Abstract
Our previous studies demonstrated that the histone deacetylase inhibitor, trichostatin A (TSA), induces derepression of the human luteinizing hormone receptor (LHR) gene by de-recruitment of the pRB homologue p107 repressor from the promoter in JAR and MCF-7 cancer cells. TSA initiates a mechanism whereby the phosphatidylinositol 3-kinase/protein kinase ζ (PKCζ) cascade phosphorylates Sp1 at Ser-641, which is essential for the release of the repression of LHR transcription. The present studies have revealed that dissociation of serine/threonine protein phosphatases PP2A and PP1 from the LHR promoter mediates TSA-induced activation of LHR gene transcription in a cell-specific manner. Changes in chromatin structure induced by TSA cause the release of PP2A in JAR cells or of PP1 in MCF-7 cells, which is associated with Sp1 directly or through histone deacetylase 1/2, respectively, at the promoter. This favors the phosphorylation of Sp1 mediated by the phosphatidylinositol 3-kinase/PKCζ pathway, which in turn causes the release of the p107 inhibitor from Sp1 and marked transcriptional activation of the LHR. These findings reveal the importance of phosphatases in the control of LHR transcription, where the balance between phosphatidylinositol 3-kinase/PKCζ and phosphatases could be critical for up- and down-regulation of LHR gene expression in physiological and pathological settings.
The luteinizing hormone receptor (LHR),2 whose expression is controlled by hormones, growth factors, and other activators during development (1), has an essential role in mammalian reproduction. Characterization of the regulatory mechanisms of LHR gene transcription has revealed complex modulation at both genetic and epigenetic levels. LHR expression is subject to various modes of regulation, with impact centered on an activating Sp1/Sp3 binding domain within the promoter (2-10). To date, the regulatory aspects identified are all within the promoter and include an EAR2/EAR3 and TR4 nuclear orphan receptor binding motif upstream of the Sp1 sites that participates in repression/derepression of the LHR during the estrus cycle in rodents (5, 6, 8). Also, histone modifications, cell-specific CpG island methylation status at the promoter, changes in association of components of basal transcriptional machinery (TFIIB, polymerase II), corepressors (mSin3A and p107), and the phosphatidylinositol/PKCζ (PI3K/PKCζ) signal transduction pathways are participants in trichostatin A (TSA)-induced derepression of the LHR in cancer cells (7, 9, 10). Coordination and interaction among these diverse regulatory effectors are crucial for induction of silencing and activation of LHR expression.
TSA-induced LHR gene activation prevailed in the absence of changes in Sp1 protein expression level and its binding activity to the promoter Sp1-I site (7). These results indicated that modification of Sp1 might serve as a mechanism for LHR gene derepression by TSA. However, in the case of the LH receptor gene promoter, Sp1 acetylation was not observed (in previous work3 and in this study) in TSA-treated or nontreated JAR and MCF-7 cells. This was in contrast to studies where acetylation of transcription factors affects the expression of some target genes (14-18). Subsequent studies demonstrated that PI3K/PKCζ-mediated phosphorylation of Sp1 at serine 641 is crucial for the marked activation of the LHR gene induced by TSA in JAR and MCF-7 cells (10). These findings revealed that the phosphorylation status of Sp1 operates as a regulatory factor for repression/derepression of the LHR gene expression in response to TSA treatment. The phosphorylation state of a target protein at a given biological condition is, thus, the net result of the opposing activities of relevant kinase(s) and phosphatase(s) (19, 20).
Our recent findings have demonstrated that PI3K/PKCζ have an essential role in the regulation of LHR gene transcription in human JAR choriocarcinoma and MCF-7 breast cancer cells (10). PI3K/PKCζ-mediated Sp1 phosphorylation at serine 641 accounts for the derepression of LHR gene transcription induced by TSA. Blockade of PI3K or PKCζ activity abolished Sp1 phosphorylation and the marked activation of LHR gene expression induced by TSA. Moreover, PKCζ was found to associate with Sp1, and this association was enhanced by TSA. Sp1 phosphorylation was required for the release of pRB homologue p107 inhibitor protein from the LHR gene promoter, where it acts as a repressor of LHR gene transcription. Collectively, these findings have revealed a novel mechanism of TSA-regulated gene expression through de-recruitment of a repressor from the LHR gene promoter in a PI3K/PKCζ-induced Sp1 phosphorylation-dependent manner. Changes in chromatin structure due to histone acetylation, independent of the DNA methylation status, were required for this activation. These studies, which indicated that changes in the equilibrium between Sp1 phosphorylation and dephosphorylation, are of significant importance for LHR transcription, suggested that phosphatases could have a role in this regulation.
Although several lines of investigation have addressed the roles of kinases in TSA-regulated gene expression (11, 12), the participation of protein phosphatase(s) in this process has not been explored. Our observations have demonstrated the participation of protein phosphatase(s) PP1 and PP2A in the negative control of LHR gene transcription. The coordinated shift to a viable PI3K/PKCς cascade due to TSA-induced release of phosphatase(s) is critical for the derepression of LHR gene transcription determined by the Sp1 phosphorylation status.
EXPERIMENTAL PROCEDURES
Reagents, Expression Vectors, and Antibodies—TSA was obtained from Calbiochem. The human LHR promoter/luciferase reporter gene wild type and Sp1-I site (-79 bp to ATG) mutant constructs have been described in our previous studies (4). The expression plasmids of pCMV-PP1 catalytic subunit and pCMV-PP2A catalytic subunit were obtained from Ori-Gene (Rockville, MD). The antibodies for Sp1, HDAC1, HDAC2, and pan-acetylated antibody (pan-Acetyl-sc8649) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The PP1C and PP2AC antibodies were obtained from Cell Signaling (Danvers, MA). Antibody recognizing the phosphorylated serine residue(s) was obtained from Sigma. Antibodies for histone H4 and acetylated H4 were purchased from Upstate Biotechnology (Lake Placid, NY).
Cell Lines and Transfection—Human choriocarcinoma JAR cells and breast tumor MCF-7 cells from American Type Culture Collection (Manassas, VA) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic solution (Invitrogen). Transfections were performed using Lipofectamine and Plus reagents (Invitrogen) according to the procedures recommended by the manufacture. For reporter gene analyses in cells transfected with expression plasmids, the DNA amount used in each well was adjusted with empty vector plasmid so that every well contained equal amount of DNA. At 24 h post-transfection, indicated doses of TSA (JAR cells, 0-100 ng/ml (Fig. 1) and 100 ng/ml in subsequent experiments; MCF7 cells, 0-500 ng/ml (Fig. 1 and 500 ng/ml in subsequent experiments)) were added to cells 16 h before termination. The dose range and maximal effective TSA dose for the individual cell types were optimized in our previous studies (7, 10). Luciferase activities were normalized to light units per microgram of protein and expressed as the means ± S.E. All experiments were carried out three times in triplicate.
FIGURE 1.
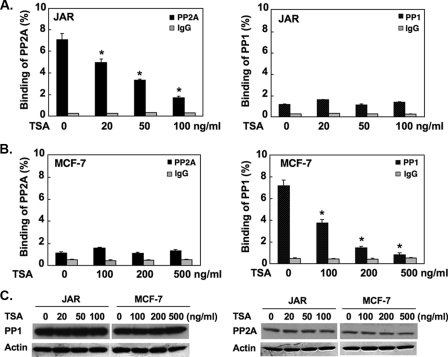
Cell-specific release of PP1 and PP2A from the LHR gene promoter mediates TSA-induced gene activation. A and B, TSA caused release of PP1 and PP2A from the LHR promoter. JAR or MCF-7 cells were treated with TSA at the indicated doses followed by ChIP analyses of the association of PP1 or PP2A catalytic subunit (PP1C, PP2AC) to the LHR gene promoter. The results are expressed as percentage to total input DNA (*, p < 0.01). C, Western blot analyses of endogenous PP1C and PP2AC expression in JAR and MCF-7 cells treated with the indicated doses of TSA. The expression level of β-actin at these conditions are shown. Results are expressed as the mean ± S.E. of three independent experiments carried out in triplicate. This applies also to all figures below.
PI3K Assay—JAR cells or MCF-7 cells treated with or without TSA at optimal doses for 16 h were washed twice with ice-cold phosphate-buffered saline, then lysed in lysis buffer (137 mm NaCl, 20 mm Tris-HCl (pH 7.4), 1 mm CaCl2, 1 mm MgCl2, 1% Nonidet P-40, 0.1 mm sodium orthovanadate, 1× Complete™ protease inhibitor mixture (Roche Applied Science)). PI3K activity was measured using a PI3K enzyme-linked immunosorbent assay kit (Echelon Biosciences Inc, Salt Lake City, UT) according to procedures recommended by the manufacture. Briefly, PI3K was immunoprecipitated by incubation of 200 μg of cell lysates with 2 μg of antibody specific for PI3K p110α catalytic subunit for 16 h at 4 °C followed by incubation with 40 μl of protein A-agarose beads (Santa Cruz) for 1 h at 4 °C. The immunoprecipitated complex was incubated with phosphatidylinositol-4,5-bisphosphate substrate in reaction buffer for 2 h at room temperature, and the supernatant recovered was subjected to competitive enzyme-linked immunosorbent assay. The colorimetric signals generated were inversely proportional to the amount of phosphatidylinositol-3,4,5-trisphosphate catalyzed from phosphatidylinositol-4,5-bisphosphate by immunoprecipitated PI3K activity, which was expressed as pmol/mg protein/2 h.
Chromatin Immunoprecipitation (ChIP) and Re-ChIP—ChIP experiments were carried out as previously described (9, 10, 13) using ChIP assay kit from Upstate Biotechnology. The cells used for each ChIP reaction were increased 2 × 106 (suggested by the manufacture) to 6 × 106. The ChIP-precipitated DNA and input DNA were analyzed by real-time PCR with SYBR green master mix in an ABI 7500 sequence detection system. The primers used for amplification of the human LHR gene promoter region were described previously (9, 10). For Re-ChIP analyses, complexes obtained from the primary immunoprecipitation were eluted from protein A-agarose beads by incubation of samples with 5 mm dithiothreitol at 37 °C for 20 min. Samples were mixed well by gentle vortex at every 5-min interval. The supernatant containing eluted protein complexes was recovered by brief centrifugation then diluted in ChIP dilution buffer (1% Triton X-100, 2 mm EDTA, 150 mm NaCl, 20 mm Tris-HCl at pH 8.1). The second round of immunoprecipitation was performed with the specified antibodies. The protocol applied in subsequent steps of the Re-ChIP assay was the same as that used in the single ChIP assay.
Whole Cell Lysate and Nuclear Protein Preparation—Whole cell lysates were prepared using M-PER mammalian protein extraction reagent of Pierce. The cytosolic and nuclear fractions from the treated and nontreated cells were isolated using Pierce N-PER nuclear protein extraction kit. All the preparations were performed in the presence of 1X Complete™ protease inhibitor mixture (Roche Applied Science) and 1× Halt™ phosphatase inhibitor mixture (Pierce).
Immunoprecipitation (IP) Analyses—250 μg of nuclear proteins or 1 mg of whole cell lysates in 1 ml of IP buffer (20 mm Tris-HCl (pH 8.0), 100 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, 1× protease inhibitor mixture, and 1× phosphatase inhibitor mixture) were used for each IP assay. The proteins were initially precleared with 0.5 μg of normal rabbit or mouse IgG and 40 μl of protein A/G-agarose beads (Santa Cruz) at 4 °C for 20 min. The precleared supernatant was incubated with 1 μg of specific antibody of interest at 4 °C for overnight with gentle shaking. This was followed by the addition of 36 μl of protein A/G-agarose beads and incubation at 4 °C for another 1 h. The protein-antibody-agarose complex was recovered by brief centrifugation and washed three times with 1 ml of IP buffer for 5 min each time at 4 °C. The complex was resuspended in 30 μl of 2× SDS protein sample buffer containing 2.5% β-mercaptoethanol and boiled for 3 min before being subjected to the Western blot analyses with the indicated antibodies. For protein acetylation studies, IP was performed using TSA-treated or nontreated JAR or MCF-7 whole cell extracts with respective antibodies followed by immunoblotting (IB) with a pan-acetylated antibody (1:500 dilution) that specifically recognizes acetylated protein. As positive control for the pan-acetylated antibody, histone proteins from TSA-treated (500 ng/ml for 16 h) or nontreated MCF-7 cells were immunoprecipitated with H4 antibody followed by Western blot analyses using pan-acetylated antibody (1:500) or an antibody to acetylated H4. To confirm the efficacy of immunoprecipitation, antibodies to Sp1, p107, PP1C, PP2AC, HDAC1 and -2, and H4 were utilized.
Statistical Analysis—Statistical significance of the data were evaluated by variance test analysis with computer programs Statview (Abacus Concepts, Berkeley, CA) and Superanova (Abacus Concept).
RESULTS
Release of Protein Phosphatases from the TSA-activated LHR Gene Promoter—By using competitive enzyme-linked immunosorbent assay, we observed that both JAR and MCF-7 cells exhibited a constitutive basal level of PI3K activity (Table 1). No significant changes of the PI3K activity were, thus, demonstrated after TSA treatment in these cell types. These results favored a functional participation of protein phosphatase(s) in the regulation of the LHR gene expression by preventing Sp1 phosphorylation from PI3K/PKCζ.
TABLE 1.
Values of phosphatidylinositol-3,4,5-trisphosphate (PIP3) catalyzed from phosphatidylinositol-4,5-bisphosphate by immunoprecipitated PI3K activity expressed as pmol/mg of protein per 2 h
|
PIP3 |
||
|---|---|---|
| −TSA | +TSA | |
| pmol/mg | ||
| JAR cells | 52.4 ± 1.2 | 53.9 ± 1.4 |
| MCF-7 cells | 70.6 ± 1.3 | 68.8 ± 1.4 |
To investigate the possible impact of protein phosphatases(s) on the Sp1 phosphorylation status, the association of two serine/threonine phosphatases, PP1 and PP2A, with the LHR gene promoter was initially assessed by ChIP assays in both JAR and MCF-7 cells (Fig. 1, A and B). Significant binding of the PP2A catalytic subunit (PP2AC) to a silenced-state LHR gene promoter was detected in JAR cells in the absence of TSA (Fig. 1A, left). During activation of LHR gene transcription by TSA, PP2AC was released in a TSA dose-dependent manner from the promoter, where about 80% reduction of PP2AC association was observed at 100 ng/ml TSA. This was the optimal TSA dose that caused maximal induction (40-fold) of LHR gene transcription in JAR cells in the absence of the DNA demethylating reagent 5-AzaCytidine (9). On the other hand, only minimal binding of PP1 catalytic subunit (PP1C) to the LHR promoter was observed regardless of the TSA treatment. This implies that PP1C does not play a significant role in LHR gene expression in JAR cells (Fig. 1A, right). In contrast, in MCF-7 cells, PP1C but not PP2AC exhibited prominent association with the LHR gene promoter under basal non-TSA treated condition (Fig. 1B, left). TSA caused dose-dependent reduction of PP1C binding in association with LHR gene activation (Fig. 1B, right). Moreover, the basal minimal binding of PP2AC was not influenced by TSA treatment (Fig. 1B, left). The 3′ end of the LHR gene coding region was also included in the ChIP analysis as a negative control to verify the phosphatase binding specificity at the gene promoter region (data not shown).
The observed phosphatase release was not attributable to a change of endogenous PP1C or PP2A protein expression level upon TSA treatment (Fig. 1C). Both phosphatases were expressed in JAR and MCF-7 cell lines. Each phosphatase expression was comparable in both cell types, and no changes were observed in presence of increasing concentration of TSA. Taken together these results indicate cell-specific recruitment of protein phosphatases PP2A and PP1 to the silenced state of the LHR gene promoter in JAR and MCF-7 cells, respectively, from which they were selectively released upon LHR gene promoter activation by increasing doses of TSA. Thus, PP2A and PP1 could participate in the regulation of LHR gene transcription in JAR and MCF-7 cells in a cell type-selective manner.
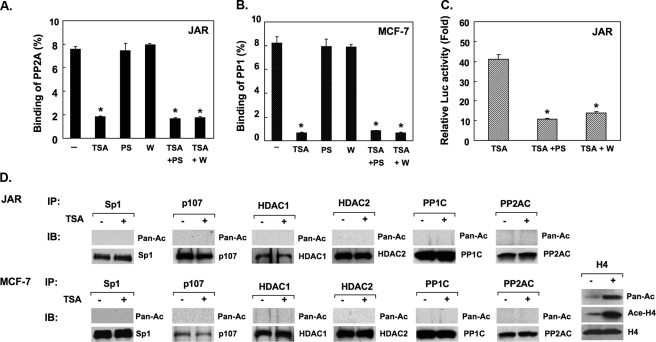
The association of PP1 or PP2A to the LHR gene promoter was further assessed in cells treated with TSA in the absence or presence of PKCζ or PI3K inhibitor to evaluate whether the kinase activities might affect the binding of the phosphatases (Fig. 1C). In contrast to our previous findings showing that the PKCζ/PI3K activity is required for the p107 release, blockade of the PKCζ or PI3K activity did not cause a change of the binding of PP1 or PP2A to the LHR gene promoter. The efficacy of the inhibitor compounds used was confirmed by their negative effects on TSA-induced LHR gene promoter activation (Fig. 2C). These results have demonstrated that the release of PP1 or PP2A caused by TSA is independent of Sp1 phosphorylation induced by PI3K/PKCζ.
FIGURE 2.
Phosphatase release is independent of PI3K/PKCζ activity and acetylation of components of the complexes. A and B, release of PP1 or PP2A is independent of the PI3K or PKCζ activity. ChIP analyses of PP2AC or PP1C to the LHR promoter in JAR or MCF-7 cells treated with or without TSA, PKCζ inhibitor (PS), PI3K inhibitor wortmannin (w), or in combination. Results are expressed as percentages of the total input DNA (*, p < 0.01). C, reporter analysis of LHR gene promoter activity in JAR and MCF-7 cells treated with TSA in the absence or presence of PS or wortmannin. The relative luciferase activity is expressed as -fold induction of the activity in the presence of treatment over the activity observed in the absence of treatment (*, p < 0.01). D, TSA-treated or nontreated JAR or MCF-7 whole cell lysates was immunoprecipitated with Sp1, p107, PP1C, PP2AC, HDAC1, and HDAC2 antibodies followed by IB with pan-acetylated (Pan-Ac) antibody or individual antibodies to these proteins. As a positive control the histone H4 acetylation status in TSA-treated or nontreated MCF-7 cells was assessed using Pan-Ac, acetylated H4, or H4 antibodies. In these experiments cells were treated with TSA concentrations of 100 ng/ml (JAR cells) and 500 ng/ml (MCF-7) cells.
We further addressed whether the observed release could result from TSA-induced acetylation of Sp1, p107, PP1C, PP2AC, HDAC1, or HDAC2, which may favor dissociation of the protein complex at the LHR gene promoter. TSA treatment did not cause acetylation of the components of the complexes (Fig. 2D), ruling out this modification for the changes induced by TSA on the phosphatase association with the LHR gene promoter. The efficiency of the pan-acetylation antibody (positive control) used in these assays was validated by IP analysis of histone H4 acetylation in MCF-7-untreated cells and after treatment with TSA (Fig. 2D). As expected, minor but detectable endogenous acetylation of H4 was observed in untreated cells, whereas major increases were induced by the TSA treatment. Taken together these studies have indicated the participation of TSA-mediated chromatin changes in the dissociation of PP1 and PP2A from the LHR promoter.
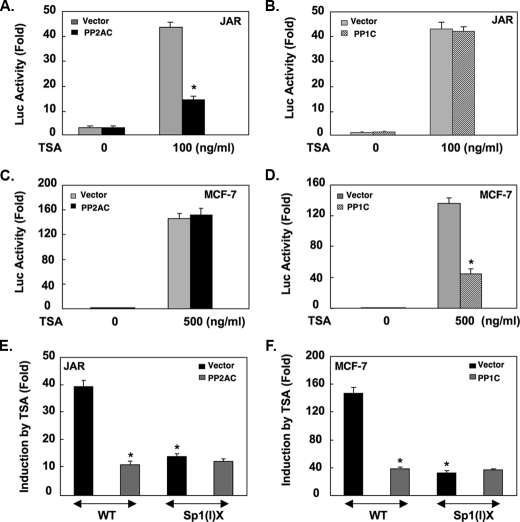
Functional Participation of PP1 and PP2A in the Control of the LHR Gene Expression—The regulatory function of PP1 or PP2A in the control of the LHR gene expression was next examined by cotransfection studies of PP1C or PP2AC expression plasmid with the LHR promoter/reporter gene construct in JAR and MCF-7 cells. Overexpression of PP2AC in JAR cells had no effect on the basal LHR promoter activity in the absence of TSA (Fig. 3A). However, PP2AC prevented the derepression of the LHR promoter induced by TSA in JAR cells. In contrast, PP1C had no functional impact on the LHR gene promoter activity regardless of TSA treatment (Fig. 3B). On the other hand, in MCF-7 cells, overexpression of PP1C largely decreased the LHR gene activation elicited by TSA, whereas no effect of PP2AC was detected in these cells Fig. 3, D and C). These results are consistent with the binding characteristic of these two phosphatases to the LHR gene promoter in JAR and MCF-7 cells. Also, our studies using a promoter construct bearing mutation at the proximal Sp1-1 site (-79 bp relative to ATG) excluded that protein phosphatases may affect other aspects of LHR gene transcription (i.e. RNA polymerase II, Fig. 3, E and F). Taken together, these studies further demonstrate that PP1 and PP2A are negative regulators of the LHR gene expression operating in a cell type-dependent manner. The release of the inhibitory effect of these phosphatases by TSA, thus, contributed to the LHR gene derepression in JAR and MCF-7 cells.
FIGURE 3.
PP1 and PP2A inhibit TSA-induced LHR gene activation dependent on the proximal Sp1 site. Reporter gene analyses of the LHR gene promoter activity in JAR (A and B) and MCF-7 cells (C and D) cotransfected with PP2AC or PP1C or empty vector plasmid followed by TSA treatment. Relative promoter activities are expressed as -fold induction over the activity in the absence of treatment (1-fold) (*, p < 0.01). Reporter gene analyses of the wild type (WT) or Sp1(I) site mutant (Sp1(I)X) LHR promoter construct in JAR cells (E) cotransfected with PP2AC or in MCF-7 cells (F) cotransfected with PP1C followed by TSA treatment. The relative luciferase activity is expressed as -fold induction of the activity in the presence of TSA over the activity observed in the absence of TSA (*, p < 0.01).
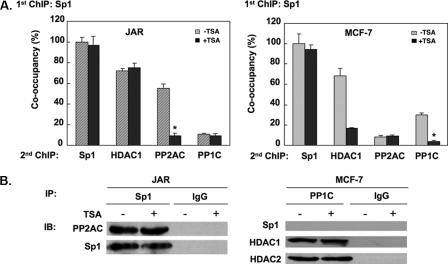
PP1 and PP2A Associated with Sp1 at the LHR Gene Promoter in a Cell-type-dependent Manner—The findings of PP1 or PP2A binding to the LHR gene promoter prompted us to investigate whether these phosphatases might associate with Sp1 to exert the regulation of its phosphorylation state. Sequential ChIP analyses were carried out in TSA-treated or nontreated cells in which Sp1 antibody was utilized for the initial IP followed by a second IP (Re-ChIP) with specific PP1C or PP2AC antibodies. Based on the known association of Sp1 with HDAC (7), Re-ChIP with an HDAC1 antibody was also included as a control. As expected, substantial binding of HDAC1 to the silenced LHR gene promoter was observed after primary precipitation by the Sp1 antibody in untreated JAR and MCF-7 cells (Fig. 4A). This was in agreement with our previous findings that Sp1 serves as an anchor protein to recruit HDAC1/2 to the LHR gene promoter. TSA treatment caused no changes of HDAC association in JAR cells, whereas marked reduction was observed in MCF-7 cells. This differential reduction of HDAC is related to the methylation state of the LHR promoter (9), which in JAR cells is methylated even after treatment with TSA. However, in MCF-7 cells the promoter is basally unmethylated, and the histone acetylation induced by TSA causes dissociation of the HDAC1/2-mSin3A complex from Sp1. These differences are also reflected in the degree of activation of the LHR gene by TSA (40-fold, JAR versus 160-fold, MCF-7 cells).
FIGURE 4.
Association of PP1 or PP2A with the LHR promoter. A, sequential ChIP analyses were performed in TSA-treated or nontreated JAR or MCF-7 cells using Sp1 antibody in the first IP followed by the second IP with the indicated antibodies. Results are expressed as the percentage of co-occupancy over the Sp1-Sp1 set in the absence of treatment (100%) (*, p < 0.01). B, coimmunoprecipitation assays of the association of PP1 and PP2A with Sp1 or HDAC1/2. Left, nuclear extracts isolated from TSA-treated (100 ng/ml) or untreated JAR cells were IP with Sp1 antibody or IgG followed by IB with antibodies to PP2AC or Sp1. Right, nuclear extracts isolated from TSA-treated (500 ng/ml) or untreated MCF-7 cells were immunoprecipitated with PP1C antibody or IgG followed by IB with Sp1, HDAC1, or HDAC2 antibodies.
In regard to the phosphatases, in the absence of TSA, significant association of Sp1 with PP2AC was detected in JAR cells, whereas the signal for a Sp1-PP1C complex was minimal and comparable with the background binding obtained by assessing supernatant derived from the first round of precipitation. In MCF-7 cells, Sp1 exhibited a strong association with PP1C but not PP2AC at the LHR promoter in basal state (before the TSA treatment). However, the marked binding of PP2AC with Sp1 in JAR cells or PP1C with Sp1 in MCF-7 cells was absent from the TSA-activated LHR gene promoter, reinforcing the notion that PP1 and PP2A were repressors for the LHR gene expression.
Coimmunoprecipitation analyses were also performed to evaluate the putative complex of phosphatase(s) with Sp1 in solution in the absence of the LHR gene promoter, utilizing nuclear extracts from control and TSA-treated JAR and MCF-7 cells. PP2AC and Sp1 readily associated with each other in JAR cell nuclear extracts, and this was not affected by TSA treatment (Fig. 4B, left). However, coimmunoprecipitation failed to detect a similar complex of PP1C-Sp1 in nuclear extracts from MCF-7 and JAR cells, implying lack of association of PP1C with Sp1. In contrast, a strong interaction of PP1C with HDAC1 or HDAC2 was present, revealing an indirect link of PPIC to Sp1 protein through HDACs (Fig. 4B, right). Also, as noted for PP2AC, the association of PP1C with HDAC1 or HDAC2 remained unchanged regardless of TSA treatment. These results demonstrated a difference in the TSA effect on the Sp1-PP1 or Sp1-PP2A complex in the absence versus presence of the LHR gene promoter. The evidence that the release of PP1 or PP2A from Sp1 by TSA occurred only in the context of the LHR gene promoter indicated the importance of changes in chromatin structure at the promoter induced by TSA during this process. Other studies (not shown) revealed that both Sp1 N-terminal and C-terminal segments were able to interact with PP2AC. Taken together these results have revealed that the observed cell type-selective binding of PP2A and PP1 to the LHR gene promoter in JAR and MCF-7 cells occurred in Sp1 or its proximity, respectively, where the association of Sp1 protein with PP2A in JAR or with PP1 in MCF-7 cells enables these phosphatases to exert their regulatory effects on Sp1 phosphorylation levels in the control of silencing or activation of the LHR gene expression.
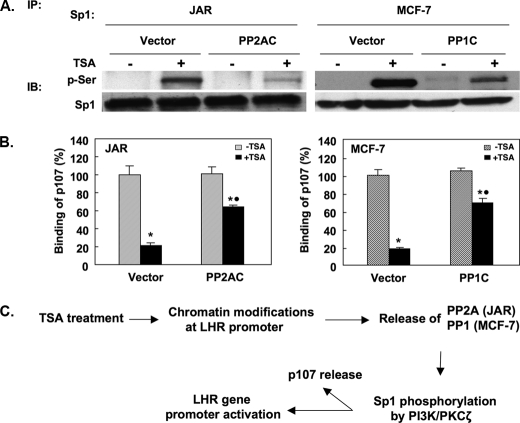
PP1 and PP2A Reduced TSA-induced Sp1 Phosphorylation—The regulation of the Sp1 phosphorylation level by PP1 or PP2A was investigated by overexpression of PP1C or PP2AC constructs in MCF-7 or JAR cells, respectively. The Sp1 phosphorylation status was assayed by IP using a Sp1 antibody followed by immunodetection using an antibody recognizing phosphorylated serines. Because phosphor-Sp1 at serine (PI3K/PKC-mediated) is not discernable from the unphosphorylated form as a retarded band upon IP by Sp1 antibody, the subsequent use of specific phospho-Ser antibody was required to assess the effect of PP2A or PP1 on the Sp1 phosphorylation status. Consistent with our previous findings, TSA treatment caused marked Sp1 phosphorylation in both JAR and MCF-7 cells (Fig. 5A). However, overexpression of PP2AC significantly reduced the level of Sp1 phosphorylation induced by TSA in JAR cells. Similarly, overexpression of PP1C also largely abolished TSA-mediated Sp1 phosphorylation in MCF-7 cells. These results have demonstrated that the negative regulation of PP1 or PP2A on the LHR gene transcription was achieved through maintaining a dephosphorylated state of Sp1 protein, thus causing the silencing of this gene. We previously reported that the Sp1 phosphorylation status is critical for p107 repressor protein release, thus contributing to the TSA-induced LHR gene activation. Overexpression of PP1C or PP2AC in MCF-7 or JAR cells, respectively, significantly antagonized TSA-induced p107 release from the LHR promoter (Fig. 5B). The reduction of p107 release was consistent with the mayor reduction of Sp1 phosphorylation observed by overexpression of the PP1C or PP2AC in the individual cell types (Fig. 5A). These results further confirm that the negative regulation of PP1 or PP2A on the LHR gene expression is mediated through their dephosphorylation effect on Sp1, which cause the release of repressor p107.
FIGURE 5.
PP1 and PP2A antagonize TSA-induced Sp1 phosphorylation and p107 release. A, JAR or MCF-7 cells were overexpressed with PP2AC or PP1C expression plasmid, respectively, followed by treatment with or without TSA. Empty vector was the control. Whole cell lysates were IP with Sp1 antibody followed by IB using anti-phosphoserine antibody (p-Ser) or Sp1 antibody. B, ChIP assays of p107 association with the LHR gene promoter in TSA-treated or nontreated JAR (100 ng/ml) or MCF-7 cells (500 ng/ml) with co-transfection of empty vector, PP1C, or PP2AC expression plasmids. Results are expressed as the percentage of occupancy of p107 in the absence of treatment (100%) (JAR cells: *, +TSA (vector) versus -TSA (vector), p < 0.01; *, +TSA (PP2AC) versus -TSA (PP2AC), p < 0.01; •, +TSA (vector) versus +TSA (PP2AC), p < 0.01; *, MCF-7 cells: +TSA (vector) versus -TSA (vector), p < 0.01; *, +TSA (PP1C) versus -TSA PP1C, p < 0.01; •, +TSA (vector) versus + TSA (PP1C) p < 0.01. C, schematic representation of functional roles of PP1 and PP2A in TSA-induced LHR gene activation. The TSA-induced changes of the chromatin modification status at the LHR promoter favor the release of PP2A or PP1 from the promoter in JAR or MCF-7 cells, respectively. This in turn facilitates the Sp1 phosphorylation by PI3K/PKCζ, which triggers the dissociation of p107 from the promoter and the LHR gene activation.
DISCUSSION
These studies have revealed that protein phosphatases PP1 and PP2A are key participants in the control of silencing and activation of the LHR gene expression. PP1 and PP2A negatively regulate the LHR gene transcription by promoting Sp1 dephosphorylation in a cell type-dependent manner. TSA treatment caused release of PP1 and PP2A from the silenced LHR promoter in MCF-7 and JAR cells, respectively. This favors a shift to the Sp1 phosphorylated state via the PI3K/PKCς pathway which causes release of corepressor p107 and LHR gene activation. These results have demonstrated a novel mechanism of gene regulation in which fine-tuning of Sp1 phosphorylation through changes in the recruitment of PP1and PP2A at the LHR promoter region contribute to derepression of the expression of this gene in response to TSA.
Uncoiling and relaxation of local chromatin structure resulting from histone hyperacetylation at a target gene promoter region is a well recognized epigenetic mechanism that participates in the regulation of gene expression induced by TSA (21). However, several studies have revealed strict dependence on certain sequence-specific cis elements for the TSA effect exemplified by the relevance of Sp1/Sp3 binding site(s) identified for LHR gene and a number of other genes (7, 22, 23), indicating that a mechanism(s) other than histone hyperacetylation also participates in the TSA effect. This has been further supported by our recent findings showing that phosphorylation of Sp1 at Ser-641 by PI3K/PKCς is essential for the activation of this gene by TSA (10). The participation of signaling pathway(s) in TSA-regulated gene expression has been shown also in studies where the inhibition of the activities of several kinases including PI3K, PKCε, and mitogen-activated protein abrogated the TSA responses (24, 25). All these studies further supported the concept that changes in the phosphorylation status of a transcription regulator(s) in concert with the changes at histone levels mediate the TSA effect on target gene expression. However, the participation and functional assessment of protein phosphatase(s) during this process has not been previously addressed. The mechanistic characterization of PP1 and PP2A in the regulation of LHR gene expression together with the findings of PI3K/PKCς participation have provided evidence delineating how a signaling pathway is integrated into the Sp1 site-dependent derepression event triggered by TSA.
The phosphorylation level of a protein determined by the equilibrium of relevant kinase(s) and phosphatase(s) is subject to regulation by changes of the activity, abundance, and availability of the kinase(s) and phosphatase(s) involved. The activities of several kinases have been demonstrated to be increased by TSA in some cell types. For example, TSA mediated-PI3K/AKT induction after epidermal growth factor receptor activation was required for survivin gene expression in ovarian cancer Caov3 cells (26). Analysis of the activities of 15 different protein kinases in LT23 lung adenocarcinoma cells showed that TSA induced the activities of calcium/calmodulin-dependent kinase II and PKC in parallel with the activation of several pro-apoptotic genes (27). Regulation of the activity of a phosphatase, on the other hand, could affect the activation of a relevant kinase pathway. Suppression of PP2A activity by the SV40 small T antigen stimulated mitogen-activated protein kinase pathway in CV-1 cells or PKCς activity in both CV-1 and NIH3T3 cells for NF-κB-dependent gene transcription (28). In this study we have identified a novel regulatory mode whereby TSA reduces the availability of a phosphatase(s) within the LHR gene promoter region which relieves repression and causes activation of this gene.
TSA-induced release of PP1 or PP2A from the LHR gene promoter caused PIK3/PKCς-mediated Sp1 phosphorylation. Overexpression of the constitutively active catalytic subunit of PP1 or PP2A strongly antagonized TSA-induced Sp1 phosphorylation causing release of p107 in MCF-7 or JAR cells, respectively. Elimination of the dephosphorylating effect upon release of a phosphatase could impact on PI3K, PKCς, and Sp1, any of which would cause increased Sp1 phosphorylation. The observation that both JAR and MCF-7 cells possess constitutive PI3K activity that was unchanged by TSA treatment indicated a lack of effect on PI3K activity upon phosphatase release.
On the other hand, in both JAR and MCF-7 cells we observed that TSA dose-dependently activated PKCς and that such activation was abrogated by the PIK3 inhibitors wortmannin and LY294002 (10). Thus, phosphatase release could facilitate the PI3K-mediated PKCς activation by preventing the antagonistic effect of a phosphatase on the progression of downstream signaling, which consequently enhanced Sp1 phosphorylation. Moreover, phosphatase release abolished association of Sp1 with PP2A or PP1 bridged by HDAC1/2, thus shifting the balance to a phosphorylated state of Sp1. Specific binding of PP1 to HDAC1/2 but not Sp1 could be favored by the presence of a PP1-binding site (898KVTF901) recently identified in breast and ovarian tumor suppressor protein BRCA1 (29). Amino acid sequence analyses of HDAC1/2 and Sp1 demonstrated that both HDAC1 and -2 possess at their N termini a single motif (10KVCY13 at HDAC1, 100KVCY103 at HDAC2) with 75% similarity/50% identity to the PP1-binding site characterized for BRCA1. In contrast, no similar site was revealed for Sp1. Complex formation of PP1 with HDACs was also supported by other studies in which PP1 attachment with HDAC1 dephosphorylated CREB at Ser-133 and silenced its activity (30). The direct and indirect association of PP1 or PP2A with HDAC1/2, respectively, raises the possibility that the phosphorylation status of HDAC1/2 is another aspect of regulation of LHR gene expression by TSA. A change of this kind might contribute to release of p107 in JAR and MCF-7 cells and of mSin3A in MCF-7 cells (9) and/or recruitment/de-recruitment of other regulatory effectors during this process whose identity has yet to be determined.
The selective binding of PP1 and PP2A to the LHR promoter in JAR and MCF-7 cells, respectively, and their repressive function in promoter activity demonstrated nonredundant roles of these phosphatases in LHR gene transcription. Although PP1 and PP2A belong to the same subfamily, based on their structural homology significant diversities have been observed in terms of their substrate specificities, subcellular localizations, and physiological functions (20). These differences are in part due to the presence of numerous phosphatase regulatory subunits in most mammalian cells as well their association with a single catalytic subunit to form heterodimeric or trimeric holoenzymes at a particular cellular condition (31). Although differential interactions of the PP1/PP2A catalytic subunit with their regulatory subunits might be a mechanism contributing to their individual association with the LHR promoter in JAR and MCF-7 cells, the chromatin context at the LHR promoter region undoubtedly has a critical role in the release of PP1 and PP2A from the promoter. The observed TSA dose-dependent release pattern of the phosphatase indicated that dissociation of PP1 and PP2A was concordant with the gradual transit to an uncompacted chromatin structure at the LHR promoter. Moreover, the finding that the phosphatase-Sp1complex was disrupted by TSA only in the presence of the LHR gene promoter further indicates that changes in both phosphatase recruitment and histone acetylation are required to achieve LHR gene activation by TSA.
Taken together these studies have defined a novel mechanism whereby TSA-induced changes in chromatin structure (9) cause a cell-specific release of a phosphatase that is associated with Sp1 directly (PP2A) or through HDAC (PP1), respectively. This favors the phosphorylation of Sp1 mediated by the PI3K/PKCζ pathway (constitutively active in these cancer cells) which in turn causes the release of the p107 inhibitor from Sp1 and the marked transcriptional activation of the human LHR (Fig. 5C).
This work was supported, in whole or in part, by National Institutes of Health (Intramural Research Program of the NICHD). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LHR, luteinizing hormone receptor; ChIP, chromatin immunoprecipitation; IB, immunoblotting; PP2AC, PP2A catalytic subunit; PP1C, PP1 catalytic subunit; PKC, protein kinase C; PI3K, phosphatidylinositol 3-kinase; TSA, trichostatin A; IP, immunoprecipitate; HDAC, histone deacetylase.
Y. Zhang, M. Liao, and M. L. Dufau, unpublished data.
References
- 1.Dufau, M. L. (1998) Annu. Rev. Physiol. 60 461-496 [DOI] [PubMed] [Google Scholar]
- 2.Tsai-Morris, C. H., Xie, X., Wang, W., Buczko, E., and Dufau, M. L. (1993) J. Biol. Chem. 268 4447-4452 [PubMed] [Google Scholar]
- 3.Tsai-Morris, C. H., Geng, Y., Xie, X. Z., Buczko, E., and Dufau, M. L. (1994) J. Biol. Chem. 269 15868-15875 [PubMed] [Google Scholar]
- 4.Geng, Y., Tsai-Morris, C. H., Zhang, Y., and Dufau, M. L. (1999) Biochem. Biophys. Res. Commun. 263 366-371 [DOI] [PubMed] [Google Scholar]
- 5.Zhang, Y., and Dufau, M. L. (2000) J. Biol. Chem. 275 2763-2770 [DOI] [PubMed] [Google Scholar]
- 6.Zhang, Y., and Dufau, M. L. (2001) Mol. Endocrinol. 15 1891-1905 [DOI] [PubMed] [Google Scholar]
- 7.Zhang, Y., and Dufau, M. L. (2002) J. Biol. Chem. 277 33431-33438 [DOI] [PubMed] [Google Scholar]
- 8.Zhang, Y., and Dufau, M. L. (2003) Mol. Cell. Biol. 23 6958-6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, Y., Fatima, N., and Dufau, M. L. (2005) Mol. Cell. Biol. 25 7929-7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, Y., Liao, M., and Dufau, M. L. (2006) Mol. Cell. Biol. 26 6748-6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, Y. K., Han, J. W., Woo, Y. N., Chun, J. K., Yoo, J. Y., Cho, E. J., Hong, S., Lee, H. Y., Lee, Y. W., and Lee, H. W. (2003) Oncogene 22 6023-6031 [DOI] [PubMed] [Google Scholar]
- 12.Yang, J., Kawai, Y., Hanson, R. W., and Arinze, I. J. (2001) J. Biol. Chem. 276 25742-25752 [DOI] [PubMed] [Google Scholar]
- 13.Dong, J., Tsai-Morris, C. H., and Dufau, M. L. (2006) J. Biol. Chem. 281 18825-18836 [DOI] [PubMed] [Google Scholar]
- 14.Li, A. G., Piluso, L. G., Cai, X., Gadd, B. J., Ladurner, A. G., and Liu, X. (2007) Mol. Cell 28 408-421 [DOI] [PubMed] [Google Scholar]
- 15.Ivanov, G. S., Ivanova, T., Kurash, J., Ivanov, A., Chuikov, S., Gizatullin, F., Herrera-Medina, E. M., Rauscher, F., III, Reinberg, D., and Barlev, N. A. (2007) Mol. Cell. Biol. 27 6756-6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy, S., and Tenniswood, M. (2007) J. Biol. Chem. 282 4765-4771 [DOI] [PubMed] [Google Scholar]
- 17.Huang, W., Zhao, S., Ammanamanchi, S., Brattain, M., Venkatasubbarao, K., and Freeman, J. W. (2005) J. Biol. Chem. 280 10047-10054 [DOI] [PubMed] [Google Scholar]
- 18.Ammanamanchi, S., Freeman, J. W., and Brattain, M. G. (2003) J. Biol. Chem. 278 35775-35780 [DOI] [PubMed] [Google Scholar]
- 19.Chou, M. M., Hou, W., Johnson, J., Graham, L. K., Lee, M. H., Chen, C. S., Newton, A. C., Schaffhausen, B. S., and Toker, A. (1998) Curr. Biol. 8 1069-1077 [DOI] [PubMed] [Google Scholar]
- 20.Mustelin, T. (2007) Methods Mol. Biol. 365 9-22 [DOI] [PubMed] [Google Scholar]
- 21.Jaskelioff, M., and Peterson, C. L. (2003) Nat. Cell Biol. 5 395-399 [DOI] [PubMed] [Google Scholar]
- 22.Sowa, Y., Orita, T., Minamikawa-Hiranabe, S., Mizuno, T., Nomura, H., and Sakai, T. (1999) Cancer Res. 59 4266-4270 [PubMed] [Google Scholar]
- 23.Camarero, N., Nadal, A., Barrero, M. J., Haro, D., and Marrero, P. F. (2003) Nucleic Acids Res. 31 1693-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, S. H., Vickers, E., Brehm, A., Kouzarides, T., and Sharrocks, A. D. (2001) Mol. Cell. Biol. 21 2802-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinos, E., Le Van Thai, A., Pomies, C., and Weber, M. J. (1999) Mol. Cell. Biol. 19 3474-3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, C., Qiu, L., Sun, Y., Healey, S., Wanebo, H., Kouttab, N., Di, W., Yan, B., and Wan, Y. (2006) Int. J. Oncol. 29 269-278 [PubMed] [Google Scholar]
- 27.Eickhoff, B., Ruller, S., Laue, T., Kohler, G., Stahl, C., Schlaak, M., and van der Bosch, J. (2000) Biol. Chem. 381 107-112 [DOI] [PubMed] [Google Scholar]
- 28.Sontag, E., Sontag, J. M., and Garcia, A. (1997) EMBO J. 16 5662-5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu, L.-C. (2007) Biochem. Biophys. Res. Commun. 360 507-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canettieri, G., Morantte, I., Guzman, E., Asahara, H., Herzig, S., Anderson, S. D., Yates, J. R., III, and Montminy, M. (2003) Nat. Struct. Biol. 10 175-181 [DOI] [PubMed] [Google Scholar]
- 31.Ceulemans, H., and Bollen, M. (2004) Physiol. Rev. 84 1-39 [DOI] [PubMed] [Google Scholar]