Abstract
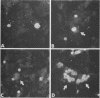
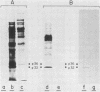
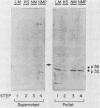
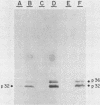
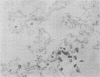
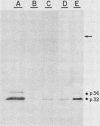
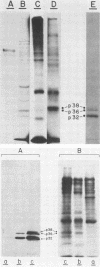
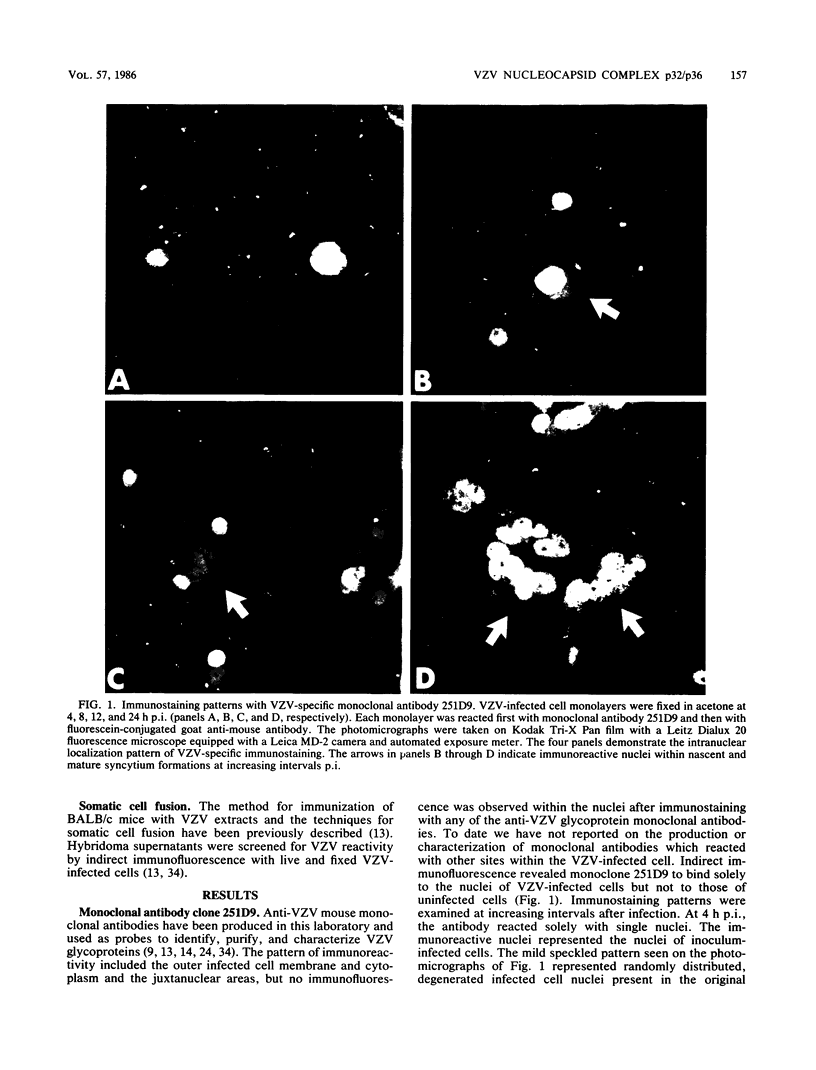
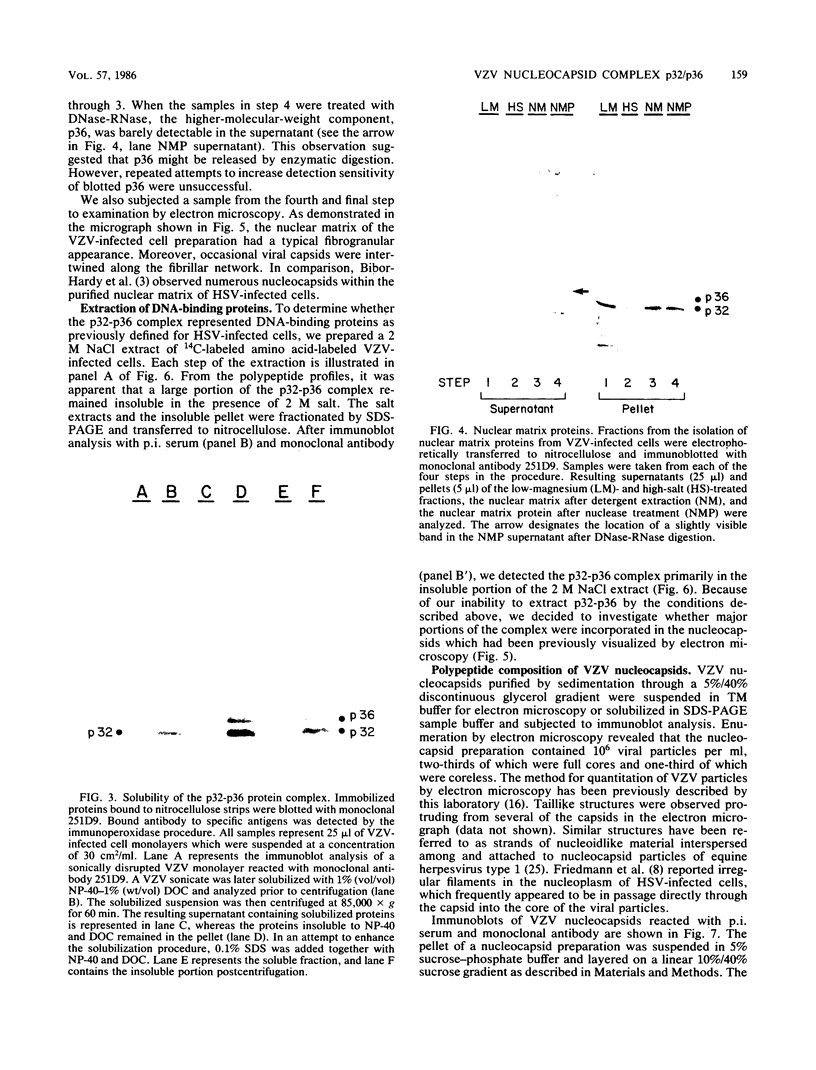
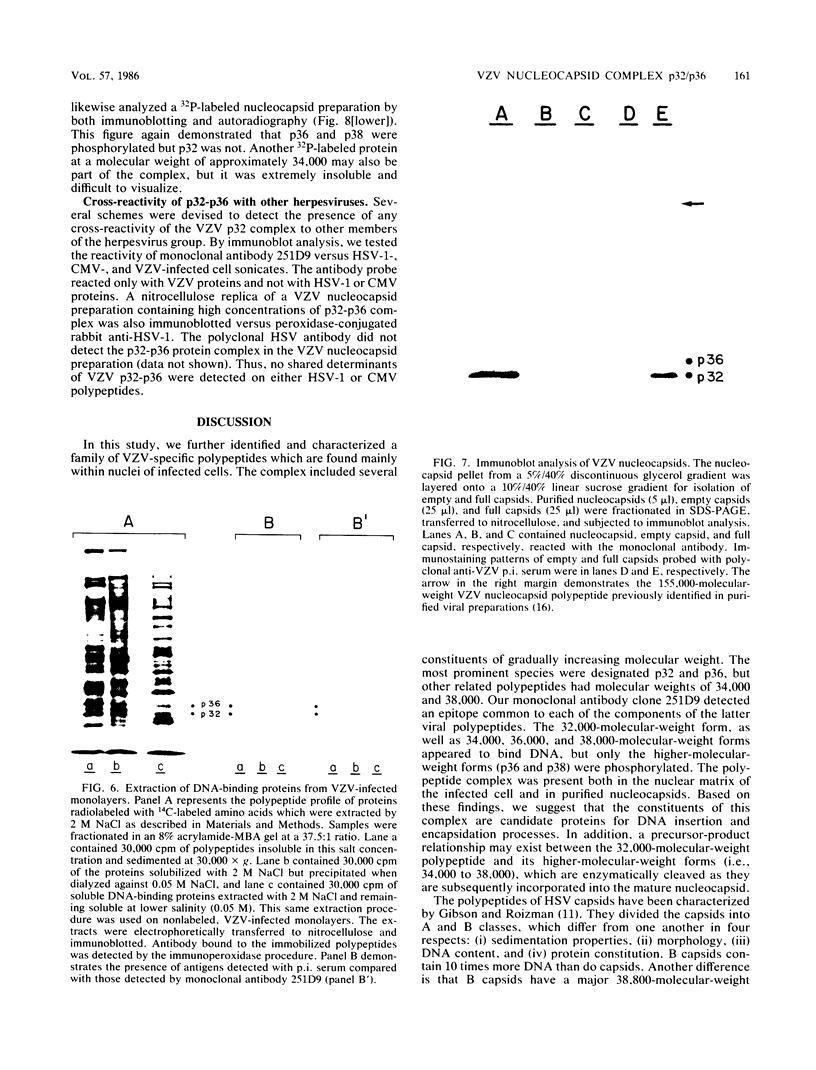
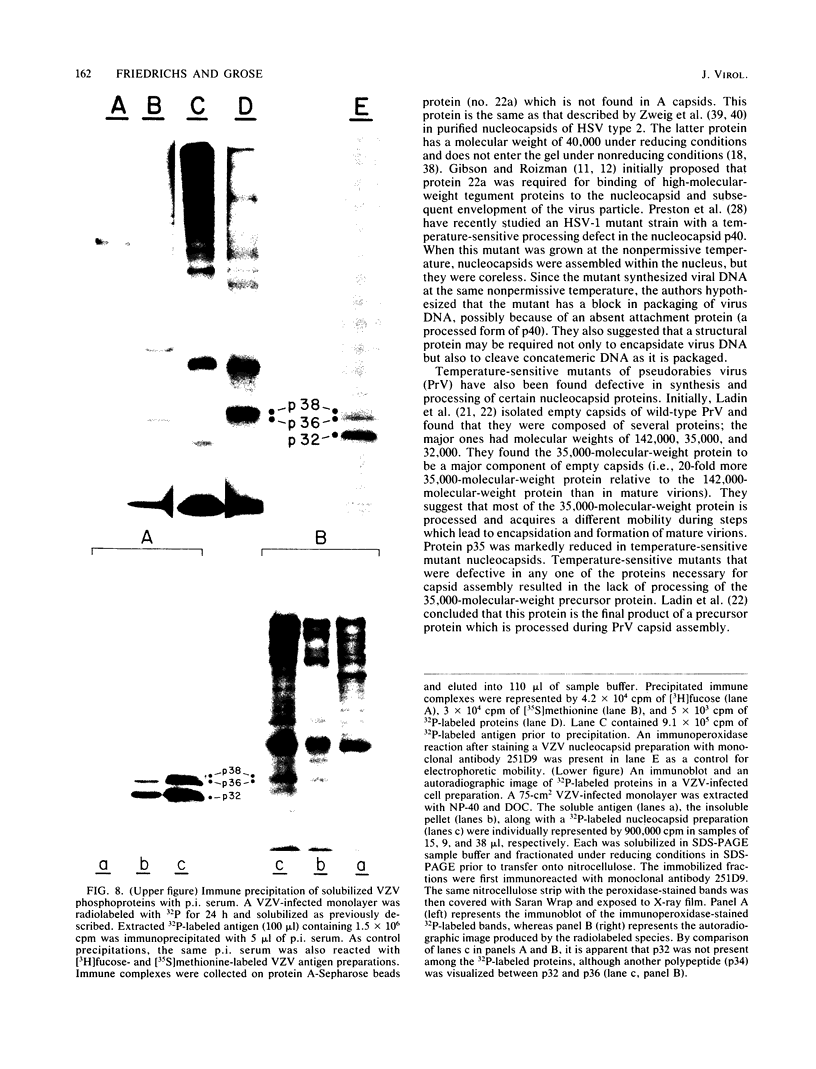
Varicella-zoster virus (VZV) directs the synthesis of numerous glycosylated and nonglycosylated infected-cell-specific proteins, many of which are later incorporated into the virion as structural components. In this study, we characterized a nonglycosylated polypeptide complex with the aid of a VZV-specific murine monoclonal antibody clone, 251D9. As detected by indirect immunofluorescence, the antibody bound mainly to antigens located within the nuclei of infected cells and did not attach to an uninfected cell substrate. The polypeptide specificity of the monoclonal antibody was determined by immunoblot analysis of electrophoretically separated infected cell extracts to react with a 32,000-molecular-weight VZV-specific protein (p32); in addition, the antibody also bound to a 36,000-molecular-weight polypeptide. The synthesis of these antigens was unaffected by inhibitors of glycosylation. Nonionic or ionic detergents were only marginally effective in solubilization of the p32-p36 complex, and relatively small amounts were eluted from nuclei by high salt concentrations (2 M NaCl). The same proteins remained associated with the nuclear matrix of VZV-infected cells. We also demonstrated that the protein complex was a major component of purified VZV nucleocapsids; p32 was especially prominent in both full and empty capsids. Immunoblot analysis of the nucleocapsid preparation revealed two additional species (p34 and p38) in the p32-p36 complex. Phosphorylation was a distinctive feature of some of the constituents. In summary, these results indicate that the p32-p36 complex represents a family of structural proteins closely associated with the assembly of VZV nucleocapsids and the encapsidation of viral DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Veach R. A., Blankenship M. L., Kaplan A. S. Differential association with cellular substructures of pseudorabies virus DNA during early and late phases of replication. Virology. 1984 Dec;139(2):205–222. doi: 10.1016/0042-6822(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibor-Hardy V., Pouchelet M., St-Pierre E., Herzberg M., Simard R. The nuclear matrix is involved in herpes simplex virogenesis. Virology. 1982 Sep;121(2):296–306. doi: 10.1016/0042-6822(82)90169-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braun D. K., Roizman B., Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984 Jan;49(1):142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Denniston K. J., Madden M. J., Enquist L. W., Vande Woude G. Characterization of coliphage lambda hybrids carrying DNA fragments from Herpes simplex virus type 1 defective interfering particles. Gene. 1981 Dec;15(4):365–378. doi: 10.1016/0378-1119(81)90180-3. [DOI] [PubMed] [Google Scholar]
- Friedmann A., Coward J. E., Rosenkranz H. S., Morgan C. Electron microscopic studies on assembly of herpes simplex virus upon removal of hydroxyurea block. J Gen Virol. 1975 Feb;26(2):171–181. doi: 10.1099/0022-1317-26-2-171. [DOI] [PubMed] [Google Scholar]
- Friedrichs W. E., Grose C. Glycoprotein gp118 of varicella-zoster virus: purification by serial affinity chromatography. J Virol. 1984 Mar;49(3):992–996. doi: 10.1128/jvi.49.3.992-996.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E. Immunoprecipitable polypeptides specified by varicella-zoster virus. Virology. 1982 Apr 15;118(1):86–95. doi: 10.1016/0042-6822(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E., Smith G. C. Purification and molecular anatomy of the varicella-zoster virion. Biken J. 1983 Mar;26(1):1–15. [PubMed] [Google Scholar]
- Hay R. T., Hay J. Properties of herpesvirus-induced "immediate early" polypeptides. Virology. 1980 Jul 15;104(1):230–234. doi: 10.1016/0042-6822(80)90381-5. [DOI] [PubMed] [Google Scholar]
- Heilman C. J., Jr, Zweig M., Stephenson J. R., Hampar B. Isolation of a nucleocapsid polypeptide of herpes simplex virus types 1 and 2 possessing immunologically type-specific and cross-reactive determinants. J Virol. 1979 Jan;29(1):34–42. doi: 10.1128/jvi.29.1.34-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Ihara T., Grose C., Starr S. Human leukocytes kill varicella-zoster virus-infected fibroblasts in the presence of murine monoclonal antibodies to virus-specific glycoproteins. J Virol. 1985 Apr;54(1):98–103. doi: 10.1128/jvi.54.1.98-103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Spang A. E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982 Jul;43(1):314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladin B. F., Blankenship M. L., Ben-Porat T. Replication of herpesvirus DNA. V. Maturation of concatemeric DNA of pseudorabies virus to genome length is related to capsid formation. J Virol. 1980 Mar;33(3):1151–1164. doi: 10.1128/jvi.33.3.1151-1164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. Thermolabile in vivo DNA-binding activity associated with a protein encoded by mutants of herpes simplex virus type 1. J Virol. 1983 Jun;46(3):909–919. doi: 10.1128/jvi.46.3.909-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. DNA-binding proteins of cells infected by herpes simplex virus type 1 and type 2. Intervirology. 1976;7(4-5):225–239. doi: 10.1159/000149955. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer Y., Leventon-Kriss S., Sarov I. Isolation and polypeptide characterization of varicella-zoster virus. Virology. 1980 Oct 15;106(1):133–140. doi: 10.1016/0042-6822(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Okuno T., Yamanishi K., Takahashi M. Polypeptides of varicella-zoster virus (VZV) and immunological relationship of VZV and herpes simplex virus (HSV). J Gen Virol. 1982 Aug;61(Pt 2):255–269. doi: 10.1099/0022-1317-61-2-255. [DOI] [PubMed] [Google Scholar]
- Spang A. E., Godowski P. J., Knipe D. M. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983 Jan;45(1):332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med. 1953 Jun;83(2):340–346. doi: 10.3181/00379727-83-20354. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983 Oct;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Molecular dissection of the humoral immune response to individual varicella-zoster viral proteins during chickenpox, quiescence, reinfection, and reactivation. J Infect Dis. 1984 May;149(5):741–749. doi: 10.1093/infdis/149.5.741. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Hampar B. Identification of disulfide-linked protein complexes in the nucleocapsids of herpes simplex virus type 2. Virology. 1979 Apr 30;94(2):442–450. doi: 10.1016/0042-6822(79)90474-4. [DOI] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Rabin H., Hampar B. Shared antigenic determinants between two distinct classes of proteins in cells infected with herpes simplex virus. J Virol. 1980 Sep;35(3):644–652. doi: 10.1128/jvi.35.3.644-652.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Rabin H., Hopkins R. F., 3rd, Neubauer R. H., Hampar B. Production of monoclonal antibodies against nucleocapsid proteins of herpes simplex virus types 1 and 2. J Virol. 1979 Nov;32(2):676–678. doi: 10.1128/jvi.32.2.676-678.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]