Abstract
Effects of the direct NMDA agonist (tetrazol-5-yl)glycine (TZG) were examined in a genetic mouse model of reduced NMDA receptor function. In this model, expression of the NR1 subunit is reduced but not eliminated and the mice are therefore designated as NR1 hypomorphic. Previous work suggested that the reduced NR1 subunit expression produced a functional subsensitivity as judged by a blunted Fos induction response to a sub-seizure dose of TZG. In the present study seizure threshold doses of TZG were tested in the wild type and mutant mice. Surprisingly, there was no difference in the seizure sensitivity between the wild type mice and mice presumed to express very low levels of the NR1 subunit. An extensive neuroanatomical analysis of Fos induction was conducted after the threshold seizure doses of TZG. The results demonstrate that some brain regions of the NR1 -/- mice exhibit much lower Fos induction in comparison to the NR1 +/+ mice. These regions include hippocampus, amygdala, and cerebral cortical regions. However, in other regions, similar induction of Fos was observed in both genotypes in response to the NMDA agonist. Regions showing similar Fos induction in the NR1 +/+ and NR1 -/- mice include the lateral septum, nucleus of the solitary tract, and medial hypothalamic regions. The results suggest that the NMDA receptor hypofunction in the NR1 -/- mice is not global but regionally specific and that subcortical structures are responsible for the seizure-inducing effects of TZG.
Section 3
Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
Keywords: NMDA receptor, Fos, hippocampus, hypothalamus, brain stem, seizures
1. Introduction
The psychotomimetic actions of NMDA antagonists suggest that endogenously reduced NMDA hypofunction could contribute to the pathophysiology of schizophrenia (Javitt and Zukin, 1991; Krystal et al., 1994; Lahti et al., 1995; Olney and Farber, 1995; Vollenweider et al., 1997; Lahti et al., 2001). A mouse line expressing low levels of the NR1 subunit of the NMDA receptor has been developed that provides a model of endogenous NMDA receptor hypofunction (Mohn et al., 1999). This mutant line was created by insertion of a neomyosin resistance gene into an intron of the NR1 locus, which results in marked under-expression of the gene. The mice are referred to as NR1 hypomorphic since expression of the gene is reduced but not eliminated. Estimates of the magnitude of the reduced NR1 expression vary between 90%-70% depending on the assessment method and brain region examined (Mohn et al., 1999; Duncan et al., 2002).
The NR hypomorphic mice (NR1 -/-) exhibit a number of phenotypes that support their utility to model certain characteristics of schizophrenia patients. These altered phenotypes include reduced locomotor habituation (Mohn et al., 1999), reduced metabolic activity in the anterior cingulate cortex and the hippocampus (Duncan et al., 2002), deficits in social interactions (Mohn et al., 1999; Duncan et al., 2004), and deficits in prepulse inhibition of acoustic startle (PPI) (Duncan et al., 2004; Fradley et al., 2005; Duncan et al., 2006). In addition, the NR1 -/- mice show enhanced sensitivity to amphetamine-induced PPI disruption (Moy et al., 2006) but not to the locomotor stimulatory effects of amphetamine (Miyamoto et al., 2004).
Previous work indicated that the reduced expression of the NR1 subunit in the NR1 -/- mice is associated with reduced functional activation of the NMDA receptor (Inada et al., 2007). In wild type mice, a sub-seizure dose of the direct NMDA agonist (tetrazol-5-yl)glycine (TZG) induced Fos protein in a limited and neuroanatomically selective manner (Inada et al., 2007). TZG is a highly selective and potent direct NMDA agonist that readily penetrates the blood brain barrier(Schoepp et al., 1991). The most robust induction of the protein after TZG was found in the hippocampal cellular layers (CA1-CA4) and in the granule cell layer of the dentate gyrus. In the NR1 -/- mice, TZG-induced Fos was greatly attenuated in all regions examined (Inada et al., 2007).
The 0.75 mg/kg dose of TZG tested in NR1 -/- and NR1 +/+ mice in the previous study (Inada et al., 2007) did not induce any behavioral signs of seizure activity in either genotype. However, TZG can induce tonic-clonic seizures and exhibits a very steep dose response for seizure induction (Schoepp et al., 1991; Lunn et al., 1992). Consitent with the study of Inada et al. (2007), Schoepp et al. (1991) found that a TZG dose of 0.75 mg/kg did not induce seizures in any mice tested. However, at 1.25 mg/kg, TZG induced tonic-clonic convulsions in approximately 30% of mice and at 2.5 mg/kg seizures were induced in 100% of the animals (Schoepp et al., 1991). TZG-induced tonic-clonic seizures were lethal in all mice. In the present study, TZG was given in threshold seizure doses of 1.25 and 1.5 mg/kg to wild type and NR1 deficient mice to determine if the mutant mice would exhibit altered seizure sensitivity to this direct NMDA receptor agonist.
2. Results
2.1 Behavior
An initial experiment tested TZG at 1.5 mg/kg in 5 NR1 +/+ and 5 NR1 -/- mice. For both genotypes, 3/5 mice in each group exhibited tonic clonic seizures 10-15 minutes after injection of 1.5 mg/kg TZG (Table 1). The seizures were lethal in all cases. The mice that did not show tonic-clonic convulsive activity at the 1.5 mg/kg dose of TZG displayed some locomotor hyperactivity but no obvious seizure activity.
Table 1.
Number of mice exhibiting seizures in NR1 +/+ and NR1 -/- genotypes after injection of TZG. Numerator values are the number of mice that exhibited tonic-clonic seizures and denominators are the total number of mice tested. Mice that did not exhibit tonic-clonic seizures expressed no obvious form of seizure activity. All tonic-clonic seizures were fatal within 1 minute or less after initiation
| Treatment | NR1 +/+ | NR1 -/- |
|---|---|---|
| Saline | 0/6 | 0/7 |
| TZG 1.25 mg/kg | 3/19 | 5/14 |
| TZG 1.5 mg/kg | 3/5 | 3/5 |
For the 1.25 mg/kg dose of TZG, 3/19 of the NR1 +/+ mice and 5/14 of the NR1 -/- mice exhibited lethal tonic-clonic seizures within 15 minutes after injection (Table 1). For mice that did not exhibit tonic-clonic seizures at the 1.25 mg/kg TZG dose, there was considerable variability of behavioral responses for both NR1 +/+ and NR1 -/- genotypes. Some mice showed no obvious behavioral effects and others showed modest locomotor hyperactivity.
A two-by-two logistic regression for the TZG treated mice showed a significant dose effect (P < .05) indicating that the two doses produced different proportions of seizures. There was no significant effect of genotype (P<0.48) and a non-significant interaction effect (P<0.48).
2.2 Induction of Fos Protein
TZG induced robust induction of Fos in select brain regions. In some regions the Fos response was markedly blunted in the NR1 -/- mice (Figure 1 A). However, in other regions the induction of Fos in the mutant mice in response to TZG was comparable to the wild type controls (Figure 1 B). Photomicrographs of sections immunocytochemically stained for Fos are shown in Figures 2-5 and demonstrate the striking differences and similarities in Fos staining in specific regions between the NR1 +/+ and NR1-/- mice.
Figure 1.
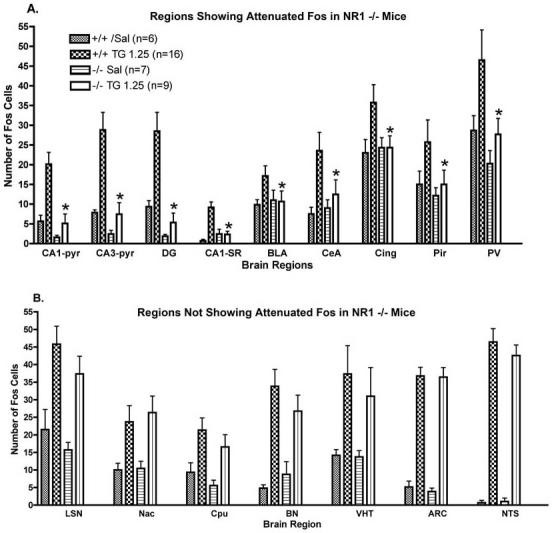
Counts of Fos immunoreactive cells in NR1 +/+ and NR1 -/- mice after administration TZG (1.25 mg/kg). Data are mean ± SEM and the number of mice in each group is indicated in the top panel. Mice were injected i.p. with TZG and perfused 90 minutes latter. Abbreviations: CA1-P, hippocampus CA1 pyramidal cell layer; CA3-P, hippocampus CA3 pyramidal cell layer; DG, granule cell layer of dentate gyrus; CA1-SR, CA1 stratum radiatum; BLA, basolateral nucleus of amygdala, CeA, central nucleus of amygdala; Cing, cingulate cortex; Pir, pyriform cortex; PV paraventricular nucleus of thalamus; LSN, lateral septal nucleus; Cpu, caudate putamen; BN bed nucleus of stria terminalis; VHT, hypothalamus ventral medial nucleus; ARC; hypothalamic arcuate nucleus.
* Significantly different from NR1 +/+ group, p < .05.
Figure 2.
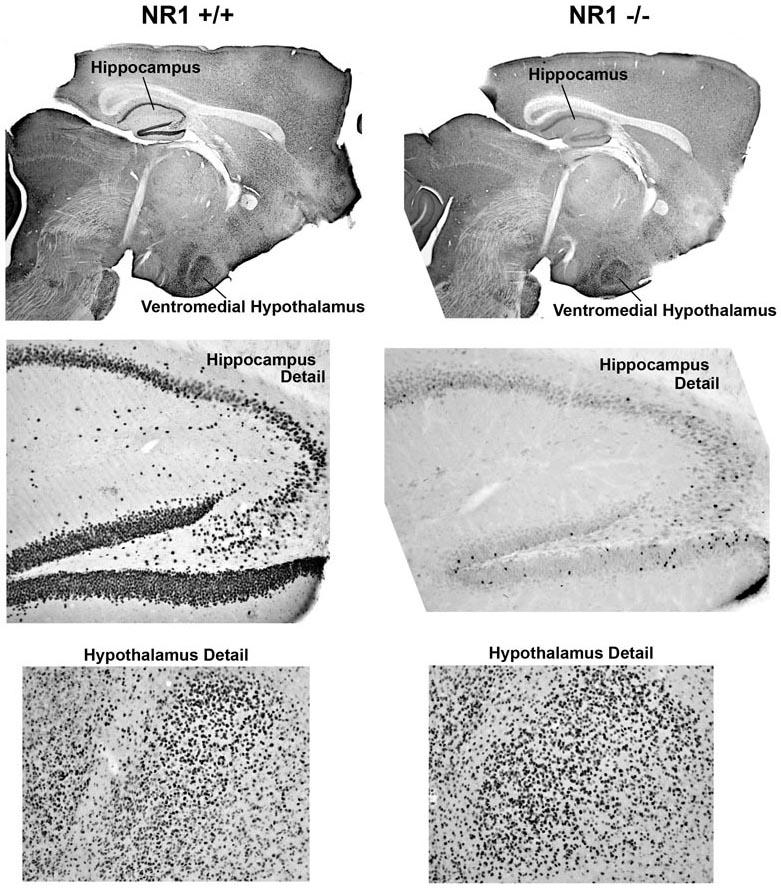
Sections immunocytochemically stained for Fos from NR1 +/+ and NR1 -/- mice after injection of TZG (1.5 mg/kg).
Figure 5.
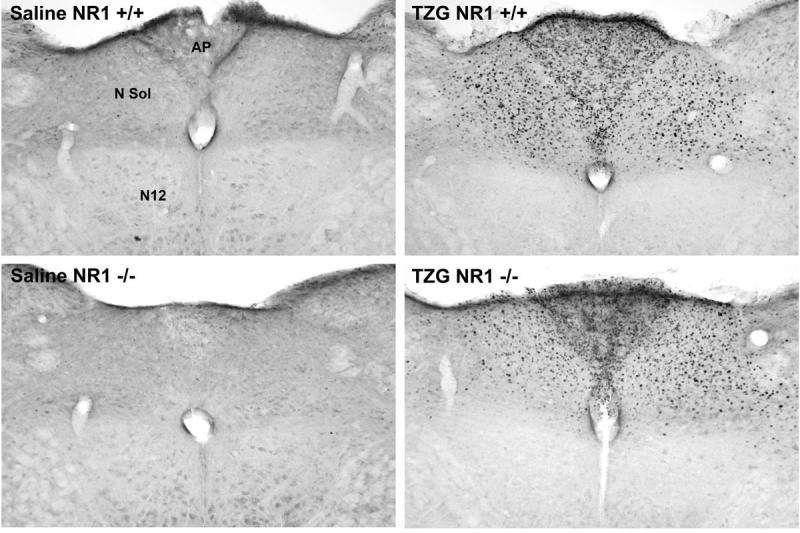
Sections immunocytochemically stained for Fos of the brain stem for NR1 +/+ and NR1 -/- mice after injection of saline or TZG. Abbreviations: AP, area postrema; N Sol, nucleus of the solitary tract; N12, motor nucleus of the 12th cranial nerve.
In NR1 +/+ mice robust induction of Fos by TZG was found in the hippocampal formation, hypothalamus, lateral septum, nucleus accumbens, and nucleus of the solitary tract. Within the hypothalamus the response was regionally selective with the most intense staining seen in the dorsal medial, ventromedial nuclei and arcuate nucleus.
In the NR1 -/- mice, induction of Fos was markedly attenuated in comparison to the NR1 +/+ in the hippocampal formation, cerebral cortical regions, and amygdala after administration TZG (Figure 1 A). However, for the NR -/- mice, as observed for the NR1 +/+ mice, robust induction of Fos was found in the lateral septal nucleus, sub-regions of the hypothalamus, bed nucleus of the stria terminalis, nucleus accumbens, and nucleus of the solitary tract (Figure 1 B).
3. Discussion
The NR1 hypomorphic mice developed by Mohn et al. (1999) provide a valuable model to study endogenous NMDA receptor hypofunction. Autoradiographic assessment of regional 3H-MK-801 binding in the mutant mice suggested a global reduction in ligand binding with 85% reductions found in the hippocampus and approximately 70% reductions in other brain regions examined (Duncan et al., 2002). Previous work indicated that the reduced NR1 subunit expression was accompanied by reduced functional sensitivity of the NMDA receptor, as judged by a markedly blunted response to a sub-seizure dose of TZG (0.75 mg/kg) (Inada et al., 2007).
In the present study there was surprisingly no reduction in seizure responses to threshold seizure doses of TZG in the NR1 -/- mice compared to wild type controls. With regard to Fos, induction of the protein by the threshold seizure doses of TZG was markedly reduced in the hippocampus, amygdala, and cerebral cortex in the NR1 -/- mice. However, many subcortical structures showed equivalent expression of TZG-induced Fos in the mutant and control mice. These data indicate that the NMDA receptor hypofunction in the NR1 -/- mice is not global but regionally specific and suggest that subcortical structures are responsible for seizures induced by the NMDA agonist.
It was not possible to examine Fos expression in the mice that exhibited tonic-clonic convulsions because those animals died within 15 min after injection of TZG. There was thus insufficient time to allow synthesis of Fos protein in those animals. It is possible that additional brain regions not identified in the Fos mapping studies were involved in mice that responded to TZG with convulsive activity. However the specific neuroanatomical regions activated by TZG at the threshold seizure doses suggest that those regions could be involved in the initiation of the seizures.
Previous work that studied effects of amphetamine on Fos induction in the NR1 -/- and NR1 +/+ mice showed similar activation in subcortical structures for both genotypes (Miyamoto et al., 2004). For example, the caudate putamen and nucleus accumbens showed similar intense Fos expression in the NR1 -/- and NR1 +/+ mice in response to amphetamine. In contrast, in cerebral cortical regions exhibited greatly reduced Fos responses after amphetamine administration in the NR1 -/- mice in comparison to controls. Since NMDA receptor activation has been implicated in the Fos response to amphetamine in the striatum (Snyderkeller, 1991; Ohno et al., 1994; Konradi et al., 1996), the findings of Miyamoto et al.(Miyamoto et al., 2004) are consistent with the interpretation of the present study that NMDA hypofunction in the NR1 -/- mice is neuroanatomically selective.
The most prominently activated subcortical regions by TZG were medial hypothalamic structures, nucleus of the solitary tract, and lateral septum. Induction of Fos by systemic administration of NMDA in those brain regions has also been reported by other investigators (Knapp et al., 2001; Velisek et al., 2007). Furthermore, induction of seizures with PTZ and electroshock induces a massive Fos response in the VMH and nucleus of the solitary tract (Kanter et al., 1996; Andre et al., 1998; Barton et al., 2001; Eells et al., 2004). Additional support for a role of the VMH in seizures is that lesioning the region disrupts propagation of seizure activity from the forebrain to the brain stem (Ferland and Applegate, 1998). Further work is necessary to determine if seizures could be induced by direct application of NMDA agonists to the hypothalamus or other structures activated by systemic administration of TZG.
Stimulation of the nucleus of the solitary tract via vagal nerve stimulation (VNS) has been shown to suppress seizure activity in experimental animals and in humans (for reviews see (Cohen-Gadol et al., 2003; Vonck et al., 2004). It is therefore curious that this region was prominently activated by TZG in the present work and by NMDA in the study of Velisek et al. (2007) in infant rats. The induction of Fos in the nucleus of the solitary tract in response to NMDA agonists raises the possibility that the region could participate in the induction of tonic-clonic seizures. Behavioral assessment after site injection of TZG into this brainstem nucleus could test that possibility.
The neurobiological basis for therapeutic effects of VNS are unknown. Current hypotheses propose that VNS activates neurons in the nucleus of the solitary tract that project to forebrain structures to produce the therapeutic effects (Groves and Brown, 2005; Nemeroff et al., 2006). However, if the NTS is directly involved in seizure induction by NMDA agonists (or in epilepsy) an alternative mechanism could be disruption of seizure-related activity directly in the region. NMDA receptor antagonists are highly effective in animal models of epilepsy but are not used in humans because they induce psychotomimetic effects (Dingledine et al., 1990; Klitgaard, 2005). If NMDA receptors in hypothalamic and brain stem regions are directly involved in genesis of seizures, NMDA antagonists that selectively target such regions could be effective antiepileptic agents with out producing psychotomimetic side effects that limit the use of currently available drugs that block NMDA receptors.
4. Experimental Procedure
4.1 Animals
The NR1 deficient mice were generated by incorporating a neomysin resistance gene in to an intron of the NR1 locus. The initial mutation was inserted in mice of a mixed genetic background consisting of alleles derived from 129/SvEv, C57BL/6, and DBA/2 (Mohn et al., 1999). This insertion mutation greatly reduces expression of the NR1 gene in all brain regions thus far examined. Modifier alleles present in various inbred mouse lines can dramatically alter the impact of primary genetic lesions. Therefore, to obtain populations of mice that differ genetically only at the NR1 locus, a strategy was devised to generate NR1 hypomorphic mice and genetically identical wild type populations. C57BL/6 heterozygous animals were intercrossed with 129-SvEv heterozygous animals. All of the F1 offspring of these litters are genetically identical at all loci except at the NR1 gene. The 129-SvEv mice were used as moms and were co-isogenic. C57BL/6 mice were backcrossed > 14 generations from C57BL/6 mice purchased from Jackson Laboratories. Analysis of the F1 hybrid litters showed that mice homozygous for the mutation were present at the expected frequency and, furthermore, that these NR1 hypomorphic mice were very close in size to the wild type littermates. The NR1 mutation was maintained on the C57BL/6 and 129/SvEv genetic backgrounds by breeding heterozygous animals. Resulting heterozygous offspring from these crosses were used to maintain the lines and to provide heterozygous breeders for the generation of the F1 hybrid homozygous mice (NR1 -/-) and their control populations (NR1 +/+).
Both male and female mice were used in these studies. Genders were balanced to the extent possible among the treatments to obtain approximately equal numbers of male and female mice in each group. The sample size available for study was not sufficient to test for gender effects in a statistically meaningful way but no obvious gender differences were apparent in the behavioral activation or Fos induction in response to TZG.
4.2 Drug Treatments
Mice were injected i.p with D,L-(tetrazol-5-yl)glycine (TZG) [Tocris] (dissolved in saline) at doses of 1.25 or 1.5 mg/kg. Mice were perfused for immunocytochemistry (see below) 90 minutes after the injections. The doses were chosen from preliminary studies to be threshold seizure-inducing. The survival period was chosen because it is near the peak of the Fos protein expression found in previous work (Inada et al., 2007).
4.3 Immunohistochemistry
Immunocytochemical procedures were performed according to the previously published protocols (Duncan et al., 1993; Miyamoto et al., 2004). Mice were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and then perfused through the left cardiac ventricle with ice-cold 100mM sodium phosphate-buffered saline (PBS, pH=7.4) for 1 min, at a rate of 3ml/min, followed by 4% paraformaldehyde for 3 min at the same rate of perfusion. The brains were removed after perfusion and fixed with 4% paraformaldehyde overnight. Coronal sections (50 μm) were cut from each brain using a vibratome and placed in PBS. Sections were treated with 5% normal goat serum (Vector Laboratories, Burlingame, CA) and 0.1% Triton X-100 in PBS for 30 min. and then were incubated for 72 hours at 4 degrees C with a Fos antibody [gift from Dr. Peter Petruz]. After incubation with the Fos antibody, sections were incubated for 1h with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA). Sections were then incubated with avidin-biotin complex [Vecstain Elite ABC kit; Vector Laboratories] for 1h. Sections were then placed in a solution containing 0.05% 3,3′-diamino-benzidene tetrahydrochloride, 0.005% cobalt chloride, 0.008% nickel ammonium sulfate, and 0.02% hydrogen peroxide.
4.4 Quantification of Fos expression
Cells exhibiting nuclear staining for Fos in selected brain regions were counted at a magnification of 200× by an experimenter blind to the treatment group. Four treatment groups were evaluated: NR1 +/+ saline (n=6), NR1 +/+ TZG 1.25 mg/kg (n=16), NR1 -/- saline (n=7), and NR1 -/- TZG 1.25 mg/kg (n=9). From preliminary studies, regions of interest were chosen based on consistent and robust induction of Fos in response to TZG. The location of the areas for counting within each brain region were guided by the atlas of Franklin and Paxinos (Franklin and Paxinos, 1997). Counts were taken bilaterally in two sections per mouse for each region. For the hippocampal sub-regions, arcuate nucleus, and nucleus of the solitary tract, cells were counted in 50×250 μm, 100×100 μm, and 100×200 μm areas, respectively, due to the small size of the regions. For other regions cell counts were made in 250×500 μm fields. Since only 2 mice in each genotype survived for the 1.5 mg/kg dose, these mice were not included in the quantitative analysis but were processed for immunocytochemistry and qualitative analysis.
4.5 Statistical Analyses
Analysis of Variance (ANOVA) was used to assess treatment effects for each brain region. When the ANOVAs indicated a significant group effect for a brain region (p < .05), post-hoc pair-wise comparisons were made between the individual treatment groups using Tukey’s multiple comparison tests. For data on the proportions of NR1 +/+ and NR1 -/- mice exhibiting seizures we performed a two-by-two factorial logistic regression analysis to examine effect of treatment and genotype on proportion of mice that seized after the 2 doses of TZG (1.25 and 1.5 mg/kg). Because both cells in the saline condition had zero mice with seizures, including those cells in the logistic regression was problematic computationally and those groups were not included in the analysis.
Figure 3.
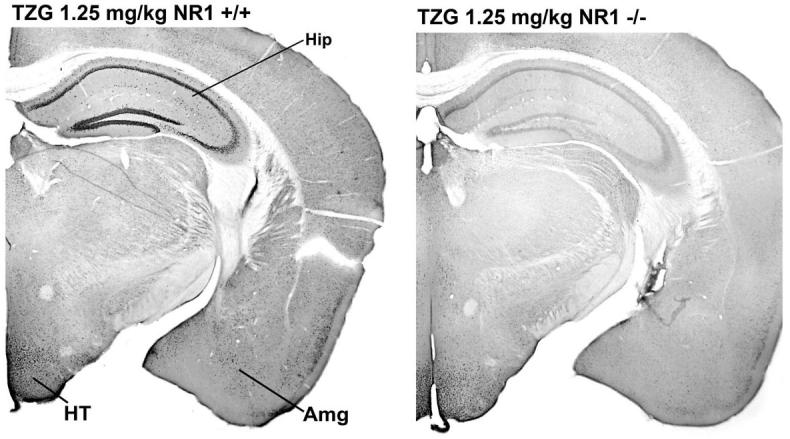
Sections immunocytochemically stained for Fos from NR1 +/+ and NR1 -/- mice after injection of TZG (1.25 mg/kg). Abbreviations: Hip, hippocampus; HT, hypothalamus; Amg, amygdala. Higher magnification of the hypothalamic region is shown in Figure 4.
Figure 4.
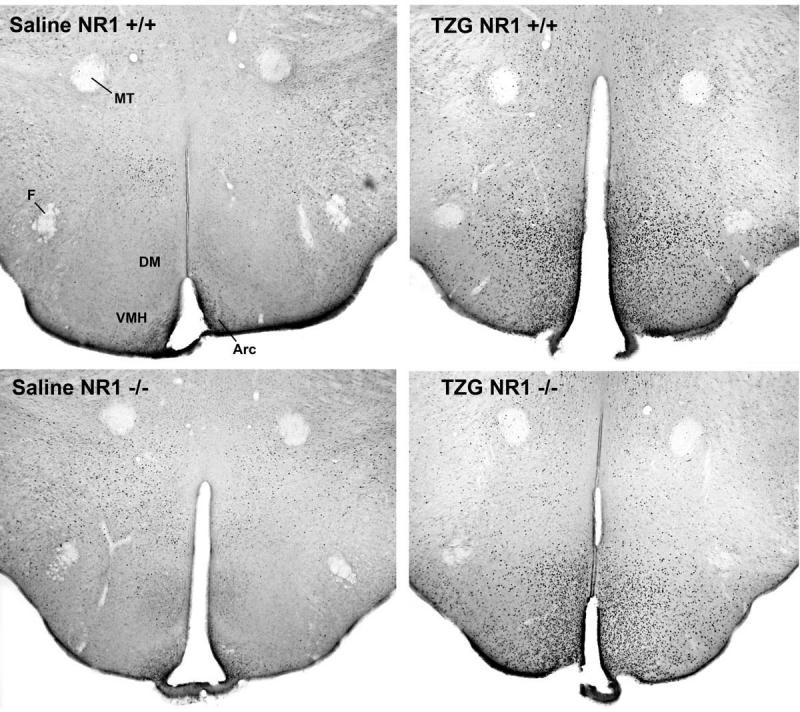
Sections immunocytochemically stained for Fos in the hypothalamus for NR1 +/+ and NR1 -/- mice after injection of saline or TZG. Abbreviations: MT, mammalothalamic tract; DM, dorsomedial nucleus; VMH, ventromedial nucleus; Arc, arcuate nucleus.
Acknowledgements
This research was supported by MH063398 from NIMH. We thank Dr. Robert Hamer for statistical consultation.
References
- Andre V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. European Journal of Neuroscience. 1998;10:2094–2106. doi: 10.1046/j.1460-9568.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, Steve W. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Research. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Brffton JW, Wetjen NM, Marsh WR, Meyer FB, Raffel C. Neurostimulation therapy for epilepsy: Current modalities and future directions. Mayo Clinic Proceedings. 2003;78:238–248. doi: 10.4065/78.2.238. [DOI] [PubMed] [Google Scholar]
- Dingledine R, McBain CJ, McNamara JO. Excitatory Amino-Acid Receptors in Epilepsy. Trends in Pharmacological Sciences. 1990;11:334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GRT. Topographic patterns of brain activity in response to swim stress: Assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J.Neuroscience. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Gu HB, Lieberman JA, Koller BH, Snouwaert JN. Alterations in regional brain metabolism in genetic and pharmacological models of reduced NMDA receptor function. Brain Research. 2002;951:166–176. doi: 10.1016/s0006-8993(02)03156-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav.Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Eells JB, Clough RW, Browning RA, Jobe PC. Comparative Fos immunoreactivity in the brain after forebrain, brainstem, or combined seizures induced by electroshock, pentylenetetrazol, focally induced and audiogenic seizures in rats. Neuroscience. 2004;123:279–292. doi: 10.1016/j.neuroscience.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Applegate CD. The role of the ventromedial nucleus of the hypothalamus in epileptogenesis. Neuroreport. 1998;9:3623–3629. doi: 10.1097/00001756-199811160-00013. [DOI] [PubMed] [Google Scholar]
- Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behavioural Brain Research. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and Biobehavioral Reviews. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Inada K, Farrington JS, Moy SS, Koller BH, Duncan GE. Assessment of NMDA receptor activation in vivo by Fos induction after challenge with the direct NMDA agonist (tetrazol-5-yl)glycine: effects of clozapine and haloperidol. Journal of Neural Transmission. 2007;114:899–908. doi: 10.1007/s00702-007-0628-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kanter RK, Strauss JA, Sauro MD. Comparison of neurons in rat medulla oblongata with Fos immunoreactivity evoked by seizures, chemoreceptor, or baroreceptor stimulation. Neuroscience. 1996;73:807–816. doi: 10.1016/0306-4522(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Antiepileptic drug discovery: lessons from the past and future challenges. Acta Neurologica Scandinavica. 2005;112:68–72. doi: 10.1111/j.1600-0404.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcoholism: Clinical & Experimental Research. 2001;25:1662–1672. [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. Journal of Neuroscience. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara MB, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lunn WHW, Schoepp DD, Calligaro DO, Vasileff RT, Heinz LJ, Salhoff CR, Omalley PJ. Dl-Tetrazol-5-Ylglycine, a Highly Potent Nmda Agonist - Its Synthesis and Nmda Receptor Efficacy. Journal of Medicinal Chemistry. 1992;35:4608–4612. doi: 10.1021/jm00102a015. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Snouwaert JN, Koller BH, Moy SS, Lieberman JA, Duncan GE. Amphetamine-induced Fos is reduced in limbic cortical regions but not in the caudate or accumbens in a genetic model of NMDA receptor hypofunction. Neuropsychopharmacology. 2004;29:2180–2188. doi: 10.1038/sj.npp.1300548. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Research. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yoshida H, Watanabe S. Nmda Receptor-Mediated Expression of Fos Protein in the Rat Striatum Following Methamphetamine Administration - Relation to Behavioral Sensitization. Brain Research. 1994;665:135–140. doi: 10.1016/0006-8993(94)91163-0. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Archives of General Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Smith CL, Lodge D, Millar JD, Leander JD, Sacaan AI, Lunn WHW. D,L-(Tetrazol-5-Yl) Glycine - a Novel and Highly Potent Nmda Receptor Agonist. European Journal of Pharmacology. 1991;203:237–243. doi: 10.1016/0014-2999(91)90719-7. [DOI] [PubMed] [Google Scholar]
- Snyderkeller AM. Striatal C-Fos Induction by Drugs and Stress in Neonatally Dopamine-Depleted Rats Given Nigral Transplants - Importance of Nmda Activation and Relevance to Sensitization Phenomena. Experimental Neurology. 1991;113:155–165. doi: 10.1016/0014-4886(91)90171-8. [DOI] [PubMed] [Google Scholar]
- Velisek L, Jehle K, Asche S, Veliskova J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Annals of Neurology. 2007;61:109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) European Neuropsychopharmacology. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Vonck K, Thadani VY, Gilbert K, Dedeurwaerdere S, De Groote L, De Herdt V, Goossens L, Gossiaux F, Achten E, Thiery E, Vingerhoets G, Van Roost D, Caemaert J, De Reuck J, Roberts D, Williamson P, Boon P. Vagus nerve stimulation for refractory epilepsy: A transatlantic experience. Journal of Clinical Neurophysiology. 2004;21:283–289. doi: 10.1097/01.wnp.0000139654.32974.4e. [DOI] [PubMed] [Google Scholar]


