Abstract
Injection drug use and associated hepatitis C virus (HCV) and HIV infections are on the rise in Russia and the republics of the former Soviet Union. While small targeted studies have found widespread drug use and disease among at-risk populations, there have been few attempts to comprehensively evaluate the extent of these epidemics in general post-Soviet societies. We conducted a two-stage cluster randomized survey of the entire adult population of T'bilisi, Republic of Georgia and assessed the burden of HCV, HIV, and risk behaviors for blood-borne infections in 2,000 study participants. Of the 2,000 surveyed individuals, 162 (8.1%) had injected illicit drugs during their lifetimes. Of the individuals who had injected illicit drugs, 138 (85.2%) reported sharing needles with injection partners. HCV was found in 134 (6.7%) of the total surveyed population, but in 114 (70.4%) of those who had injected illicit drugs. We found HIV in only three (0.2%) individuals, all of whom had injected illicit drugs. Injection drug use and high-risk injection practices are very common in Georgia and may be harbingers of a large burden of HCV-associated liver diseases and a potentially serious HIV epidemic in the years to come.
Keywords: Hepatitis C virus, HIV, Injection drug use, Needle sharing, Republic of Georgia
Introduction
Current estimates suggest that about 2.2%, or approximately 140 million people worldwide are infected with the hepatitis C virus (HCV).1 Although the magnitude of the epidemic is well characterized in industrialized countries, little is known about the burden of disease in many developing countries, including in many of the republics of the former Soviet Union. Recent reports from Russia and other former Soviet republics have documented high but heterogeneous HCV prevalence rates among injection drug users (IDU) and other non-IDU groups2–10 suggesting the presence of an ongoing, but as yet poorly characterized, HCV epidemic.
While the scale of the HCV epidemic in much of the former Soviet Union remains to be elucidated, the epidemic of injection drug abuse, the principal risk factor for HCV infection, is being followed with great interest in the region. Since the breakup of the Soviet Union the number of individuals who are using injection drugs in its former republics has increased markedly11 resulting in widespread concern that large epidemics of HCV and HIV could follow in the wake of this drug use epidemic.12,13
The Republic of Georgia is situated south of the Russian Federation in the Caucasus region between the Black and Caspian Seas (see Fig. 1). Georgia has approximately 4.5 million citizens, a quarter of whom live in the capital city of T'bilisi. Though politically transformed by the recent ‘Rose Revolution,’ the Georgian economy remains stagnant, and healthcare resources are scarce.14
Figure 1.
Republic of Georgia.
The first epidemiologic window into the Georgian HCV situation came in a 1998 survey of 4,970 commercial blood donors.8 Of these individuals, 343 (6.9%) were positive for HCV antibody, but only three (0.06%) were HIV positive. Subsequently, the Georgian AIDS and Clinical Immunology Research Center surveyed 552 commercial blood donors9 and found an anti-HCV prevalence of 7.8%. This survey additionally found that 1.5% of surveyed donors reported lifetime injection drug use. While providing good assessments of both the HCV and HIV epidemics in Georgia, both of these surveys evaluated commercial blood donors, a group often noted to have higher rates of blood-borne pathogen infections than individuals in the general population.15–17
In the same time period, the Georgian AIDS and Clinical Immunology Research Center surveyed 926 high-risk injection drug users and found that 58.2% of them were HCV positive, but only 0.54% were HIV positive. The survey found that 72.9% of these high-risk individuals reported sharing needles.10
In this article, we report the results of a population-based cross-sectional survey of the adult population of T'bilisi that assessed the prevalence of HCV, HIV, and risk factors for blood-borne infections.
Materials and Methods
Population and Study Design
The goal of this study was to obtain a random sample of 2,000 adults willing to participate in our survey from the entire population of T'bilisi, a city of approximately 1.5 million people. In order to accomplish this goal, we utilized a two-stage cluster sample design, with explicit over sampling to account for participation refusals.
The city of T'bilisi is comprised of ten administrative districts, each of which has an official estimate of population size based on voter eligibility records. Within each administrative district are found hospitals and polyclinics (out-patient clinics) which serve as the principal effectors of a centrally organized health care system. Polyclinics serve small jurisdictions of city residents with primary care and hospital referrals, and maintain continuously updated rolls of individuals within their jurisdictions.
In order to implement the survey, we first calculated a target number of individuals to be sampled from each administrative district based on the relative population sizes of each district. We subsequently inflated the target number of individuals to be sampled by 20% to account for potential participation refusals and difficult to locate individuals. In the first sampling stage, we randomly selected three polyclinics from each administrative district. In the second sampling stage, we visited sampled polyclinics and randomly selected a number of individuals between the ages of 18 and 65 corresponding to a third of our inflated target number for each jurisdiction from polyclinic rolls of individuals within their jurisdictions. We noted names and addresses of sampled individuals from polyclinic rolls and prepared for the door-to-door phase of our survey.
Between October, 2001 and June, 2002, our survey team visited residences of sampled individuals in all ten Tbilisi districts. Our team asked for the sampled individual at each noted residence, and returned to the residence two times if the sampled individual was not available. Upon meeting sampled individuals, our team explained the purpose of the survey, and invited sampled individuals to participate. Potential study participants were informed of the strict measures undertaken by the survey team to maintain their confidentiality should they decide to participate. Individuals agreeing to participate in the survey were asked to sign an informed consent form, administered a behavioral questionnaire, and underwent venipuncture. The design and conduct of this study were approved by the Georgia HIV/AIDS Patients Support Foundation and Johns Hopkins Bloomberg School of Public Health institutional review boards prior to initiation.
Behavioral Questionnaire
Sampled individuals consenting to participate in the survey were asked if a private space was available in their residence for the administration of the behavioral questionnaire. If a private space was not available the person was asked to be accompanied to the National AIDS Center's ambulance car for questionnaire administration. Blood drawing for all cases was conducted at the individuals' apartments.
The survey questionnaire included questions related to individual demographics, as well as histories of invasive medical procedures, non-invasive medical procedures, blood transfusions, sexual behaviors, sexually transmitted infections (STIs), and injection drug use. Individuals who reported a history of injection drug use were asked additional questions regarding their age at first injection, whether they were injecting at present, and whether they shared needles and drug preparation equipment with others. The survey questionnaire also assessed whether the study participant was jaundiced at the time of the study interview. Jaundiced individuals were given referrals to medical care providers with viral hepatitis experience.
Laboratory Methods
Blood samples drawn from participating individuals were assayed for anti-HCV using an Ortho HCV 3.0 ELISA (Ortho Diagnostic Systems, New Jersey, USA). Blood samples from individuals with positive HCV ELISA results and from individuals who reported jaundice at the time of the study interview were additionally tested with a Chiron RIBA HCV 3.0 (Chiron Corp., California, USA). We defined individuals as HCV seropositive if they had both positive ELISA and RIBA test results, or if they reported jaundice at the time of the study interview and had positive RIBA test results. Blood samples drawn from participating individuals were also tested for anti-HIV using a Vironostika HIV Uni-Form II Ag/Ab Microelisa System (bioMerieux, Marcy L'Etoile, France). All laboratory testing was conducted at the AIDS and Clinical Immunology Research Center in T'bilisi.
All individuals who agreed to participate in this survey were provided with the results of their HCV and HIV test results approximately 3 weeks after venipuncture. Test result communication was conducted in a private space or in the National AIDS Center's ambulance car. Individuals were counseled as to the meaning of their test result, and HCV or HIV positive individuals were given referrals to medical care providers with viral hepatitis or HIV/AIDS experience.
Statistical Methods
We calculated descriptive statistics for laboratory results and questionnaire variables in the population as a whole, and among various population sub-strata. We used t-tests and asymptotic chi-square tests to test for differences between injection drug using and non-injection drug using populations. We used multiple logistic regression models to assess risk factors for HCV seropositivity in the population as a whole, and separately among individuals who did and did not indicate a history of injection drug use during the behavioral questionnaire. We grouped age into quartiles when age trends were themselves of interest, and included age as a continuous variable when included to control for confounding. We additionally included gender in our analyses of the total population and among individuals who did not indicate a history of injection drug use. All analyses were conducted using SAS 8.2 (SAS Institute, North Carolina, USA).
Reported Cases of HIV/AIDS in the Republic of Georgia
The Georgian AIDS and Clinical Immunology Research Center in T'bilisi is charged with monitoring and surveillance of the HIV/AIDS epidemic in the Republic of Georgia. HIV and AIDS, reportable diagnoses in the Republic of Georgia, are reported by health professionals to the Georgian AIDS and Clinical Immunology Research Center where they are archived for surveillance and policy purposes. We queried reported cases of HIV/AIDS through October, 2005 to produce a picture of the known scope of the Georgian HIV/AIDS epidemic.
Results
Survey Administration
Between October, 2001 and June, 2002 our survey team visited 2,200 residences of individuals selected by our two-stage cluster sampling mechanism. The selected individual could not be found at 74 visited residences, and 126 (5.9%) selected individuals who could be located at their residences refused to participate in our survey. A total of 2,000 individuals between the ages of 18 and 65 were administered the behavioral questionnaire and underwent venipuncture.
Questionnaire Results
Characteristics of the 2,000 study participants are listed in Table 1. Study participants were on average 39 years old and two thirds of them were unmarried. Seventy percent of participants had received non-invasive medical care such as dental work or obstetric and gynecological care, while less than 10% had undergone invasive medical procedures such as surgery. Only 2% of the surveyed population had received a blood transfusion. Despite an average of less than three lifetime sexual partners and regular condom use in a fifth of those surveyed, 10% of those surveyed reported STIs at some time in their lives. We additionally found that 8% of the 2,000 surveyed individuals had engaged in injection drug use at some point in their lifetimes.
Table 1.
Characteristics of the survey participants (N = 2,000)
| Characteristic | Number | Percenta |
|---|---|---|
| Male | 962 | 48.1 |
| Unmarried | 1356 | 67.8 |
| Regularly use condoms | 416 | 20.8 |
| Ever sexually transmitted infection (STI) | 212 | 10.6 |
| Non-invasive medical procedure | 1,402 | 70.1 |
| Invasive medical procedure | 165 | 8.3 |
| Blood transfusion | 46 | 2.3 |
| Ever injection drug use | 162 | 8.1 |
| Current jaundice | 11 | 0.6 |
| Mean | SDb | |
| Age | 39.1 | 12.7 |
| Number of lifetime sexual partners | 2.6 | 2.5 |
aPercentages are rounded to one decimal place.
bSD Standard deviation
Characteristics and injection drug use practices of the 162 individuals reporting injection drug use are listed in Table 2. Injection drug users (IDU) were almost exclusively male, were significantly younger (p < 0.01), had more sexual partners (p < 0.01), and were more likely to report a lifetime STI (p < 0.01) than individuals who never used injection drugs. In contrast, IDU were more likely to report regular use of condoms than non-IDU (p < 0.01). Ninety eight percent of individuals reporting lifetime injection drug use also reported current injection drug use, and 85% of the IDU also reported sharing needles with injection partners.
Table 2.
Characteristics of ever injection drug users (N = 162)
| Characteristic | Number | Percenta |
|---|---|---|
| Male | 160 | 98.8 |
| Unmarried | 117 | 72.2 |
| Regularly use condoms | 49 | 30.2 |
| Ever sexually transmitted infection (STI) | 41 | 25.3 |
| Non-invasive medical procedure | 57 | 35.2 |
| Invasive medical procedure | 15 | 9.3 |
| Blood transfusion | 2 | 1.2 |
| Using injection drugs at present | 158 | 97.5 |
| Age at first injection drug use | ||
| <21 | 52 | 32.7 |
| 21–25 | 75 | 46.3 |
| 26–30 | 29 | 17.9 |
| >30 | 6 | 3.7 |
| Ever shared needles with partners | 138 | 85.2 |
| Ever share drug preparation equipment with partners | 122 | 75.3 |
| Current jaundice | 2 | 1.2 |
| Mean | SDb | |
| Age | 29.7 | 7.2 |
| Number of lifetime sexual partners | 4.3 | 2.2 |
aPercentages are rounded to one decimal place.
bSD Standard deviation.
HCV and HIV Prevalence
In this survey, we found that 134 (6.7%) of the 2,000 surveyed individuals were HCV seropositive, but only three (0.15%) were HIV positive. Of the individuals who had ever injected drugs, we found that 114 (70.4%) of them were HCV seropositive, versus only 20 (1.1%) individuals who had never used injection drugs. All three HIV seropositive individuals reported using injection drugs during their lifetimes.
Risk Factors for HCV Seropositivity
Risk factors for HCV infection in the total population, and among individuals who had, and had not, used injection drugs are presented in Table 3. The risk of HCV seropositivity was significantly greater for men among both the total population and non-IDU groups. Risk of HCV seropositivity was significantly higher for IDU aged 25–28 compared to those aged 18–24. Among non-IDUs, the risk of HCV seropositivity did not differ significantly across quartiles of age. IDU who used condoms regularly were significantly less likely to be HCV seropositive, but experience with STIs was not associated with HCV seropositivity in either group. IDU who reported a moderate number of sexual partners were less likely to be HCV seropositive compared to those with few partners, and receipt of non-invasive medical procedures was associated with less risk of HCV seropositivity among IDU. Non-IDUs who had undergone invasive medical procedures or blood transfusions were at significantly greater risk of HCV seropositivity than those who had not.
Table 3.
Risk factors for HCV seropositivity among IDU, non-IDU, and in the total population
| IDU (N = 162) | Non-IDU (N = 1,838) | Total (N=2,000) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Male | –a | 3.9 (1.4, 10.9) | 22.40 (10.4, 48.2) |
| Ageb | |||
| Youngest quartile | Referent | Referent | Referent |
| Quartile 2 | 10.6 (2.8, 40.2) | 2.6 (0.7, 9.8) | 0.9 (0.6, 1.3) |
| Quartile 3 | 2.3 (0.9, 5.9) | 1.7 (0.4, 7.1) | 0.2 (0.1, 0.3) |
| Oldest quartile | 1.2 (0.5, 2.9) | 1.2 (0.3, 5.3) | 0.1 (0.0, 0.2) |
| OR (95% CI)c | OR (95% CI)d | OR (95% CI)d | |
| Regular condom use | 0.1 (0.1, 0.3) | 1.4 (0.5, 4.0) | 0.4 (0.2, 0.6) |
| Ever sexually transmitted infection (STI) | 1.0 (0.5, 2.2) | 1.8 (0.6, 5.5) | 1.7 (1.1, 2.7) |
| Number lifetime sex partners | |||
| 0–3 | Referent | Referent | Referent |
| 4–6 | 0.4 (0.2, 0.8) | 0.4 (0.1, 1.7) | 0.7 (0.5, 1.1) |
| >6 | 0.8 (0.2, 2.9) | 3.2 (0.9, 11.7) | 1.7 (0.9, 3.3) |
| Non-invasive medical procedure | 0.2 (0.1, 0.4) | 0.9 (0.2, 3.7) | 0.3 (0.2, 0.5) |
| Invasive medical procedure | 0.6 (0.2, 1.7) | 3.1 (1.0, 9.4) | 1.1 (0.6, 2.1) |
| Ever blood transfusion | 0.5 (0.0, 8.1) | 6.2 (1.7, 23.1) | 1.6 (0.5, 4.8) |
| Ever injection drug use | – | – | 122.1 (62.9, 237.0) |
a98.8% (n = 160) of individuals who reported drug use were male.
bThe age quartiles for; IDU: (18–24, 25–28, 29–33, >33), non-IDU: (18–28, 29–40, 41–48, >48),and total population: (18–28, 29–39, 40–48, >48).
c OR(95%CI)=odds ratio, 95% confidence interval.
Reported Cases of HIV/AIDS in the Republic of Georgia
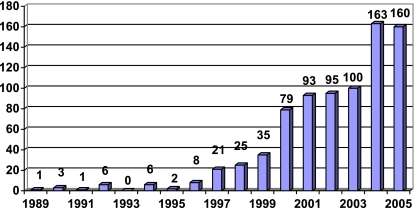
A histogram of the HIV/AIDS cases reported to the Georgian AIDS and Clinical Immunology Research Center as of October, 2005 is shown in Fig. 2. As seen in the figure, a total of 798 cases of HIV/AIDS have been officially reported in the Republic of Georgia. The figure shows an increasing trend of HIV/AIDS case reporting, and a likely record number of reported cases in 2005 given that the number of cases reported in the first 9 months of 2005 is equal to that reported in all of 2004.
Figure 2.
Cases of HIV/AIDS reported the Georgian AIDS and Clinical Immunology Research Center, 1989–October, 2005.
Discussion
Russia and the republics of the former Soviet Union are viewed with increasing concern as being in the early stages of potentially explosive HIV epidemics,18 and additionally at risk for widespread epidemics of HCV and other blood-borne pathogens. As is common in the early stages of any epidemic, these scenarios are largely derived from study of sentinel populations, and represent a best guess as to the experience of populations as a whole.19,20
In this report, we move beyond sentinel surveillance and give one of the most comprehensive pictures available in any former Soviet republic of HCV, HIV, and risk behaviors for blood borne infections in a general population. Our survey found a widespread epidemic of injection drug use among young men, and found that over two thirds of this drug using population was HCV seropositive. These men commonly reported needle and drug preparation equipment sharing, and moderately high numbers of sexual partners. Both the HCV and needle sharing prevalences seen in this survey among IDU were increased compared to an earlier study of high-risk IDU in Georgia.10
The prevalence of HIV seen in this survey, 0.15%, is within the range of estimates (0.1–0.4%) made by UNAIDS for Georgia at the end of 2003.21 Although the absolute number of individuals infected with HIV in Georgia was likely fairly low at the time of this survey, it is the rate of increase in the number of HIV infections in Georgia and the surrounding countries that is most worrisome (see Fig. 2).
We additionally found widespread experience with STIs in the total population despite a relatively low average number of sexual partners. A low proportion of all individuals surveyed reported regular condom use, but those with an IDU history reported more regular condom use than non-IDUs. This association disappeared however (data not shown) when this comparison was limited exclusively to men, suggesting that men were more likely to report regular condom use than women. This finding is of concern, and suggests that women in T'bilisi will be especially at risk should HIV spread significantly.
In contrast to these findings, our study found that non-IDU had a low overall prevalence of HCV and no identifiable HIV at the time of this survey. Among these individuals, the HCV epidemic is likely driven by nosocomial- and transfusion-associated factors, but the introduction in 1997 of screening for the general Georgian blood supply8,10 should help to minimize these risks in the future.
Although the sampling design used for this study minimized sampling biases to a great extent, the use of polyclinic rolls as a sampling frame meant we had no access to populations not represented on polyclinic rolls. These populations; the homeless, foreign workers, and the institutionalized may differ from the general T'bilisi population. Our study was additionally contingent on the validity of self-reported behaviors, and although we made every effort to assure participants of the confidentiality of their responses, fear of disclosure and social taboos may have stifled honesty in some study participants.
This report gives a comprehensive picture of HCV, HIV, and risk behaviors for blood-borne infections among the residents of Tbilisi, but the extent to which rural Georgians and residents of neighboring republics share this experience remains less well understood. The driving force behind the HCV epidemic seen in this survey, injection drug use, is generally on the rise in the former Soviet republics but is dependent on both opiate supply and the effectiveness of anti-drug governmental interventions within individual republics. Since Georgia and the Caucasus region remain key transit points for drug trafficking into Turkey and Russia,22 the epidemic of HCV is likely to spread in the region. The future HCV-associated heath care burden in Georgia and neighboring republics could be substantial.
Acknowledgements
We thank colleagues at the Georgian AIDS and Clinical Immunology Research Center for their hard work and dedication. This work is supported in part by the Biotechnology Engagement Program (BTEP) (G-616P to K.N.).
Footnotes
Stvilia, Tsertsvadze, Sharvadze, and Aladashvili are with the Georgian AIDS and Clinical Immunology Research Center, T'bilisi, Republic of Georgia; Rio is with the Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA; Kuniholm and Nelson are with the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe St., Baltimore, MD 21205, USA; Nelson is with the Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
References
- 1.Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. [DOI] [PubMed]
- 2.Abdala N, Carney JM, Durante AJ, et al. Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St Petersburg, Russia. Int J STD AIDS. 2003;14:697–703. [DOI] [PubMed]
- 3.Reshetnikov OV, Khryanin AA, Teinina TR, Krivenchuk NA, Zimina IY. Hepatitis B and C seroprevalence in Novosibirsk, western Siberia. Sex Transm Infect. 2001;77:463. [DOI] [PMC free article] [PubMed]
- 4.Mikhailov MI, Gomberg MA, Dolzhanskaya NA, Koubanova AA. Significance of sexual route of transmission of hepatitis B and C in Russia. Int J STD AIDS. 2002;13(Suppl 2):9–11. [DOI] [PubMed]
- 5.Ambrozaitis A, KS ZA, Balc IG, Widell A. Hepatitis C in Lithuania: incidence, prevalence, risk factors and viral genotypes. Clin Diagn Virol. 1995;4:273–284. [DOI] [PubMed]
- 6.Lvov DK, Samokhvalov EI, Tsuda F, et al. Prevalence of hepatitis C virus and distribution of its genotypes in Northern Eurasia. Arch Virol. 1996;141:1613–1622. [DOI] [PubMed]
- 7.Drobeniuc J, Hutin YJ, Harpaz R, et al. Prevalence of hepatitis B, D and C virus infections among children and pregnant women in Moldova: additional evidence supporting the need for routine hepatitis B vaccination of infants. Epidemiol Infect. 1999;123:463–467. [DOI] [PMC free article] [PubMed]
- 8.Butsashvili M, Tsertsvadze T, McNutt LA, Kamkamidze G, Gvetadze R, Badridze N. Prevalence of hepatitis B, hepatitis C, syphilis and HIV in Georgian blood donors. Eur J Epidemiol. 2001;17:693–695. [DOI] [PubMed]
- 9.Zaller N, Nelson KE, Aladashvili M, Badridze N, del Rio C, Tsertsvadze T. Risk factors for hepatitis C virus infection among blood donors in Georgia. Eur J Epidemiol. 2004;19:547–553. [DOI] [PubMed]
- 10.Tkeshelashvilli-Kessler A, del Rio C, Nelson K, Tsertsvadze T. The emerging HIV/AIDS epidemic in Georgia. Int J STD AIDS. 2005;16:61–67. [DOI] [PubMed]
- 11.Koshkina EA. The prevalence of the use of narcotics and other psychoactive substances in Russia today. Zh Mikrobiol Epidemiol Immunobiol. 2000;15–19. [PubMed]
- 12.Lowndes CM, Alary M, Platt L. Injection drug use, commercial sex work, and the HIV/STI epidemic in the Russian Federation. Sex Transm Dis. 2003;30:46–48. [DOI] [PubMed]
- 13.Kelly JA, Amirkhanian YA. The newest epidemic: a review of HIV/AIDS in Central and Eastern Europe. Int J STD AIDS. 2003;14:361–371. [DOI] [PubMed]
- 14.Skarbinski J, Walker HK, Baker LC, Kobaladze A, Kirtava Z, Raffin TA. The burden of out-of-pocket payments for health care in Tbilisi, Republic of Georgia. JAMA. 2002;287:1043–1049. [DOI] [PubMed]
- 15.Domen RE. Paid-versus-volunteer blood donation in the United States: a historical review. Transfus Med Rev. 1995;9:53–59. [DOI] [PubMed]
- 16.Strauss RG. Blood donations, safety, and incentives. Transfusion. 2001;41:165–167. [DOI] [PubMed]
- 17.van der Poel CL, Seifried E, Schaasberg WP. Paying for blood donations: still a risk? Vox Sang. 2002;83:285–293. [DOI] [PubMed]
- 18.Aris B. Russia confronts the threat of AIDS. The Lancet. 2004;364:2007–2008. [DOI] [PubMed]
- 19.Arita I, Nakane M, Kojima K, Yoshihara N, Nakano T, El Gohary A. Role of a sentinel surveillance system in the context of global surveillance of infectious diseases. Lancet Infect Dis. 2004;4:171–177. [DOI] [PMC free article] [PubMed]
- 20.Rehle T, Lazzari S, Dallabetta G, Asamoah-Odei E. Second-generation HIV surveillance: better data for decision-making. Bull World Health Organ. 2004;82:121–127. [PMC free article] [PubMed]
- 21.UNAIDS. Global HIV/AIDS and STD Surveillance: Epidemiological Factsheets. 11-23-2004.
- 22.Ryabochkin A. Azerbaijan remains the key territory for transit of drugs from Afganistan, the interior minister states. Turan news agency (Baku). November 25, 2004.