potentially because of differences in compensatory mechanisms. CMA inhibition leads to an accumulation of soluble high molecular weight and detergent-insoluble species of ASYN, suggesting that CMA dysfunction may play a role in the generation of such aberrant species in PD. ASYN and Lamp2a are developmentally regulated in parallel in cortical neuron cultures and in vivo in the central nervous system, and they physically interact as indicated by co-immunoprecipitation. In contrast to previous reports, inhibition of macroautophagy, but not the proteasome, also leads to WT ASYN accumulation, suggesting that this lysosomal pathway is also involved in normal ASYN turnover. These results indicate that CMA and macroautophagy are important pathways for WT ASYN degradation in neurons and underline the importance of CMA as degradation machinery in the nervous system.
α-Synuclein (ASYN)3 is central in Parkinson disease (PD) pathogenesis. Point mutations in the gene encoding ASYN, as well as multiplications of the gene locus, are identified in rare cases of familial PD (1–3). Genetic polymorphic variants within the ASYN locus that may be associated with increased production of ASYN, confer an increased risk for sporadic PD (4). These data suggest that modulation of wild type (WT) ASYN levels is critical for PD pathogenesis. Control of protein levels is in part achieved by differential degradation that modulates cellular protein half-life. The subject of ASYN degradation is controversial.
Initial studies showed that ASYN accumulated in cells upon proteasomal inhibition, suggesting that the proteasome was responsible for ASYN degradation (5, 6). This has also been shown in more recent work (7, 8). Other studies however, including our own, failed to detect accumulation of endogenous or overexpressed ASYN with proteasomal inhibition (9–12). In contrast, ASYN appeared to accumulate with general lysosomal inhibition (12, 13). In lysosomes, degradation of cytoplasmic components is achieved through distinct types of autophagic pathways as follows: chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy (14, 15). CMA involves the selective targeting of proteins containing a KFERQ peptide motif to lysosomes. This requires binding to the lysosomal receptor, Lamp2a, the rate-limiting step in CMA (16–18). Microautophagy involves the pinocytosis of small quantities of cytosol directly by lysosomes (18, 19). Macroautophagy involves the sequestration of cytosolic regions into autophagosomes that deliver their contents to late endosomal and lysosomal compartments for degradation (20).
Direct assessment of the contribution of macroautophagy to WT ASYN degradation was performed in two studies using the selective macroautophagy inhibitor 3-methyladenine (3-MA). In both cases (7, 8), 3-MA application failed to enhance ASYN levels, suggesting the lack of involvement of macroautophagy in normal ASYN turnover. ASYN contains the pentapeptide motif KFERQ that could target it to the CMA pathway. Analysis in an in vitro system of purified liver lysosomes confirmed that ASYN can be degraded by CMA. Coupled with cellular data, which indicated that rat ASYN is degraded in ventral midbrain cultures by a lysosomal pathway, we had proposed that CMA may be the major pathway used for WT ASYN degradation (13). However, there is no direct proof that CMA is responsible for ASYN degradation in cells, and in particular neuronal cells; rather this hypothesis is based on the in vitro data with purified liver lysosomes and the exclusion of other degradation pathways. Therefore, molecular techniques targeted specifically toward CMA are needed to prove or disprove the hypothesis that CMA represents a major route for WT ASYN degradation in cellular systems. Ideally, such experiments should be performed in neuronal cells, and particularly those that are most relevant to PD. This is all the more prescient, as the rate-limiting step in CMA, the levels of Lamp2a, have been reported to be very low in the CNS (21, 22). Accordingly, we have undertaken the present study to ascertain whether CMA is indeed responsible for WT ASYN degradation in neuronal cells. Because of the controversy surrounding the issues of proteasomal and macroautophagy-dependent degradation of WT ASYN, we have also examined the contribution of these pathways in various neuronal cell culture systems.
EXPERIMENTAL PROCEDURES
Animals—Wistar rats and wild type control or double transgenic C57BI/C3H mice expressing human A53T ASYN under the control of the prion promoter were used. The generation and phenotype of these mice have been described previously (23). The mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and were housed in the animal facility of the Biomedical Research Foundation of the Academy of Athens in a room with a controlled light-dark cycle (12 h light-12 h dark) and free access to food and water. For immunoprecipitation and Western immunoblotting experiments, the animals were sacrificed by cervical dislocation; brains were harvested, dissected on ice to obtain the region of interest, and immediately frozen. All animals were processed in a similar manner. Tissue was stored at –80 °C until further use. Genotyping was performed by quantitative Southern dot blot analysis with a 32P-labeled oligonucleotide-primed ASYN DNA probe as described previously (23). All efforts were made to minimize animal suffering and to reduce the number of the animals used, according to the European Communities Council Directive (86/609/EEC) guidelines for the care and use of laboratory animals. All animal experiments were approved by the Institutional Animal Care and Use Committee of Biomedical Research Foundation of the Academy of Athens.
Generation of Stable Cell Lines and Transfections—We generated the ΔDQ mutation in the ASYN open reading frame by substituting the amino acids DQ at positions 98–99 with AA, using PCR-based site-directed mutagenesis, as described previously (13). Human ASYN in pcDNA3 was used as template (24). Naive SH-SY5Y cells were transfected overnight with the Tet-Off vector (10 μg) (Clontech) using the Lipofectamine 2000 reagent (Invitrogen). Selection was performed with 500 μg/ml G418 (Calbiochem). Growth medium was changed every 2 days. G418-resistant colonies were picked. Inducibility of each clone was determined by transient transfection of a pTRE-LUC vector, in the presence or absence of doxycycline (dox, 2 μg/ml) TET-approved medium (Clontech). Two clones were finally chosen for tight regulation. Maintenance of the clones was in 250 μg/ml G418. One of the clones was further used for generation of stable pTRE-ASYN expression as described below. Mutant ΔDQ/WT ASYN was generated by PCR from human WT ASYN. PCR products were then cloned into a TOPO-pCRII vector (Invitrogen). WT, ΔDQ/WT ASYN were subcloned into the HindIII and XbaI sites of the pTRE-tight vector (Clontech) and transfected along with the pTK-hygromycin vector (Clontech) using Lipofectamine 2000, following the manufacturer's recommendations. Selection was with 200 μg/ml G418 and 25 μg/ml (for PC12 cells) or 50 μg/ml (for SH-SY5Y cells) hygromycin B (Roche Diagnostics). Single colonies were isolated and maintained in medium with/without dox (2 μg/ml for PC12 or 3 μg/ml for SH-SY5Y cells) for 4 days. The clones were tested for ASYN expression by immunocytochemistry and Western blot analysis. Resistant clones were picked, and ASYN inducibility was examined by immunofluorescence in the presence or absence of dox (2 μg/ml added for 4 days) in Tet-Off approved medium using the monoclonal antibody Syn 211 sc-12767 (Santa Cruz Biotechnology, Santa Cruz, CA). Clones with the tightest regulation were further confirmed by Western immunoblotting with the same antibody.
Cell Culture—PC12 cells were cultured in RPMI 1640 medium (Invitrogen) with 10% horse serum (Biowest, Nuaillé, France) and 5% fetal bovine serum (FBS, Biowest, France) on rat tail collagen-coated plates. SH-SY5Y cells were cultured in RPMI 1640 medium with 10% FBS. Stable cell lines were co-cultured with 200 μg/ml G418 and 25 μg/ml (for PC12 cells) or 50 μg/ml (for SH-SY5Y cells) hygromycin B. For pharmacological studies, 3-methyladenine (3-MA, Sigma), NH4Cl (Sigma), epoxomicin (epx, Sigma), and dox (Clontech) were added at indicated times and concentrations. Serum deprivation in SH-SY5Y cells was in RPMI 1640 medium + 0.5% FBS. All plasticware was from Greiner (Greiner, Bio One GmbH, Germany).
RNAi—A 21-nucleotide, small interfering RNA was designed against the rat Lamp2a mRNA (GenBank™ accession number NM_017068) according to the criteria of Elbashir et al. (25) and Reynolds et al. (26). The nucleotide sequence targeting rat Lamp2a was 5′-AAGCGCCATCATACTGGATAT-3′ (L1) and was subjected to a BLAST search to verify specificity. As a control, we used a scrambled (scr) siRNA containing the 21-nucleotide sequence 5′-AATTTAGCCGATACTGCCTAG-3′. Briefly, PC12 cells were grown in 12-well dishes, and siRNAs (L1 and scr) at a concentration of 25 nm were delivered with Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Six hours later, the medium was removed and replaced with normal culture medium. Lamp2a down-regulation was assessed 24 and 48 h post-transfection.
Primary Neuronal Cultures—Cultures of rat (embryonic day 18, E18) or mouse (E16) cortical neurons were prepared as described previously (27, 28). Dissociated cells were plated onto poly-d-lysine-coated 6-well or 12-well dishes at a density of ∼150,000–200,000/cm2. Cells were maintained in Neurobasal medium (Invitrogen), with B27 serum-free supplements (Invitrogen), l-glutamine (0.5 mm), and penicillin/streptomycin (1%). More than 98% of the cells cultured under these conditions represent post-mitotic neurons (11).
Rat midbrain cultures derived from postnatal day 1 were prepared using standard procedures (13) with modifications. Briefly, material dissected form the ventral portion of the midbrain was cleaned free of meningeal tissue, minced, and enzymatically dissociated in a mixture of trypsin/DNase. Dissociated cells were plated at a density of ∼200,000 cells per cm2 on poly-d-lysine-coated plates. The neurons were maintained in neurobasal A medium with B27 serum-free supplements.
Intracellular Protein Degradation—Total protein degradation in cultured cells (PC12 cells, cortical neurons) was measured by pulse-chase experiments (13, 29) with modifications. Briefly, confluent PC12 cells or cortical neurons (day 7 in culture) were labeled with [3H]leucine (2 μCi/ml) (leucine, L-3,4,5; PerkinElmer Life Sciences) at 37 °C for 48 or 24 h respectively. The cultures were then extensively washed with medium and returned in complete growth medium containing 2 mm of unlabeled leucine for 6 h. This medium containing mainly short lived proteins was removed and replaced with fresh medium containing cold leucine, and/or the general lysosomal inhibitor NH4Cl (30), or the inhibitor of macroautophagy 3-MA (31) at indicated concentrations. Aliquots of the medium were taken at different times (14 h for PC12 cells and 12 and 24 h after labeling for cortical neurons), and proteins in the medium were precipitated with 20% trichloroacetic acid for 20 min on ice and centrifuged (10,000 × g, 10 min, 4 °C). Radioactivity in the supernatant (representing degraded proteins) and pellet (representing undegraded proteins) was measured in a liquid scintillation counter (Wallac T414, PerkinElmer Life Sciences). At the last time point, cells were lysed with 0.1% NaOH. Proteolysis was expressed as the percentage of the initial total acid-precipitable radioactivity (protein) in the cell lysates transformed to acid-soluble radioactivity (amino acids and small peptides) in the medium during the incubation. Total radioactivity incorporated in cellular proteins was determined in triplicate samples as the amount of acid-precipitable radioactivity in labeled cells.
Measurement of Half-lives of WT (Endogenous and Over-expressed) and ΔDQ/WT Synucleins—85% confluent cell cultures were grown in methionine/cysteine-deprived RPMI 1640 medium (Sigma) for 10 min and then labeled with [35S]methionine/cysteine mixture (0.2 mCi/ml) (Express Labeling Mix, PerkinElmer Life Sciences) for 2 h. For cortical neurons the radiolabeling was performed for 24 h in Neurobasal medium without previous amino acid starvation. After extensive washing with medium, cells were maintained in complete or serum-deprived medium (0.5% FBS) and at the indicated times lysed in RIPA buffer (150 mm NaCl, 50 mm Tris, pH 7.6, 0.1% SDS, 1% Triton X-100, 2 mm EDTA, and 0.1% deoxycholate) with protease inhibitors (complete mini, Roche Diagnostics) and subjected to immunoprecipitation with an antibody against ASYN. The antibodies used were the sc-7011 (C-20) rabbit polyclonal antibody (Santa Cruz Biotechnology) for PC12 and SH-SY5Y cells and the monoclonal Syn 1 antibody (BD Biosciences) for cortical cultures. Protein G+-agarose beads were from Santa Cruz Biotechnology. Immunoprecipitates were resolved by SDS-PAGE (12%), and the gels were dried and then exposed on a PhosphorImager Screen and quantified using Gel Analyzer version 1.0 software (Biosure, Greece).
Western Immunoblotting—PC12 cells, SH-SY5Y cells, and primary neurons were washed twice in cold PBS and then harvested in lysis buffer (150 mm NaCl, 50 mm Tris, pH 7.6, 0.1% SDS, 1% Triton X-100, 2 mm EDTA) with protease inhibitors. Lysates were centrifuged at 10,000 × g for 10 min at 4 °C. The detergent-insoluble pellets were washed twice in PBS and resuspended in 2× Laemmli buffer. Protein concentrations in soluble fractions were determined using the Bradford or Lowry methods (Bio-Rad). Proteins were resolved on 12% SDS-polyacrylamide gels or 4–12% BisTris NuPAGE gels (Invitrogen) and transferred onto nitrocellulose membranes. Blots were probed with antibodies directed against the following: 1) ASYN, monoclonal Syn 1 (1:1000; BD Biosciences), polyclonal C20 (1:1000; Santa Cruz Biotechnology); 2) polyclonal Lamp2a (Igp96), (1:1000; Zymed Laboratories Inc.); 3) monoclonal Lamp1 (1:1000; Santa Cruz Biotechnology); 4) polyclonal ERK (loading control; 1:5000; Santa Cruz Biotechnology); 5) monoclonal GAPDH (1:1000; Chemicon); 6) monoclonal ubiquitin (1:750; Chemicon); 7) monoclonal GFP (1:500, Santa Cruz Biotechnology); 8) monoclonal β-actin (1:20,000; Sigma); and 9) polyclonal TH (1:1000, Chemicon). Blots were probed with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch), visualized with Western Lightning® (PerkinElmer Life Sciences), and exposed to Super RX film (Fuji Film). After scanning the images, the intensity of each immunoreactive band was estimated by densitometric quantification using the Gel Analyzer version 1.0 software.
Immunocytochemistry—Cortical or ventral midbrain neurons grown on 24-well plates were fixed in freshly prepared 3.7% formaldehyde for 45 min. Blocking was with 10% normal goat serum, 0.4% Triton X-100 for 1 h at room temperature. Mouse anti-ASYN (1:200; BD Biosciences) and rabbit anti-tyrosine hydroxylase (TH, 1:500; Calbiochem) antibodies were applied overnight at 4 °C. Fluorescent secondary antibodies (mouse Cy3, 1:250; rabbit Cy2, 1:150, Jackson ImmunoResearch) were added for 1 h. The fluorescent marker Hoechst 33258 (1 μm; Sigma) was used to assess cell nuclei.
RT-PCR—Total RNA was extracted from cortical neurons 48 and 72 h after infection with the lentiviruses bearing the L1 or the scrambled siRNAs using TRIzol (Invitrogen), and cDNA was generated with the reverse transcription system (Invitrogen), according to the manufacturer's instructions. RT-PCR was performed using the cDNA as template. All primer pairs were optimized to be in the log -exponential phase of amplification. The following primers were used: 1) ASYN-forward, ttctgcggaagcctagagag, and ASYN-reverse, tcctccaacatttgtcacttgc (product size = 253 bp); 2) β-actin-forward, tcaccatggatgatgatatcgcc, and β-actin-reverse, ccacacgcagctcattgtagaagg (product size = 282 bp); all primers were from Clough and Stefanis (32). Products were subsequently resolved on 1% agarose gels and stained with ethidium bromide, and the signal intensity was quantified using Gel Analyzer version 1.0 software.
Design of siRNAs and Cloning of Small Hairpin RNAs—A stem-loop structure incorporating the 21-nucleotide targeting rat Lamp2a sequence was created based on Rubinson et al. (33), so that small hairpin RNA (shRNA) could be produced from the lentiviral vector PLL3.7 gift from Dr. Dimitrios Thanos (Laboratory for Molecular Biology, Biomedical Research Foundation of the Academy of Athens). Complementary oligonucleotides encoding the shRNAs were synthesized, annealed, and cloned into pLL3.7 vector by ligation into HpaI- and XhoI-digested vector (L1 vector). The pLL3.7 vector carries loxP sites, a cytomegalovirus promoter driving expression of EGFP, and the mouse U6 promoter with downstream restriction sites (HpaI and XhoI) to allow efficient introduction of oligonucleotides encoding shRNAs.
Lentivirus Production—The lentiviruses were generated by co-transfection of human embryonic kidney (HEK) 293T cells with three plasmids using the calcium phosphate method (34). We cultured HEK 293T cells in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. For transfection, 107 cells were plated into 150-cm2 flasks. The next day, the 293T cells were transfected with 50 μg of vector DNA (PLL3.7-L1, PLL3.7-scr), 17.5 μgof pMDG2 (env), and 32.5 μg of R8 91 (gag/pol) plasmids, using calcium-phosphate precipitation (all the plasmids for lentiviral vector packaging were kindly provided by Dr. Dimitrios Thanos). After 16 h, the medium was removed, and the cells were washed twice with PBS and returned to the normal culture medium. Medium containing recombinant lentivectors was collected at 24, 48, and 72 h post-transfection and centrifuged (400 × g, 10 min, 4 °C) to remove cellular debris. After filtration through 0.45-μm filter unit (Millipore), the supernatant from each time point was centrifuged at 75,000 × g or 90 min at 4 °C in Sorvall Discovery TH641 swing bucket rotor. The supernatant was discarded, and the virus (pellet) was resuspended in 50 μl/tube of PBS supplemented with 0.5% bovine serum albumin, aliquoted, and stored at –80 °C. Lentiviral titers for the viruses collected each day were determined by seeding HeLa cells in 12-well plates at 5 × 104 cells per well, 3–4 h before infection with serial dilutions of the concentrated viral stock. After incubation for 2 days, the medium was removed, and the EGFP-expressing cells were identified using a fluorescence-activated cell sorter. Titers ranged from 3 to 6 × 107 infectious units (IU/ml).
Statistical Analysis—All data are expressed as means ± S.E. Statistical significance of differences was evaluated either with Student's t test or with one way ANOVA followed by the Student-Newman-Keuls' test. Probability values <5% were considered significant.
RESULTS
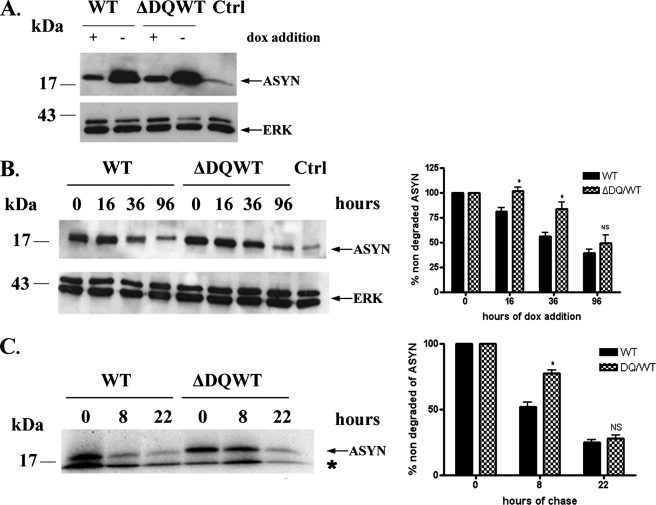
Involvement of CMA in the Degradation of ASYN in PC12 Cell Lines—To investigate whether WT ASYN is degraded through CMA in PC12 cells, we created stable inducible Tet-Off PC12 cell lines overexpressing human WT ASYN and human ΔDQ/WT that lacks the motif that targets it for CMA degradation. This ΔDQ/WT mutant, in a recombinant form, has been shown previously to lack significant CMA-dependent uptake into lysosomes in an isolated liver lysosome preparation (13). Removal of dox from the medium of these cells results in high levels of expression of the gene of interest (Fig. 1A). If ASYN is indeed degraded, at least in part, through CMA, we would expect that the ΔDQ/WT mutant form would have a slower rate of degradation compared with the WT ASYN. To investigate this, we allowed PC12 cells to fully express WT or ΔDQ/WT (cultured without dox for 5 days) ASYN and then turned the gene off with addition of dox. Samples were collected before (time point 0) addition of dox, and at various time points (16, 36, and 96 h) after the addition of dox, cells were lysed, and the levels of ASYN at each time point were examined by immunoblotting. We observed higher levels of ΔDQ/WT ASYN compared with WT/ASYN at 16 h and more clearly at 36 h after dox addition. No significant difference was observed at 96 h because of the fact that in both cases the gene was completely turned off, and the levels of ASYN approached background (Fig. 1B). This indicates that ΔDQ/WT ASYN has a slower degradation rate compared with WT ASYN. To further confirm this result, we also performed pulse-chase experiments in ΔDQ/WT or WT ASYN cell lines. We again observed that ΔDQ/WT ASYN exhibited a slower rate of degradation compared with WT ASYN (Fig. 1C). We should note that the differences observed in the rate of degradation of ASYN in the two assays depicted in Fig. 1, B and C, reflect the difference in the methods and culture conditions utilized. Taken together, the above data suggest that CMA is responsible, in part, for the degradation of WT ASYN, as, when this route of degradation is blocked for ASYN, ASYN is degraded more slowly.
FIGURE 1.
ΔDQ/WT ASYN exhibits slower turnover compared with WT ASYN in PC12 cells. A, generation of stable inducible Tet-Off PC12 cell lines overexpressing WT ASYN and ΔDQ/WT. The cells were cultured in the presence (+) or absence (–) of dox (2 μg/ml) for 4 days and assayed for ASYN expression with the C20 polyclonal Ab. ERK Ab is used as a loading control. B and C, overexpressed ΔDQ/WT ASYN displays a slower rate of degradation compared with WT ASYN. B, ΔDQ/WT and WT PC12 cell lines were cultured in the absence of dox for 5 days, and then dox (2 μg/ml) was added at the indicated times. Left, representative immunoblot of ASYN levels. Right, quantification of rate of overexpressed ΔDQ/WT and WT ASYN turnover after dox addition. Ctrl, control. C, ΔDQ/WT and WT ASYN cell lines were cultured in the absence of dox for 5 days. The cells were then labeled with [35S]cysteine/methionine and pulse-chased for 0, 8, and 22 h. ASYN was immunoprecipitated from hot lysates with C20 Ab, and its levels were assessed by autoradiography. Left, a representative pulse-chase experiment is shown. The band corresponding to ASYN is depicted by the arrow. The asterisk indicates an irrelevant band. Right, quantification of the turnover of ΔDQ/WT and WT ASYN. All data are presented as the relative OD values of each time point relative to time point 0. The graphs represent the mean ± S.E. of three independent experiments. (*, p < 0.05, Student's t test comparing ΔDQ/WT to WT ASYN).
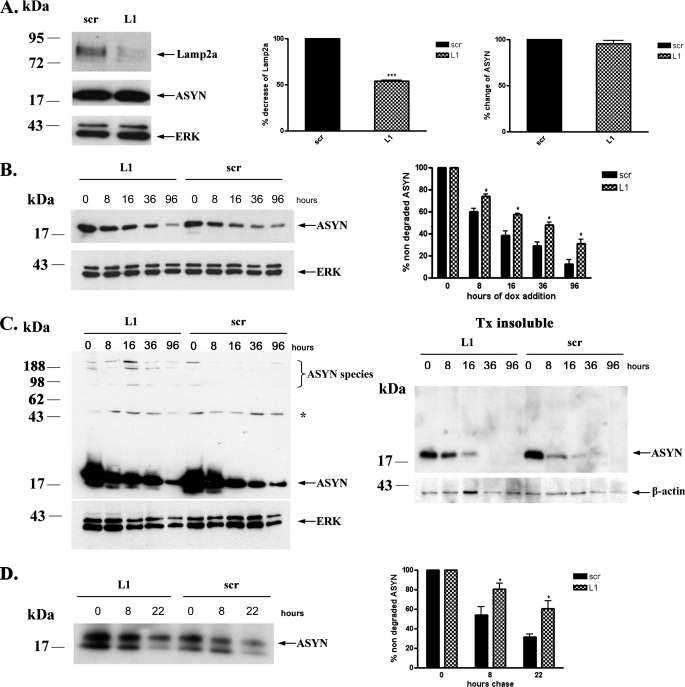
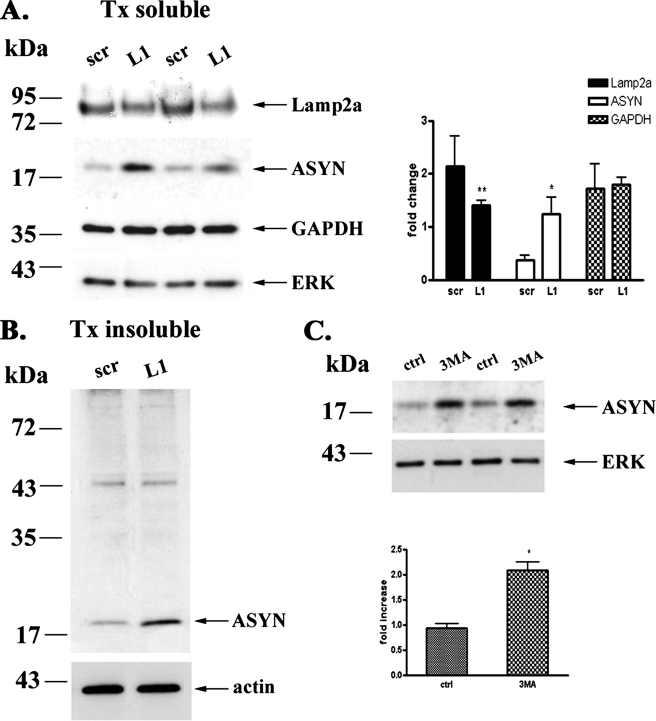
It has been shown in non-neuronal cell systems that Lamp2a is the rate-limiting step of CMA (35). To further assess whether ASYN is degraded through CMA in PC12 cells, we also used the strategy of Lamp2a down-regulation and examined its effects on ASYN levels and turnover. To down-regulate Lamp2a, we employed the siRNA technique using a siRNA molecule (L1) against rat Lamp2a and a scr siRNA as a control. When PC12 cells were transiently transfected with the L1 siRNA, we detected a significant down-regulation of endogenous Lamp2a by 50% compared with cells transfected with the scr siRNA (Fig. 2A). Transfection with the scr siRNA did not alter the expression of Lamp2a, compared with untransfected (ctrl) cells (supplemental Fig. S1). Contrary to what we expected, there was no change in the steady state levels of overexpressed WT ASYN (Fig. 2A). Therefore, we examined the effect of Lamp2a down-regulation on the rate of WT ASYN turnover. We first utilized the strategy of adding dox to WT PC12 cells previously transfected with siRNAs and following the levels of ASYN by Western immunoblotting. It should be noted that in preliminary experiments we found that the transfection procedure itself accelerated somewhat the turnover of ASYN in this assay (data not shown). Cells transfected with L1 displayed slower degradation rate than cells transfected with scrambled siRNA (Fig. 2B). Additionally, on gradient gels we observed a persistence of ASYN detergent-soluble high molecular weight species following Lamp2a down-regulation (Fig. 2C, left panel). Interestingly, detergent-insoluble ASYN species also exhibited a reduced clearance rate following Lamp2a silencing (Fig. 2C, right panel).
FIGURE 2.
Down-regulation of Lamp2a in PC12 cells leads to slower turnover of WT ASYN. A, transient transfection of rat Lamp2a siRNA (L1), effectively down-regulates endogenous Lamp2a but has no effect on the steady state levels of overexpressed WT ASYN. PC12 cells expressing WT ASYN were cultured in the absence of dox for 5 days and then were transiently transfected with L1 or scr siRNA. 48 h later, cells were lysed and assayed for ASYN and Lamp2a. Left, representative immunoblot of Lamp2a and ASYN. Middle and right, quantification of Lamp2a (middle) and ASYN (right) levels in cells transfected with L1 compared with cells transfected with scr siRNA. Results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean of + S.E. of 9 (for Lamp2a) and 6 (ASYN) independent experiments. B–D, WT ASYN displays a slower turnover rate in cells transfected with L1 compared with cells transfected with scr siRNA. B, WT ASYN cells were cultured without dox for 5 days and next transfected with L1 or scr siRNA, and 48 h later dox was added. At successive time points after dox addition, cells were examined for ASYN levels by immunoblotting. Left, representative immunoblot of ASYN. Right, quantification of turnover rate of WT ASYN after dox addition. C, WT ASYN cells were treated as in B and examined for ASYN levels by immunoblotting. Left panel, the detergent (Triton X-100 (Tx))-soluble L1 or scr siRNA-treated samples were run on 4–12% BisTris NuPAGE gels and assayed by Western blot for ASYN and ERK (loading control). High molecular weight ASYN species in the Lamp2a down-regulated (L1)-treated samples are indicated by a brace. The asterisk indicates an irrelevant band. Right panel, the detergent-insoluble pellets from the L1 or scr siRNA-treated samples were solubilized in SDS sample buffer, run on 12% gel, and assayed by Western blot for ASYN and β-actin (loading control). Representative Western blots from three separate experiments are shown. D, WT ASYN cell lines were cultured in the absence of dox for 5 days. Cultures were treated with siRNAs as in B and labeled with [35S]cysteine/methionine for pulse-chase. ASYN was immunoprecipitated from hot lysates with C20 ASYN Ab, and its levels were assessed by autoradiography (indicated by the arrow). Left, representative pulse-chase is shown. Right, quantification of the turnover of WT ASYN in cells transfected with L1 or scr siRNA. All data are presented as the relative OD values of each time point relative to time point 0. The graphs represent the mean ± S.E. of three independent experiments. (*, p < 0.05; ***, p < 0.001, Student's t test comparing L1 with the control scr).
To further confirm these findings, we also performed pulsechase experiments. Again, ASYN in cells transfected with L1 exhibited considerably slower turnover compared with cells transfected with scrambled siRNA (Fig. 2D). The above data suggest that although 50% down-regulation of endogenous Lamp2a has no obvious effect on the steady state levels of WT ASYN, it decreases its degradation rate by inactivating the CMA pathway.
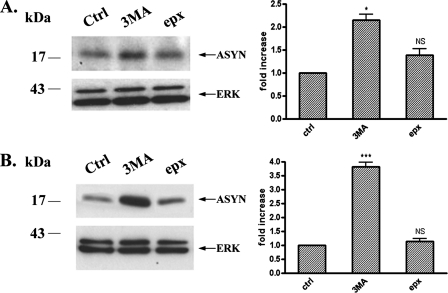
Contribution of Macroautophagy to the Degradation of Endogenous and Overexpressed ASYN Levels in PC12 Cells—Our results so far indicate that WT ASYN is in part degraded by CMA in PC12 cells. However, it is also clear that CMA cannot totally account for ASYN degradation in this setting, as substantial non-CMA-dependent degradation of ASYN appears to also occur. Two other major systems of intracellular protein degradation include another lysosomal pathway, macroautophagy, and the proteasome. In the study of Webb et al. (7) in PC12 cells inducibly expressing various forms of ASYN, both these systems appeared to be involved in mutant ASYN degradation, although in the case of the WT protein, macroautophagy inhibition via 3-MA did not have an effect. To examine this issue, we used the selective inhibitors of the proteasome and macroautophagy, epoxomicin (36) and 3-MA (31), respectively, and assessed their impact on steady state levels of either endogenous or overexpressed WT ASYN. Addition of 3-MA resulted in a significant increase of the steady state levels of endogenous or WT ASYN, as we observed an increase of 1.5- and 3.8-fold, respectively, whereas no significant difference was observed with the addition of epoxomicin (Fig. 3), despite a significant effect on the accumulation of polyubiquitinated species (data not shown). Thus, macroautophagy, but not the proteasome, also plays an important role in the degradation of endogenous or overexpressed WT ASYN in PC12 cells.
FIGURE 3.
Inhibition of macroautophagy increases endogenous (A) and human overexpressed (B) WT ASYN levels in PC12 cells. Cells were cultured in the presence of 3-MA (10 mm) or epoxomicin (1 μm) for 14 h. Untreated cells were used as a control (Ctrl). Cell lysates were assessed by Western immunoblotting for ASYN levels. Left, representative immunoblot of ASYN. Right, quantification of endogenous (A) or overexpressed (B) ASYN levels after 3-MA or epx addition, compared with control. Results are expressed as the ratio of OD values to the corresponding controls, and data are presented as mean ± S.E. of six (for 3-MA) or three (B, for epx) independent experiments. (*, p < 0.05; ***, p < 0.001, Student's t test comparing 3-MA or epx with the control).
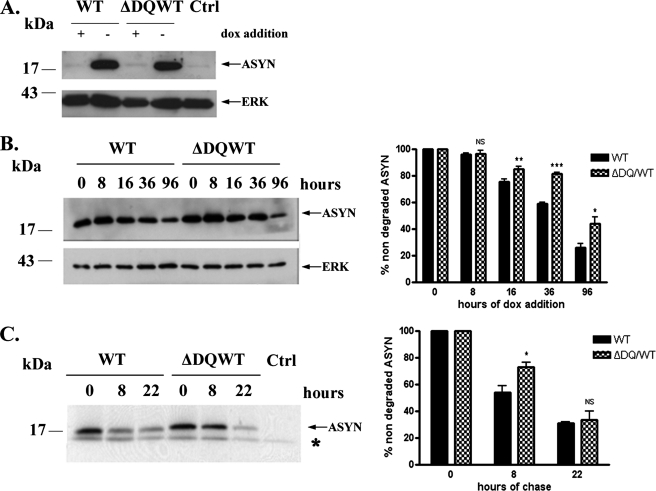
Involvement of CMA in the Degradation of WT ASYN in SH-SY5Y Cell Lines—We wished to investigate whether our findings in PC12 cells regarding participation of CMA in WT ASYN degradation could be extended to a human neuronal cell culture system. We therefore used a similar approach as in PC12 cells in human SH-SY5Y neuroblastoma cells. We created stable inducible Tet-Off SH-SY5Y cell lines overexpressing human WT ASYN and human ΔDQ/WT that lacks the motif that targets it for CMA degradation (Fig. 4A). We studied the rate of degradation of ΔDQ/WT and WT ASYN following dox addition and observed that ΔDQ/WT ASYN has a slower degradation rate compared with WT ASYN (Fig. 4B). Pulse-chase experiments confirmed this finding (Fig. 4C). Altogether, the above data provide evidence for the involvement of CMA in WT ASYN degradation in human SH-SY5Y neuronal cell lines, as shown in PC12 cell lines.
FIGURE 4.
Involvement of CMA in degradation of ASYN in SH-SY5Y cell lines. A, generation of stable inducible Tet-Off SH-SY5Y cell lines overexpressing ΔDQ/WT and WT ASYN. Cells were treated with or without 3 μg/ml dox for 4 days, and lysates were used for ASYN immunoblotting. B and C, overexpressed ΔDQ ASYN displays a slower rate of degradation compared with WT ASYN. B, ΔDQ/WT and WT ASYN SH-SY5Y cell lines were cultured in the absence of dox for 5 days, and then dox was added at the indicated times. Left, representative immunoblot of ASYN levels. Right, quantification of rate of turnover of overexpressed ΔDQ/WT and WT ASYN after dox addition. C, ΔDQ/WT and WT ASYN SH-SY5Y cell lines were cultured in the absence of dox for 5 days. The cells were then labeled with [35S]cysteine/methionine for pulse-chase. ASYN was immunoprecipitated with C20 ASYN Ab, and its levels were assessed by autoradiography. ERK immunoprecipitation was used as negative control (Ctrl). Left, representative pulse-chase is shown. Right, quantification of the turnover of ΔDQ/WT and WT ASYN. All results are expressed as the ratio of OD values to the corresponding controls, and all data are presented as a percent of each time point compared with the value at time point 0. (*, p < 0.05; **, p < 0.01; ***, p < 0.001, Student's t test comparing ΔDQ/WT to WT ASYN).
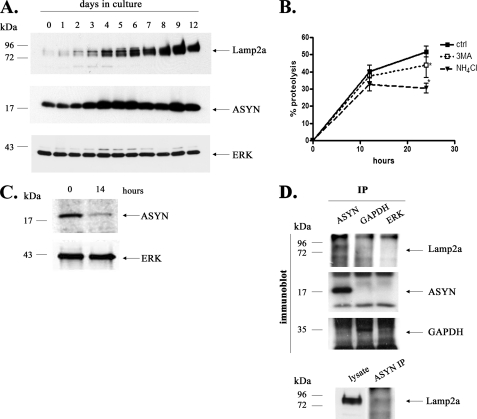
Endogenous ASYN Is Degraded via CMA in Primary Cortical Neuron Cultures—From the aforementioned data, it is obvious that CMA is responsible, in part, for WT ASYN degradation in ASYN-overexpressing rat (PC12) and human (SH-SY5Y) cell lines. To elucidate further the contribution of lysosomes and of CMA in particular to ASYN degradation in a primary post-mitotic neuronal setting, we turned to cultured rat embryonic cortical neurons. These cultures provide a rich, homogeneous source of material of CNS neurons, in which biochemical studies can be performed. Cortical neurons accumulate aberrant ASYN in PD and Diffuse LB disease, and this aberrant accumulation is widely thought to underlie the cognitive changes and in particular the dementia that occur in these disorders (37, 38). The study of ASYN degradation in these cultures may therefore be highly relevant to LB disorders. The levels of ASYN and of Lamp2a both increased during maturation of the cortical neurons in culture and at day 6 to day 7 they were both expressed sufficiently (Fig. 5A). Additionally, long lived protein degradation experiments of cortical neurons at day 7 revealed that these cells exhibit lysosomal-dependent degradation of such proteins, as defined by the significant drop of degradation in the presence of the general lysosomal inhibitor NH4Cl (30.5%, compared with the control 51.7%). Part of this lysosomal dependent degradation was inhibited by the macroautophagy inhibitor 3-MA (43.8%, compared with the control 51.7%), suggesting that non-macroautophagy pathways, such as CMA and microautophagy, also contribute to long lived protein degradation in this setting (Fig. 5B). We further examined by pulse-chase the rate of degradation of endogenous ASYN at this time juncture, and we observed that within 14 h a significant amount of endogenous ASYN is degraded (Fig. 5C). We therefore felt that cultures under these conditions would be suitable for the study of the contribution of CMA to ASYN degradation.
FIGURE 5.
Expression of ASYN and Lamp2a in rat cortical cultures. A, levels of ASYN and of Lamp2a increase in parallel during in vitro maturation of rat cortical neurons. Immunoblot probed with Syn 1 ASYN Ab, Lamp2a, and ERK (loading control) Abs from cortical cultures collected from day 0 to day 12 after plating. B, effect of different inhibitors on the degradation of long lived proteins on rat cortical cultures. [3H]Leucine-labeled cortical cultures (day 7) supplemented with or without NH4Cl (20 mm) or 3-MA (10 mm) were assayed for long lived protein degradation as described under “Experimental Procedures.” Values are the mean ± S.E. of three independent experiments, and within each experiment triplicate samples per condition were assessed (*, p < 0.05, for Student's t test, comparing 3-MA or NH4Cl with the control). C, ASYN immunoprecipitated from rat cortical cultures. Cultures (day 7) were labeled for 24 h with a [35S]methionine/cysteine and pulse-chased for 0 and 14 h. ASYN was immunoprecipitated from hot lysates with Syn 1 ASYN antibody. A representative pulsechase experiment is shown. D, ASYN interacts with Lamp2a receptor in cortical cultures. Lysates from cortical cultures (day 7) were immunoprecipitated (IP) with Syn 1 ASYN antibody and GAPDH. ERK immunoprecipitation was used as a negative control for nonspecific IgG-associated bands. The samples were immunoblotted for Lamp2a, ASYN, and GAPDH antibodies. ASYN immunoprecipitated Lamp2a from cortical cultures co-migrates with the Lamp2a-specific band in the same cortical lysate (bottom panel). Representative Western blots from three independent experiments are presented.
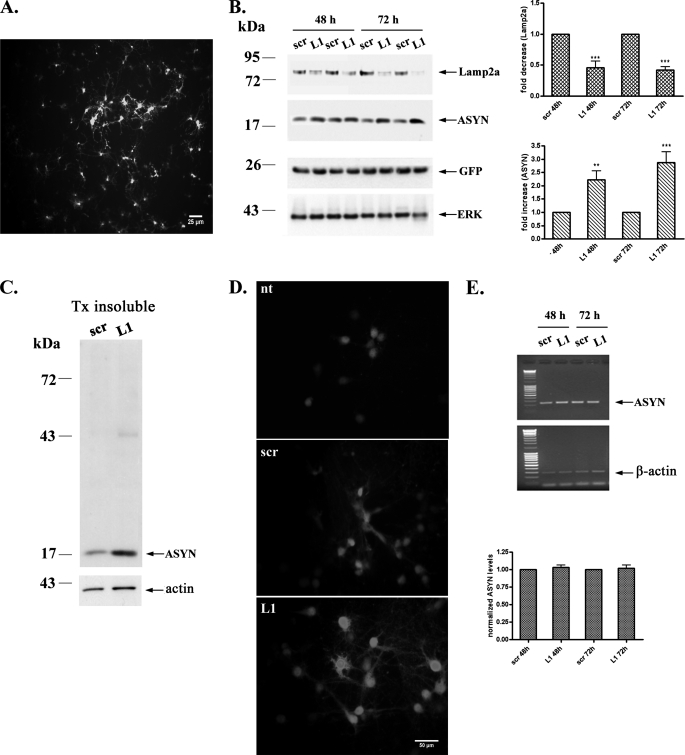
If indeed endogenous ASYN is degraded by CMA, it should interact physically with Lamp2a, as we had observed for overexpressed ASYN in PC12 cells (13). We indeed found that endogenous ASYN in cortical neurons co-immunoprecipitates with Lamp2a. Somewhat surprisingly, GAPDH, which may be degraded via CMA in other settings (13, 39), did not co-immunoprecipitate with Lamp2a (Fig. 5D). We attempted to also perform immunoprecipitation of Lamp2a, to assess whether this would also bring down ASYN, but unfortunately, the Lamp2a Ab, the only available specific Ab for this molecule, did not reliably immunoprecipitate (data not shown). To further elucidate the possible degradation of ASYN via CMA in neurons, we down-regulated the levels of Lamp2a using the siRNA approach. Because cortical neurons are post-mitotic cells, the classic method for siRNA delivery (with Lipofectamine) did not provide satisfactory results (data not shown). For this reason we constructed lentiviruses expressing either the RNAi specific for Lamp2a (L1) or the control RNAi (scr). We transduced 1-week -old cortical cultures with the L1 or scr RNAi-expressing lentiviruses with m.o.i. values ranging from 0.5 to 20, and we tested the efficiency of Lamp2a down-regulation 48, 72, 96, and 120 h after viral infection. Using lentiviruses with an m.o.i. of 5, we obtained a high percentage of infected neurons 72 h after viral infection, by monitoring EGFP expression under immunofluorescence microscope (Fig. 6A), and a substantial 50% down-regulation of Lamp2a by Western immunoblotting (Fig. 6B). Under these conditions, there was a significant increase in endogenous ASYN levels, which at 48 and 72 h reached 2.2 ± 0.33 and 2.87 ± 0.4 times the levels of the control (scr RNAi) treated cultures, respectively (Fig. 6B). On the contrary, the levels of GAPDH which, as mentioned, did not co-immunoprecipitate with Lamp2a (Fig. 5D), were not altered by Lamp2a silencing (supplemental Fig. S2A). Additionally, we found a significant increase in the Triton X-100-insoluble species of ASYN in L1-treated cortical neurons compared with scr-treated neurons (Fig. 6C). We also performed ASYN immunostaining to assess whether the accumulation of ASYN occurred in any particular cellular compartment, and whether ASYN inclusions could be demonstrated. We detected a diffuse accumulation of ASYN in cell bodies and neurites, without apparent inclusions (Fig. 6D). The fact that endogenous ASYN accumulates upon Lamp2a silencing indicates that WT ASYN is degraded in the lysosomes of cortical neurons via CMA. This hypothesis was further confirmed by the observation that Lamp2a down-regulation does not alter the mRNA levels of ASYN (Fig. 6E). To confirm the specificity of the siRNA that we used, we tested its effects on the protein levels of Lamp1, a lysosome-associated membrane protein closely related to Lamp2a. Because there is no commercially available antibody specific for the detection of rat Lamp1, we utilized mouse cortical neuron cultures and infected them with the same L1 and scr lentiviruses, as the mRNA sequence of Lamp2a targeted by L1 is identical in rat and mouse. Infection of mouse cortical neurons with the L1 siRNA (m.o.i. 2.5, 24 h) resulted in significant down-regulation of Lamp2a levels (30 ± 0.03%, compared with scr siRNA), whereas the levels of Lamp1 were not modified (supplemental Fig. S2B). This finding shows that the siRNA that we used is selective for Lamp2a, the only member of lysosome-associated membrane proteins that is known to participate in CMA. Additionally, the decreased levels of mouse Lamp2a were accompanied by an increased expression of endogenous ASYN (1.3 ± 0.1-fold compared with control scr-infected cultures) (supplemental Fig. S2B), further supporting the idea that CMA is responsible, at least in part, for WT ASYN degradation in primary mouse or rat cortical neurons.
FIGURE 6.
Lentiviral down-regulation of Lamp2a significantly increases endogenous ASYN protein levels in rat cortical neurons. CMA impairment via Lamp2a silencing results in endogenous ASYN accumulation. Primary rat cortical neurons were transduced with lentiviruses (m.o.i. 5) expressing Lamp2a (L1) or scr siRNA for 24 h. After 48 and 72 h, cells were assayed by Western blot for Lamp2a, ASYN, GFP (infection control), and ERK (loading control) levels. A, representative image of rat cortical neurons 72 h post-infection with the L1 lentivirus that contains a CMV-EGFP reporter cassette to monitor infection is shown. B, left, representative Western blots from 12 separate experiments are shown. Right, densitometric analysis of the levels of Lamp2a and ASYN. C, detergent (Triton X-100 (Tx)-insoluble pellets from the L1 or scr siRNA-treated samples were solubilized in SDS sample buffer and assayed by Western blot. Representative Western blots from three separate experiments are shown. D, ASYN immunostaining in rat cortical neurons 72 h post-infection with the scr or L1 lentiviruses. ASYN immunostaining in nontransduced (nt) neurons is also presented. The same microscopy settings were used in all cases. L1 infected neurons display increased ASYN immunofluorescence. No frank inclusions were observed. E, mRNA levels of ASYN are not altered following down-regulation of Lamp2a. 48 and 72 h post-infection RNA was extracted, and RT-PCR for ASYN and β-actin was performed. Representative images from three separate experiments are shown in the left panel. Densitometric analysis of the RT-PCR products, expressed as ASYN: β-actin ratio (arbitrary units), is presented in the right panel. All results are presented as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. (**, p < 0.01; ***, p < 0.001, one way ANOVA followed by the Student-Newman-Keuls' test, comparing L1 to scr).
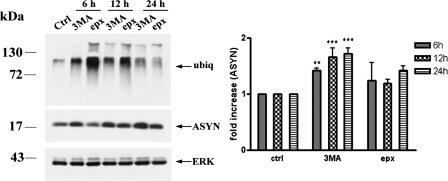
Inhibition of Macroautophagy Increases ASYN Levels in Cortical Neurons—We wished to see whether other non-CMA-dependent pathways may also be operative in the degradation of ASYN in cortical neurons. We therefore examined the effects of 3-MA and epoxomicin inhibiting macroautophagy and the proteasome, respectively. Application of 3-MA resulted in a significant increase of ASYN levels (1.72 ± 0.1-fold of nontreated control cultures after 24 h of exposure), whereas application of epoxomicin led to a very small increase that was not statistically significant (Fig. 7). These results indicate that macroautophagy also participates in the degradation of endogenous ASYN in primary cortical neurons, although its contribution in this system appears to be of a lesser magnitude compared with that of CMA.
FIGURE 7.
Macroautophagy participates in the degradation of endogenous ASYN in rat cortical neurons. Cultures were incubated with 3-MA (10 mm) or epx (50 nm). At the end of the incubation the cells were assayed for ASYN, ubiquitin (ubiq), and ERK (loading control) levels by Western blot. Ctrl, control. Representative Western blots from nine independent experiments are shown in the left panels, and densitometric analysis of the levels of ASYN is shown in the right panel. Results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. of nine independent experiments. (**, p < 0.01; ***, p < 0.001, one way ANOVA followed by the Student-Newman-Keuls' test, comparing 3-MA or epoxomicin with the control).
Endogenous ASYN Is Degraded via CMA and Macroautophagy in Post-natal Ventral Midbrain Cultures—Degeneration of dopaminergic ventral midbrain neurons represents the pathological hallmark of PD and the cause for the major motor symptoms of the disease. We therefore wished to extend our studies to this cell culture system. We used postnatal cultures to obtain a high percentage of mature dopaminergic neurons. Using a serum-free cell culture system, without a glial feeder layer, we routinely achieved at least 30% dopaminergic tyrosine hydroxylase (TH)-positive neurons (supplemental Fig. S3). As we have previously reported in embryonic ventral midbrain cultures (40), ASYN immunostaining in these cultures was essentially confined to TH-positive neurons (data not shown). Infection of midbrain cultures 3 days after plating with the L1 siRNA (m.o.i. 5, 24 h) resulted in significant down-regulation of Lamp2a levels (35 ± 0.12%, compared with scr siRNA) (Fig. 8A). This was accompanied by a significant increase in detergent-soluble (3.3 ± 0.3 times the levels of the scr RNAi) (Fig. 8A) and detergent-insoluble (Fig. 8B) ASYN levels. As in cortical neurons, the levels of GAPDH were not altered upon Lamp2a down-regulation (Fig. 8A). Inhibition of macroautophagy via 3-MA also resulted in a significant increase (2.23 ± 0.2 times the levels of the control) in ASYN levels (Fig. 8C), although this effect was less pronounced compared with that achieved with modest CMA inhibition. Therefore, ASYN is degraded by CMA and, to a lesser extent, by macroautophagy in dopaminergic ventral midbrain neurons.
FIGURE 8.
Blockade of CMA or macroautophagy increases endogenous ASYN protein levels in postnatal ventral midbrain neurons. Three days after plating, postnatal ventral midbrain neurons (P1) were transduced with lentiviruses (m.o.i. 5) expressing Lamp2a (L1) or scr siRNA for 24 h. After 72 h, cells were assayed by Western blot for Lamp2a, ASYN, GAPDH, and ERK (loading control) levels. A, left, representative Western blots from three separate experiments are shown. Right, densitometric analysis of the levels of Lamp2a, ASYN, and GAPDH. B, detergent (Triton X-100)-insoluble pellets from the L1 or scr-treated samples were solubilized in SDS sample buffer and assayed by Western blot for ASYN and β-actin (loading control) levels. Representative Western blots from three separate experiments are shown. C, cultures were incubated with 3-MA (10 mm) for 24 h. Untreated cells were used as a control (ctrl). Cell lysates were assessed by Western immunoblotting for ASYN levels. Upper panel, representative immunoblot of ASYN. Bottom panel, quantification of endogenous ASYN levels after 3-MA addition, compared with control. All results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. of three independent experiments. (*, p < 0.05; **, p < 0.01, one way ANOVA followed by the Student-Newman-Keuls' test).
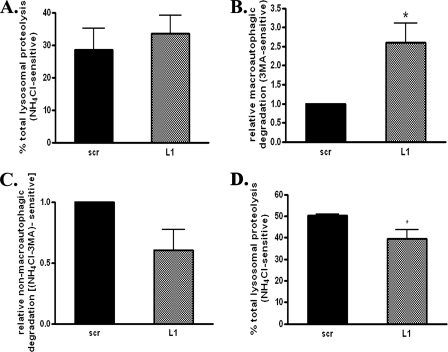
Effects of CMA Down-regulation on Macroautophagy—Our results indicate that CMA is in part responsible for ASYN degradation in a variety of neuronal cell culture systems. However, a comparison of the results achieved with the L1 siRNA in PC12 cells and in cortical or ventral midbrain neurons indicates that this strategy is much more effective in augmenting ASYN levels in the latter, suggesting that CMA may be more important in the regulation of ASYN degradation in primary neurons. An alternative or complementary explanation could be that compensatory mechanisms of degradation are activated in PC12 cells but not in primary neuronal cultures. Indeed, there is precedent in mouse fibroblasts, where down-regulation of CMA via siRNA directed against Lamp2a caused a compensatory increase in macroautophagy (39). We therefore wished to examine lysosomal function upon Lamp2a down-regulation in the two different systems. To this end, we transiently transfected PC12 cells with the L1 or scrambled siRNA molecule (as described above), and we measured long lived protein degradation in the presence or absence of various lysosomal inhibitors. Somewhat paradoxically, we found that long lived protein degradation was somewhat increased in cultures transfected with L1 compared with those transfected with the scr siRNA, although this difference was not significant (Fig. 9A). This increase was driven by a very significant increase in macroautophagic degradation, defined as long lived protein degradation that was 3-MA-inhibitable. Cultures transfected with the L1 siRNA demonstrated a 2.5-fold increase of macroautophagic degradation compared with those transfected with scr siRNA (Fig. 9B). In contrast, non-macroautophagic lysosomal degradation, defined as the difference between NH4Cl-inhibited degradation and 3-MA-inhibited degradation, showed a tendency to decrease with L1 treatment (Fig. 9C). These results indicate that down-regulation of CMA in PC12 cells causes a significant compensatory activation of macroautophagy.
FIGURE 9.
Effects of CMA down-regulation on macroautophagy. PC12 cells transiently transfected with L1 or scr siRNA were labeled with [3H]leucine for 48 h (2 μCi/ml). Cells were treated with or without NH4Cl or 3-MA and degraded proteins were assayed 14 h later. A, rate of total long lived protein degradation in PC12 cells transfected with L1 or scrambled siRNA. B, rate of macroautophagic degradation (inhibitable by 3-MA) in PC12 cells transfected with L1 or scrambled siRNA. C, rate of non-macroautophagic lysosomal degradation (defined as the difference between NH4Cl-sensitive and 3-MA-sensitive long lived protein degradation) in PC12 cells transfected with L1 or scrambled siRNA. All presented data are the mean of four independent experiments, and within each experiment triplicate samples per condition were assessed. D, Lamp2a down-regulation results in decreased long lived protein degradation. Cortical cultures were transduced with lentiviruses expressing L1 or scr siRNA for 24 h. and then labeled with [3H]leucine for 24 h. Protein degradation was assayed 12 h later. All presented data are the mean of three independent experiments, and within each experiment triplicate samples per condition were assessed. (**, p < 0.01, *, p < 0.05, for Student's t test comparing between cells transduced with the L1 or scr siRNA lentiviruses.)
We also wished to assess the effects of Lamp2a down-regulation on lysosomal function in cortical neurons. We found that long lived protein degradation was significantly decreased upon infection of the cultures with the L1 (39.6 ± 4%), compared with the scr lentivirus (50.2 ± 0.8%) (Fig. 9D). This result indicates that Lamp2a down-regulation has a significant impact on lysosomal function in this primary neuron setting. It further suggests that the compensatory changes observed in PC12 cells with the up-regulation of macroautophagy occur at a much lower extent or not at all in this system. Unfortunately, we could not directly test the extent of macroautophagy in lentivirus-infected cultures, as the combination of the lentivirus and 3-MA proved lethal to the cells. Western immunoblotting with LC3 Ab also failed to show a change in LC3 II in the two conditions, again suggesting that no gross induction of macroautophagy occurred with Lamp2a down-regulation in cultured cortical neurons (data not shown).
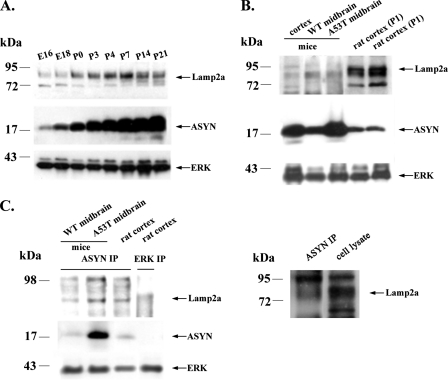
Expression and Interaction of ASYN and Lamp2a in Rats and WT and A53T Transgenic Mice—Our results indicate that WT ASYN is in part degraded via CMA in cultured neuronal cell lines and primary neurons, after binding to Lamp2a. We wished to examine whether we could detect the observed interactions between ASYN and Lamp2a also in an in vivo setting, and to this end we have used tissues derived from rats and WT and A53T transgenic mice. We first isolated cortices from rats from embryonic days 16 and 18 and then from postnatal day 0 to 21. As shown in Fig. 10A, the levels of ASYN and Lamp2a both increase during early postnatal rat CNS development, in a pattern that resembles the one observed during in vitro maturation of cortical neurons (see Fig. 5A). Additionally, Lamp2a is not only expressed in the cortex of rats but also in the cortex and the midbrain of WT and A53T transgenic mice (Fig. 10B). Moreover, co-immunoprecipitation experiments in cortices and midbrains isolated from rats and WT and A53T transgenic mice revealed that both WT and mutant ASYNs pull down Lamp2a receptor in vivo (Fig. 10C), as we had previously found in PC12 cells (13). We conclude that interactions between ASYN and Lamp2a can also be detected in vivo, indicating that CMA very likely also regulates ASYN degradation in vivo in the rodent CNS.
FIGURE 10.
Expression and interaction of ASYN and Lamp2a in rats and WT and A53T transgenic mice. A, Lamp2a and ASYN display a similar expression pattern during maturation in rat cortex. Proteins from embryonic day 16 to postnatal day 21 were lysed and immunoblotted for Lamp2a, ASYN, and ERK (loading control). B, Lamp2a and ASYN are expressed in the cortex and midbrain of WT and A53T transgenic mice. C, Lamp2a co-immunoprecipitates with rat (WT) and mouse (WT and A53T) ASYN. Lysates from rat cortex and midbrains of WT and A53T mice were immunoprecipitated (IP) with Syn 1 ASYN and ERK antibodies (negative control) and then immunoblotted with Lamp2a and Syn 1 ASYN antibodies. ASYN immunoprecipitated Lamp2a from rat brain co-migrates with the Lamp2a-specific band in the same brain extract (right panel). Representative Western blots from three independent experiments are presented.
DISCUSSION
In this study we have used two different approaches to address the contribution of CMA to ASYN degradation in neuronal cells as follows: targeting of Lamp2a, the rate-limiting step in the pathway, with RNAi; and comparison of the rate of clearance of the WT form of ASYN with that of a form (ΔDQ) that lacks the recognition motif for chaperone binding and Lamp2a presentation. Both approaches show that WT ASYN is degraded via CMA in PC12 and SH-SY5Y cell lines and in primary neurons. The experiments with ΔDQ in particular demonstrate that the KFERQ loose recognition motif is crucial in this regard. We have also detected by co-immunoprecipitation experiments a direct interaction between ASYN and Lamp2a in cultured cortical neurons, thus extending our previous findings, which were achieved in neuronal cell lines overexpressing ASYN, to the endogenous ASYN in a primary neuronal cell culture system. These results therefore represent the first convincing evidence in a cellular context of the specific degradation of ASYN by CMA, as our prior study (13) had merely shown that the purified protein of WT ASYN can be degraded by CMA in vitro, in isolated liver lysosomes. It is important to note that we have detected a CMA contribution to the degradation of either overexpressed or endogenous WT rodent or human ASYN in five different neuronal cell types, and therefore this is likely to be a universal phenomenon, at least in neuronal cell culture systems. The facts that the extent (but not the existence) of WT ASYN degradation by CMA depends on cell type and that GAPDH, a “traditional” CMA substrate based on in vitro assays (13, 35), is not altered following Lamp2a down-regulation (at the same time that ASYN levels increase by more than 3-fold) and does not appreciably associate with Lamp2a in neuronal cells indicate that the extension of previous studies performed in isolated artificial systems to living cells, and in particular those that are most relevant to PD, is crucial to assess physiological relevance.
We have also observed that inhibition of WT ASYN degradation by CMA leads to the accumulation of presumably aberrant species of ASYN, such as detergent-soluble oligomers and detergent-insoluble forms. Over the limited time course of our experiments inclusions were not readily appreciated. This may require longer periods of CMA dysfunction, more intense inhibition of CMA function, or additional factors. These results suggest the possibility that CMA dysfunction may act as a trigger or a contributing factor to the accumulation of aberrant species of ASYN, which occurs in PD and is likely to be involved in disease pathogenesis. Recently, Martinez-Vicente et al. (41) demonstrated that post-translational modifications of ASYN can impair degradation of the protein by CMA. Interestingly, dopamine-modified ASYN induced autophagic inhibition via blockade of the CMA pathway.
The lack of an effect of CMA dysfunction on GAPDH in a neuronal cell context reinforces the idea that such dysfunction may have relatively selective effects in the nervous system. CMA function is known to decrease with aging, at least in the liver (42, 43), and this may be one of the factors involved in the association of PD with aging.
The role, if any, of CMA in protein degradation in neurons has been unclear. In fact, prior studies have suggested that mRNA levels of Lamp2a are extremely low in the CNS, especially compared with Lamp2b, casting doubt on the importance of CMA in the nervous system, as Lamp2a is the rate-limiting step in this pathway (21, 22). In this study, we provide evidence that Lamp2a at the protein level is expressed at quite high levels in cortical and midbrain neurons in culture, as well as in vivo in the CNS of rats. Inhibition of CMA with Lamp2a down-regulation led to a decrease of long lived protein degradation in cortical neurons, indicating that CMA plays a role in proteolysis in primary neurons. Furthermore, we present evidence that Lamp2a is developmentally regulated, both in the primary neuronal cell culture and in vivo, and that this developmental up-regulation mirrors that of ASYN. Endogenous ASYN and Lamp2a also interact in vivo, as detected by co-immunoprecipitation experiments. Taken together, these results suggest that CMA may be an important route for ASYN degradation in vivo in the CNS and indicate for the first time directly that CMA is functionally important for protein degradation in neurons.
CMA however is not the only route for WT ASYN degradation. Application of the selective macroautophagy inhibitor 3-MA led to a considerable increase of the steady state levels of ASYN in PC12 cells and in primary cortical and ventral midbrain neurons, indicating that the clearance of ASYN is in part also mediated through macroautophagy. Two previous studies that examined this issue in PC12 cells (7) and in ventral midbrain neurons (13) failed to find an effect of macroautophagy inhibition with 3-MA on overexpressed human or endogenous rat WT ASYN, respectively. Methodological differences, such as the presence of an epitope tag in the Webb et al. study (7), different cell culture conditions, or different times of exposure to 3-MA (48 h) may underlie such discrepancies. Our data indicate that macroautophagy represents an important route of degradation not only for mutant forms of ASYN (as suggested by Webb et al.) but also for the WT form of ASYN in multiple cell types under a variety of cell culture conditions. It is important to note that in our case these results have been verified in primary neuronal cell culture systems and applied also to endogenous ASYN. Endogenous ASYN is readily soluble and is unlikely to harbor aberrant conformations under our cell culture conditions. Therefore, our data show that macroautophagy degrades normal conformations of WT ASYN constitutively, and not just aberrant conformations that evade other degradation systems. In an analogous fashion to what we have proposed for CMA dysfunction, it is thus conceivable to envision that macroautophagy dysfunction could also contribute to the gradual accumulation of endogenous WT ASYN in sporadic PD.
We failed to find a significant effect of proteasomal inhibition on the levels of ASYN in our experiments. This is consistent with our earlier results (10, 11) but contrasts with other reports in the literature (5, 6). We used relatively short term exposure of epoxomicin, the most selective proteasomal inhibitor available, and achieved a dramatic increase in polyubiquitinated proteins, indicating that the pharmacological agent utilized indeed had the desired effect. We cannot exclude the possibility that epoxomicin applied over more prolonged periods of time would have led to the detection of a small pool of ASYN species that are degraded by the proteasome; however, such experiments could not be performed because of the toxicity of the reagent. Indeed, in recent studies we have identified in PC12 cells expressing the A53T mutant form of ASYN specific ASYN species representing a very small fraction of total ASYN, which are degraded by the proteasome and are in fact responsible for proteasomal inhibition in this cell system.4
The steady state levels of ASYN were not appreciably increased with Lamp2a down-regulation in PC12 cells, whereas there was a dramatic accumulation of ASYN with a similar approach in primary cortical and ventral midbrain neurons. Half-lives of ASYN were not significantly different between the two cellular systems. One possibility for this discrepancy is that compensatory mechanisms for ASYN degradation are activated more readily in PC12 cells compared with primary neurons. Consistent with this idea, following Lamp2a down-regulation, up-regulation of macroautophagy and a slight increase of total long lived protein degradation occurred in PC12 cells, whereas a decrease in long lived protein degradation and no apparent change in macroautophagy occurred in cortical neurons. Therefore, compensatory activation of macroautophagy in PC12 cells, which as we have found could degrade WT ASYN, may account in part for the overt lack of change in steady state ASYN levels. Alternative or complementary interpretations are that compensatory changes at the mRNA level of ASYN occur in PC12 cells, but not cortical neurons, following Lamp2a down-regulation, or that ASYN may be degraded more readily by CMA in primary neurons, or that CMA is generally a more important degradation mechanism in primary neurons compared with neuronal cell lines.
At least in the two primary neuronal cell systems examined, inhibition of CMA leads to a greater induction of ASYN levels compared with the presumably complete inhibition of macroautophagy by 3-MA (as at this dose 3-MA completely inhibits the rapamycin-induced induction of LC3-II (data not shown)). Taking into account the fact that our strategy with the RNAi approach leads only to ∼50% reduction of Lamp2a levels, the contribution of CMA to α-synuclein degradation may actually be of an even greater magnitude.
In conclusion, our data directly indicate for the first time that CMA is an active proteolytic mechanism in neuronal cells, including primary cortical and ventral midbrain neurons, and can degrade, along with macroautophagy, monomeric WT ASYN. Perturbation of CMA-mediated ASYN degradation leads to accumulation of aberrant ASYN species, suggesting the possibility of the contribution of CMA dysfunction to PD pathogenesis. Furthermore, Lamp2a and ASYN interact in the rat and mouse cortex and midbrain, regions known to be affected in PD, suggesting that CMA operates in the brain as a mechanism to degrade ASYN. Our results add further importance to finding the means for reducing monomeric WT ASYN brain burden by regulating the balance of autophagic lysosomal pathways as a possible therapeutic intervention for the treatment of sporadic PD.
Supplementary Material
Acknowledgments
We thank Dr. Ana Maria Cuervo for valuable advice with setting up the long lived protein degradation assay.
This work was supported, in whole or in part, by National Institutes of Health Grant R21 NS055693 (to L. S. and K. V.). This work was also supported by a grant from the Parkinson Disease Foundation (to K. V.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: ASYN, α-synuclein; CMA, chaperone-mediated autophagy; dox, doxycycline; Lamp, lysososome-associated membrane protein; RNAi, RNA interference; WT, wild type; 3-MA, 3-methyladenine; EGFP, enhanced green fluorescent protein; PBS, phosphate-buffered saline; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; FBS, fetal bovine serum; RT, reverse transcription; siRNA, small interfering RNA; CNS, central nervous system; ANOVA, analysis of variance; scr, scrambled; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; shRNA, small hairpin RNA; m.o.i., multiplicity of infection; epx, epoxomicin; ERK, extracellular signal-regulated kinase; Ab, antibody; TH, tyrosine hydroxylase.
Emmanouilidou, E., Stefanis, L., and Vekrellis, K. (2008) Neurobiol. Aging,in press.
References
- 1.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998) Nat. Genet. 18106 –108 [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 2762045 –2047 [DOI] [PubMed] [Google Scholar]
- 3.Singleton, A., and Gwinn-Hardy, K. (2004) Lancet 3641105 –1107 [DOI] [PubMed] [Google Scholar]
- 4.Maraganore, D. M., de Andrade, M., Elbaz, A., Farrer, M. J., Ioannidis, J. P., Kruger, R., Rocca, W. A., Schneider, N. K., Lesnick, T. G., Lincoln, S. J., Hulihan, M. M., Aasly, J. O., Ashizawa, T., Chartier-Harlin, M. C., Checkoway, H., Ferrarese, C., Hadjigeorgiou, G., Hattori, N., Kawakami, H., Lambert, J. C., Lynch, T., Mellick, G. D., Papapetropoulos, S., Parsian, A., Quattrone, A., Riess, O., Tan, E. K., and Van Broeckhoven, C. (2006) J. Am. Med. Assoc. 296661 –670 [Google Scholar]
- 5.Bennett, M. C., Bishop, J. F., Leng, Y., Chock, P. B., Chase, T. N., and Mouradian, M. M. (1999) J. Biol. Chem. 27433855 –33858 [DOI] [PubMed] [Google Scholar]
- 6.Imai, Y., Soda, M., and Takahashi, R. (2000) J. Biol. Chem. 27535661 –35664 [DOI] [PubMed] [Google Scholar]
- 7.Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N., and Rubinsztein, D. C. (2003) J. Biol. Chem. 27825009 –25013 [DOI] [PubMed] [Google Scholar]
- 8.Tofaris, G. K., Layfield, R., and Spillantini, M. G. (2001) FEBS Lett. 50922 –26 [DOI] [PubMed] [Google Scholar]
- 9.Ancolio, K., Alves da Costa, C., Ueda, K., and Checler, F. (2000) Neurosci. Lett. 28579 –82 [DOI] [PubMed] [Google Scholar]
- 10.Rideout, H. J., Larsen, K. E., Sulzer, D., and Stefanis, L. (2001) J. Neurochem. 78899 –908 [DOI] [PubMed] [Google Scholar]
- 11.Rideout, H. J., and Stefanis, L. (2002) Mol. Cell. Neurosci. 21223 –238 [DOI] [PubMed] [Google Scholar]
- 12.Paxinou, E., Chen, Q., Weisse, M., Giasson, B. I., Norris, E. H., Rueter, S. M., Trojanowski, J. Q., Lee, V. M., and Ischiropoulos, H. (2001) J. Neurosci. 218053 –8061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T., and Sulzer, D. (2004) Science 3051292 –1295 [DOI] [PubMed] [Google Scholar]
- 14.Levine, B., and Klionsky, D. J. (2004) Dev. Cell 6463 –477 [DOI] [PubMed] [Google Scholar]
- 15.Cuervo, A. M. (2004) Trends Cell Biol. 1470 –77 [DOI] [PubMed] [Google Scholar]
- 16.Cuervo, A. M., and Dice, J. F. (1996) Science 273501 –503 [DOI] [PubMed] [Google Scholar]
- 17.Majeski, A. E., and Dice, J. F. (2004) Int. J. Biochem. Cell Biol. 362435 –2444 [DOI] [PubMed] [Google Scholar]
- 18.Massey, A., Kiffin, R., and Cuervo, A. M. (2004) Int. J. Biochem. Cell Biol. 362420 –2434 [DOI] [PubMed] [Google Scholar]
- 19.Muller, O., Sattler, T., Flotenmeyer, M., Schwarz, H., Plattner, H., and Mayer, A. (2000) J. Cell Biol. 151519 –528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shintani, T., and Klionsky, D. J. (2004) Science 306990 –995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konecki, D. S., Foetisch, K., Zimmer, K. P., Schlotter, M., and Lichter-Konecki, U. (1995) Biochem. Biophys. Res. Commun. 215757 –767 [DOI] [PubMed] [Google Scholar]
- 22.Furuta, K., Yang, X. L., Chen, J. S., Hamilton, S. R., and August, J. T. (1999) Arch. Biochem. Biophys. 36575 –82 [DOI] [PubMed] [Google Scholar]
- 23.Giasson, B. I., Duda, J. E., Quinn, S. M., Zhang, B., Trojanowski, J. Q., and Lee, V. M. (2002) Neuron 34521 –533 [DOI] [PubMed] [Google Scholar]
- 24.Stefanis, L., Larsen, K. E., Rideout, H. J., Sulzer, D., and Greene, L. A. (2001) J. Neurosci. 219549 –9560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. (2001) Nature 411494 –498 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds, A., Leake, D., Boese, Q., Scaringe, S., Marshall, W. S., and Khvorova, A. (2004) Nat. Biotechnol. 22326 –330 [DOI] [PubMed] [Google Scholar]
- 27.Stefanis, L., Park, D. S., Friedman, W. J., and Greene, L. A. (1999) J. Neurosci. 196235 –6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich, P., Rideout, H. J., Wang, Q., and Stefanis, L. (2003) Mol. Cell. Neurosci. 24430 –441 [DOI] [PubMed] [Google Scholar]
- 29.Franklin, J. L., and Johnson, E. M. (1998) J. Cell Biol. 1421313 –1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart, P. D., and Young, M. R. (1991) J. Exp. Med. 174881 –889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seglen, P. O., and Gordon, P. B. (1982) Proc. Natl. Acad. Sci. U. S. A. 791889 –1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clough, R. L., and Stefanis, L. (2007) FASEB J. 21596 –607 [DOI] [PubMed] [Google Scholar]
- 33.Rubinson, D. A., Dillon, C. P., Kwiatkowski, A. V., Sievers, C., Yang, L., Kopinja, J., Rooney, D. L., Zhang, M., Ihrig, M. M., McManus, M. T., Gertler, F. B., Scott, M. L., and Van Parijs, L. (2003) Nat. Genet. 33401 –406 [DOI] [PubMed] [Google Scholar]
- 34.Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D., and Naldini, L. (1998) J. Virol. 728463 –8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuervo, A. M., and Dice, J. F. (2000) J. Cell Sci. 1134441 –4450 [DOI] [PubMed] [Google Scholar]
- 36.Lee, D. H., and Goldberg, A. L. (1998) Trends Cell Biol. 8397 –403 [DOI] [PubMed] [Google Scholar]
- 37.Martin, L. J., Pan, Y., Price, A. C., Sterling, W., Copeland, N. G., Jenkins, N. A., Price, D. L., and Lee, M. K. (2006) J. Neurosci. 2641 –50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trojanowski, J. Q., Goedert, M., Iwatsubo, T., and Lee, V. M. (1998) Cell Death Differ. 5832 –837 [DOI] [PubMed] [Google Scholar]
- 39.Massey, A. C., Kaushik, S., Sovak, G., Kiffin, R., and Cuervo, A. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 1035805 –5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rideout, H. J., Dietrich, P., Savalle, M., Dauer, W. T., and Stefanis, L. (2003) J. Neurochem. 84803 –813 [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Vicente, M., Talloczy, Z., Kaushik, S., Massey, A. C., Mazzulli, J., Mosharov, E. V., Hodara, R., Fredenburg, R., Wu, D. C., Follenzi, A., Dauer, W., Przedborski, S., Ischiropoulos, H., Lansbury, P. T., Sulzer, D., and Cuervo, A. M. (2008) J. Clin. Investig. 118777 –788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiffin, R., Kaushik, S., Zeng, M., Bandyopadhyay, U., Zhang, C., Massey, A. C., Martinez-Vicente, M., and Cuervo, A. M. (2007) J. Cell Sci. 120782 –791 [DOI] [PubMed] [Google Scholar]
- 43.Cuervo, A. M., and Dice, J. F. (2000) J. Biol. Chem. 27531505 –31513 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.